Abstract
Neurons and epithelia are viewed as fundamentally different cell types, yet some sensory neurons exhibit hallmarks of epithelial cells. For example, they use tight junctions to form a diffusion barrier continuous with the skin or other epithelia and they exhibit bona fide apical-basal polarity, with an outward-facing apical surface that is biochemically and functionally distinct from their inward-facing basolateral surface. Yet they are unmistakeably neurons with axon-dendrite polarity. Examples include olfactory receptor neurons and photoreceptors. In this review, I highlight how viewing these neurons as specialized epithelial cells informs our understanding of their development and raises intriguing questions about the establishment of apical-basal and axon-dendrite polarity.
A fundamental way that a cell self-organizes is by dividing its surface into compartments that express different proteins and carry out distinct functions. For example, an epithelial cell has an outward or lumen-facing (apical) surface that is biochemically and functionally distinct from its inward-facing (basolateral) surface (Fig. 1A). Likewise, in a neuron, the axon expresses different proteins and carries out distinct functions from the dendrites or soma (Fig. 1B). Indeed, epithelial cells and neurons have provided the main experimental systems for learning how cell polarity arises and how polarized sorting of proteins creates distinct surface compartments (Mellman and Nelson, 2008).
Fig. 1. Some sensory neurons exhibit both apical-basal and axon-dendrite polarity.
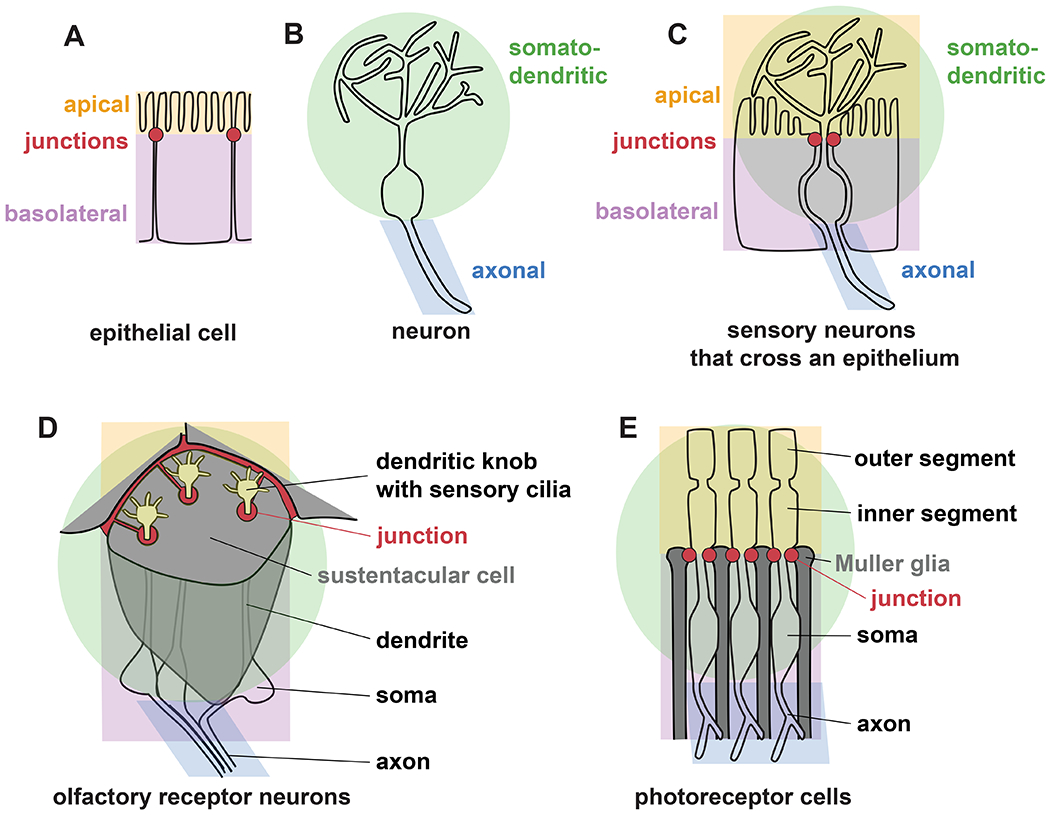
(A) Epithelial cells are polarized into distinct outward-facing (apical, orange) and inward-facing (basolateral, purple) surface compartments separated by tight and adherens junctions (red), while (B) most neurons are polarized into somato-dendritic (green) and axonal (blue) compartments. (C) Sensory neurons that cross an epithelium can exhibit apical-basal and axon-dendrite polarity simultaneously. (D) Olfactory receptor neurons protrude through an epithelium formed by glial-like sustentacular cells, with a dendritic knob and sensory cilia on the apical surface. (E) Photoreceptor cells protrude through an epithelium formed by Müller glia, with their apical domain (inner segment) and sensory cilium (outer segment) on the apical surface.
Interestingly, some sensory neurons protrude across an epithelium, implying that they may simultaneously possess apical-basal and axon-dendrite polarity (Fig. 1C). For example, mammalian olfactory receptor neurons have cell bodies that are positioned basally in the olfactory epithelium with dendrites that protrude to the external (apical) surface where they terminate in an enlarged dendritic knob bearing multiple sensory cilia (McEwen et al., 2008) (Fig. 1D). The dendrite endings are ensheathed by glial-like cells, called sustentacular cells, that form ring-shaped junctions surrounding each dendrite (Liang, 2020, 2018; Steinke et al., 2008). The junctions contain classical markers of epithelial tight junctions (claudins, occludin, and ZO1-3) and adherens junctions (E-cadherin and β-catenin) (Liang, 2020, 2018; Steinke et al., 2008). Together, these features suggest that the dendrite ending represents a functionally and biochemically distinct apical surface.
A similar arrangement is found in photoreceptors of the mammalian retina. The retinal surface, called the external (or outer) limiting membrane, is a continuous cellular sheet composed of photoreceptors and Müller glia connected through tight and adherens junctions that contain occludin, ZO1, E-cadherin and β-catenin (Mehalow, 2003; Omri et al., 2010) (Fig. 1E). The retinal surface can thus be viewed as the apical surface of an epithelium. Indeed, apical markers such as Crb1 localize there and, when disrupted, lead to retinal degeneration (Mehalow, 2003). Similar to olfactory neurons, photoreceptors protrude past the apical surface of the retina and terminate in an enlarged region, called the inner segment, that bears a sensory cilium, called the outer segment. Rather than being exposed to the external environment, the photoreceptor endings are sandwiched between the apical surfaces of the retina and another epithelium (the retinal pigmented epithelium), which together create a lumenal space that is topologically equivalent to the external environment.
In summary, mammalian olfactory neurons and photoreceptors each protrude across an epithelium and are wrapped by tight and adherens junctions, suggesting that they possess apical-basal polarity. At the same time, they are bona fide neurons with dendrites and long axons. This raises the questions of whether these cells simultaneously exhibit apical-basal and axon-dendrite polarity and how proteins are accurately sorted in such a multi-compartment cell.
Sensory neurons sequentially acquire apical-basal and axon-dendrite polarity
How does a developing sensory neuron acquire both epithelial and neuronal features? In the olfactory epithelium, new neurons are born throughout life at the basal surface, acquire their neuronal morphology first, and then extend dendrites that integrate into the existing epithelium (Cuschieri and Bannister, 1975; Rodriguez-Gil et al., 2015). As the growing dendrite tip extends toward the apical surface of the olfactory epithelium, it houses groups of migrating centrioles that will ultimately localize to the dendritic knob on the apical surface in order to nucleate sensory cilia (Ching et al., 2022), suggesting the nascent dendrite ending may already have some aspects of apical polarity. By contrast, photoreceptors form as part of the developing retinal neuroepithelium early in life. However, using organoid technology, human photoreceptors can be produced in vitro and transplanted into a mature mouse retina, where Müller glia wrap them and establish adherens junctions to them (Gasparini et al., 2022). The transplanted neurons proceed to develop neuronal morphology, including an axon and outer segment, only if they become incorporated into the epithelium (Gasparini et al., 2022), suggesting that establishing apical-basal polarity may be required to attain a mature neuronal morphology. Determining how apical-basal and axon-dendrite polarity are co-established will thus inform our fundamental understanding of development and may also guide strategies for cell replacement therapy in retinal disorders.
Lessons can be learned from invertebrate sensory neurons that protrude across an epithelium and exhibit both apical-basal and axon-dendrite polarity. The amphid is the major sense organ of C. elegans, consisting of 12 sensory neurons that extend unbranched dendrites to the nose tip, where they enter a narrow tube formed by two glial cells called the sheath and socket (Ward et al., 1975) (Fig. 2A). The dendrite endings protrude through the glial tube to directly contact the external environment (or, for four of the neurons, to terminate in a lumenal compartment of the sheath glia cell). In the mature structure, each neuron forms a ring-shaped tight junction with the sheath glial cell, which in turn forms a tight junction with the socket glial cell, which forms a tight junction to the skin (Low et al., 2019; Ward et al., 1975). Thus, despite being neurons and glia, these cells are part of a tube-shaped epithelium that is continuous with the skin, with tight junctions demarcating an outward-facing compartment (Fig. 2A). Indeed, proteins that localize to apical or basolateral compartments of other epithelial cells (e.g., the intestine) also localize respectively to the outward- or inward-facing surfaces of these neurons and glia, further showing that these cells exhibit apical-basal polarity (Low et al., 2019).
Fig. 2. Variants of canonical sorting motifs distinguish apical-basal and axon-dendrite sorting in C. elegans amphid neurons.
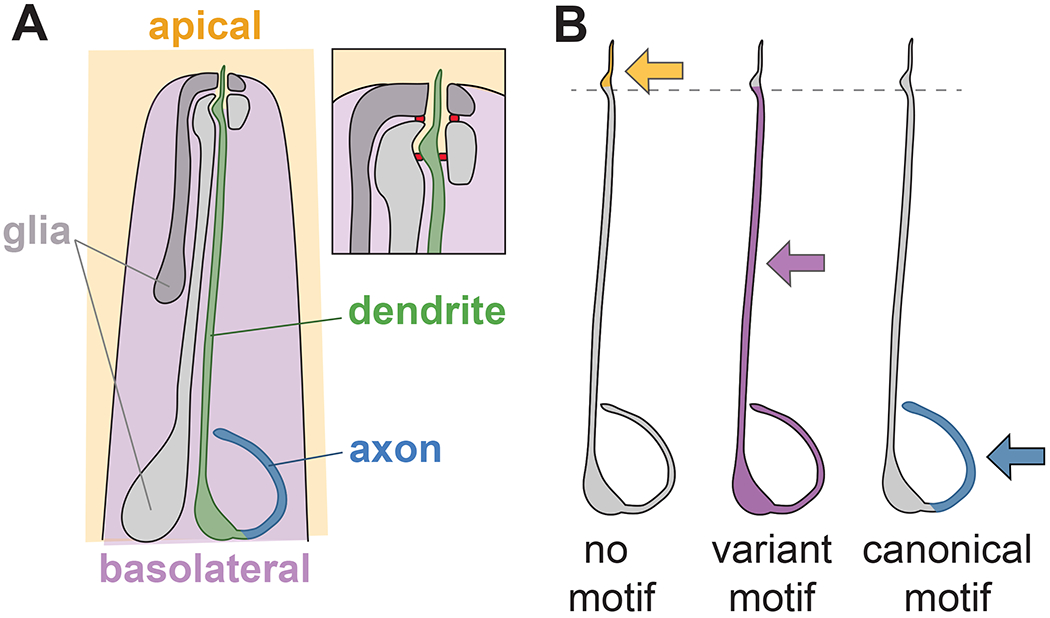
(A) Schematic of the C. elegans head showing one of the 12 amphid neurons and the two amphid glial cells. Inset shows detail of the nose tip, where the dendrite terminates in a sensory cilium that extends into the environment through a narrow epithelial tube formed by the two glia. Tight junctions (red) demarcate an outward-facing apical compartment (orange) from an inward-facing basolateral compartment (purple). The neuron’s axon, cell body, and most of its ~100 μm long dendrite face the basolateral compartment, while the distal ~5 μm of its dendrite and its sensory cilium face the apical compartment. (B) Minimal transmembrane proteins with no sorting motif localize apically (orange), those with variant dileucine-like or tyrosine motifs localize basolaterally (purple), and those with canonical dileucine or tyrosine motifs localize only to the axon (blue). Changing two amino acids in one of the variant sorting motifs (EREQGREPIL) is sufficient to re-direct it to three compartments: AA (no motif), apical; IL (variant motif), basolateral; LL (canonical motif), axon only.
We recently showed that dendrite morphogenesis depends on apical features at the dendrite ending, especially interactions with apical extracellular matrix (aECM) proteins such as DYF-7, DEX-1, and FBN-1 (Heiman and Shaham, 2009; Low et al., 2019; Mizeracka et al., unpublished). Early in development, the amphid neurons and glia assemble into a rosette with apical polarity markers such as PAR-6 localized to the rosette vertex (Fan et al., 2019). The cells then rearrange into an epithelial tube with an apical lumen that is lined with extracellular filaments visible by electron microscopy (Low et al., 2019). In the absence of DYF-7, the lumenal aECM filaments are absent and the glial tube fails to form or ruptures during morphogenesis (Low et al., 2019), reminiscent of defects seen upon disruption of lumenal aECM in other kinds of epithelial tubes (Sundaram and Cohen, 2017). When the tube ruptures, the sheath glial cell and nascent dendrite endings become detached from the socket glial cell and skin epithelium and fail to extend with the growing head, leading to severely shortened sensory dendrites that do not reach the nose tip (Heiman and Shaham, 2009; Low et al., 2019). Thus, the incorporation of these neurons within an epithelium is essential for proper dendrite morphogenesis.
The apical-basal polarity of sensory neurons can also directly instruct axon-dendrite polarization. Another C. elegans sensory neuron, PDE, develops post-embryonically from an epithelial precursor and inherits apical-basal polarity from the epithelium (Lee et al., 2021). In this case, apical proteins including PAR-6 are deposited at the outward-facing surface of the cell as the PDE neuron divides from its epithelial precursor (Lee et al., 2021). Following cell division, a centriole translocates in a dynein-dependent manner to the apical surface where it recruits GIP-2 and other γ-tubulin complex components to create a microtubule organizing center (MTOC) (Lee et al., 2021). The apical positioning of the MTOC in the newborn PDE neuron leads to the establishment of characteristically polarized microtubules in the dendrite and the formation of the sensory cilium at the dendrite ending (Lee et al., 2021). Thus, apical polarity cues inherited from its epithelial precursor establish the polarized dendritic features of the mature PDE neuron.
These examples illustrate two general principles by which apical-basal and axon-dendrite polarity can be coordinated: first, through apical cell-cell and cell-matrix interactions and, second, through apical localization of an MTOC driving overall microtubule polarity. Both of these may be at play in the polarization of photoreceptors and olfactory receptor neurons.
A single amino acid change can switch apical-basal to axon-dendrite sorting
The presence of both apical-basal and axon-dendrite polarity in a single cell raises the question of how proteins are correctly sorted among these compartments. In general, transmembrane proteins are directed to different parts of the cell surface by sequence motifs in their cytoplasmic tails that are recognized by trafficking proteins. In epithelial cells, canonical sequences for basolateral sorting include acidic dileucine motifs ([D/E]XXXL[L/I], where X is any amino acid) and tyrosine-based motifs (YXXΦ or NPXY) (Duffield et al., 2008). Remarkably, these motifs can often mediate axon-dendrite sorting in neurons as well, suggesting a surprising degree of overlap in the sorting motifs used by epithelial cells and neurons (Bentley and Banker, 2016). This poses a problem: if the same motifs direct apical-basal and axon-dendrite sorting, how can a sensory neuron simultaneously sort proteins among all of these compartments?
To address this question, we recently examined how a single amphid sensory neuron (ASER) sorts the cell adhesion molecule SAX-7 (Lillis et al., 2022), a conserved member of the L1 family of cell adhesion molecules that consists of a large extracellular adhesion module, a transmembrane segment, and a 91 amino acid (aa) cytoplasmic tail (Chen et al., 2001). SAX-7 is expressed in epithelia such as skin and also in many neurons, including amphid neurons, where it localizes to basolateral surfaces (Chen et al., 2001; Dong et al., 2013; Low et al., 2019; Salzberg et al., 2013). By replacing its extracellular or intracellular regions with GFP, we found that the SAX-7 cytoplasmic tail is necessary and sufficient for basolateral localization (Lillis et al., 2022). By contrast, apical localization occurs by default in the absence of any sorting motif.
Deletion analysis identified two motifs that are independently sufficient to drive basolateral localization of a synthetic minimal transmembrane protein (Lillis et al., 2022). The first motif, a 10-aa sequence, harbors the sequence EPIL which is reminiscent of EXXXLL basolateral sorting motifs. The dileucine-like IL motif is required for its basolateral sorting activity, as mutating IL>AA converts it to completely apical localization (Lillis et al., 2022) (Fig. 2B). Strikingly, replacing the EPIL motif with a canonical EXXXLL motif (ENVSLL) or “improving” it by mutating it to EPLL converts it to axon-only localization (Lillis et al., 2022) (Fig. 2B). Thus, a single amino acid change can switch this motif from apical-basal to axon-dendrite sorting. The second motif, a 19-aa sequence, harbors a tyrosine that is reminiscent of tyrosine-based YXXΦ or NPXY motifs. The tyrosine is important for basolateral sorting, as mutating Y>A converts it to a mixed apical-basal localization, whereas replacing it with a canonical basolateral YXXΦ motif (YQRL) converts it to axon-only localization (Lillis et al., 2022).
Thus, in these multi-compartment neurons, we find that apical-basal sorting is achieved through variants of the canonical dileucine- and tyrosine-based motifs used by epithelial cells. It is striking that even a single amino acid change can redirect cargo from apical-basal to axon-dendrite sorting (Fig. 2B). More broadly, many neurons divide their surfaces into multiple compartments with distinct protein expression and specialized functions, including distal and proximal dendrites, dendritic spines, the axon initial segment, and presynaptic active zones. Possibly, the ability to recognize and differentially localize such closely related sorting motifs could explain the evolution of diverse subcellular compartments in neurons.
In summary, while all neurons arise developmentally from a neuroepithelium, some sensory neurons retain epithelial features throughout life. These include vertebrate photoreceptors and olfactory neurons and many invertebrate neurons. The epithelial features of these cells inform our understanding of their development and function. First, during development, epithelial features play a critical role in polarized morphogenesis of sensory neurons. Second, the functions of mature sensory neurons rely on the ability to simultaneously maintain epithelial and neuronal features, such as apical-basal and axon-dendrite protein sorting. Understanding how this is achieved will reveal the relationship between epithelial and neuronal polarity and, perhaps, shed light on the origins of neuronal complexity.
ACKNOWLEDGMENTS
I thank Monique Lillis for helpful comments and discussion. I thank Dr. Yimin Zou, Dr. Huaye Zhang, and other participants in the 2020 American Society for Cell Biology minisymposium on Cell Polarity Signaling in Neurons, on which this review is based. This work is supported by NIH R01NS112343.
REFERENCES
- Bentley M, Banker G, 2016. The cellular mechanisms that maintain neuronal polarity. Nat Rev Neurosci 17, 611–622. 10.1038/nrn.2016.100 [DOI] [PubMed] [Google Scholar]
- Chen L, Ong B, Bennett V, 2001. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol 154, 841–855. 10.1083/jcb.200009004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching K, Wang JT, Stearns T, 2022. Long-range migration of centrioles to the apical surface of the olfactory epithelium. eLife 11, e74399. 10.7554/eLife.74399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri A, Bannister LH, 1975. The development of the olfactory mucosa in the mouse: electron microscopy. J Anat 119, 471–498. [PMC free article] [PubMed] [Google Scholar]
- Dong X, Liu OW, Howell AS, Shen K, 2013. An Extracellular Adhesion Molecule Complex Patterns Dendritic Branching and Morphogenesis. Cell 155, 296–307. 10.1016/j.cell.2013.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield A, Caplan MJ, Muth TR, 2008. Protein trafficking in polarized cells. Int Rev Cell Mol Biol 270, 145–179. 10.1016/S1937-6448(08)01404-4 [DOI] [PubMed] [Google Scholar]
- Fan L, Kovacevic I, Heiman MG, Bao Z, 2019. A multicellular rosette-mediated collective dendrite extension. Elife 8. 10.7554/eLife.38065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini SJ, Tessmer K, Reh M, Wieneke S, Carido M, Völkner M, Borsch O, Swiersy A, Zuzic M, Goureau O, Kurth T, Busskamp V, Zeck G, Karl MO, Ader M, 2022. Transplanted human cones incorporate and function in a murine cone degeneration model. Journal of Clinical Investigation. 10.1172/JCI154619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman MG, Shaham S, 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137, 344–355. 10.1016/j.cell.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Magescas J, Fetter RD, Feldman JL, Shen K, 2021. Inherited apicobasal polarity defines the key features of axon-dendrite polarity in a sensory neuron. Curr Biol 31, 3768–3783.e3. 10.1016/j.cub.2021.06.039 [DOI] [PubMed] [Google Scholar]
- Liang F, 2020. Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes 11, 493. 10.3390/genes11050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, 2018. Olfactory receptor neuronal dendrites become mostly intra-sustentacularly enwrapped upon maturity. J. Anat 232, 674–685. 10.1111/joa.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis M, Zaccardi NJ, Heiman MG, 2022. Axon-dendrite and apical-basolateral sorting in a single neuron. Genetics 221, iyac036. 10.1093/genetics/iyac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low IIC, Williams CR, Chong MK, McLachlan IG, Wierbowski BM, Kolotuev I, Heiman MG, 2019. Morphogenesis of neurons and glia within an epithelium. Development 146. 10.1242/dev.171124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DP, Jenkins PM, Martens JR, 2008. Chapter 12 Olfactory Cilia: Our Direct Neuronal Connection to the External World, in: Current Topics in Developmental Biology. Elsevier, pp. 333–370. 10.1016/S0070-2153(08)00812-0 [DOI] [PubMed] [Google Scholar]
- Mehalow AK, 2003. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Human Molecular Genetics 12, 2179–2189. 10.1093/hmg/ddg232 [DOI] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ, 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9, 833–845. 10.1038/nrm2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri S, Omri B, Savoldelli M, Jonet L, Thillaye-Goldenberg B, Thuret G, Gain P, Jeanny JC, Crisanti P, Behar-Cohen F, 2010. The outer limiting membrane (OLM) revisited: clinical implications. Clin Ophthalmol 4, 183–195. 10.2147/opth.s5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Bartel DL, Jaspers AW, Mobley AS, Imamura F, Greer CA, 2015. Odorant receptors regulate the final glomerular coalescence of olfactory sensory neuron axons. Proc. Natl. Acad. Sci. U.S.A 112, 5821–5826. 10.1073/pnas.1417955112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg Y, Díaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, Bülow HE, 2013. Skin-Derived Cues Control Arborization of Sensory Dendrites in Caenorhabditis elegans. Cell 155, 308–320. 10.1016/j.cell.2013.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke A, Meier-Stiegen S, Drenckhahn D, Asan E, 2008. Molecular composition of tight and adherens junctions in the rat olfactory epithelium and fila. Histochem Cell Biol 130, 339–361. 10.1007/s00418-008-0441-8 [DOI] [PubMed] [Google Scholar]
- Sundaram MV, Cohen JD, 2017. Time to make the doughnuts: Building and shaping seamless tubes. Semin Cell Dev Biol 67, 123–131. 10.1016/j.semcdb.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S, 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol 160, 313–337. 10.1002/cne.901600305 [DOI] [PubMed] [Google Scholar]


