Abstract
Purpose:
There is growing evidence that sub-symptom threshold aerobic exercise speeds recovery from sport-related concussion (SRC). It is not known whether there is a direct relationship between adherence to a personalized exercise prescription and recovery or if initial symptom burden affects adherence to the prescription.
Methods:
This study was a planned secondary analysis of one arm of a randomized controlled trial. Male and female adolescent athletes (aged 13–18 years) presenting within 10 days of SRC were given aerobic exercise prescriptions based on their heart rate threshold (HRt) at the point of exercise intolerance on a graded treadmill test. Adherence was determined objectively with HR monitors and compared against time to recovery. Participants who completed at least two-thirds of their aerobic exercise prescription were considered to be adherent.
Results:
Sixty-one percent of adolescents met adherence criterion. Those who were adherent were more symptomatic and were more exercise intolerant at their initial visit, yet they recovered faster than those who were not adherent (median recovery time 12 [Interquartile range [IQR] 9, 22] days versus 21.5 [IQR 13, 29.8] days, p = 0.016). On linear regression, adherence during week one was inversely related to recovery time (β = −0.002 [−0.003, 0.0], p=0.046) and to initial exercise tolerance (β = −0.886 [−1.385, −0.387], p <0.001), but not to initial symptom severity (β = 0.545 [−0.232, 1.323], p= 0.146). No adverse events or near misses were reported.
Conclusions:
Adherence to individualized sub-symptom threshold aerobic exercise within the first week of evaluation is associated with faster recovery from SRC. The data suggest that initial degree of exercise intolerance, but not initial symptom severity, affects adherence to aerobic exercise prescribed to adolescents within 10 days of SRC.
Keywords: CONCUSSION, ADOLESCENT, PEDIATRIC, EXERCISE, ADHERENCE, AEROBIC, SPORT
INTRODUCTION
Sport-related concussion (SRC) is a traumatic brain injury that is associated with physical, cognitive and emotional symptoms (1). It has been estimated that 1.1–1.9 million children and adolescents in the United States sustain SRC yearly (2). Patients who take longer than 4-weeks to recover are considered to have Persistent Post-Concussive Symptoms (PPCS) (3). PPCS has been associated with poor educational, social, and developmental outcomes in pediatric patients (4). The current body of literature supports the use of individualized aerobic exercise programs within 10 days of injury to speed recovery from SRC and to reduce the incidence of PPCS (5,6). Nevertheless, the efficacy of at-home exercise may depend upon the willingness of patients to follow their exercise prescription. It is therefore important to assess the role of adherence in order to understand the effect of aerobic exercise as a treatment for concussion in adolescent athletes.
An exercise prescription includes the intensity, duration, and frequency of activity, which must provide a sufficient stimulus to evoke positive adaptations (7). Adherence to an exercise intervention is generally defined as completing at least two-thirds of the prescribed volume (8,9). There is reason to believe that concussed individuals may be more likely to not adhere well to an exercise prescription since exercise reliably exacerbates concussion symptoms. (10) There is a lack of research regarding adherence to aerobic exercise prescriptions for the treatment of SRC, as well as a dearth of information regarding the influence adherence has on recovery from SRC. Initial visit symptom severity/burden (11) and initial degree of exercise intolerance (12–14) have been identified as strong predictors of recovery duration from SRC. Therefore, the aim of this study was to investigate the relationship of recovery after SRC to aerobic exercise prescription adherence. We hypothesized that participants who adhered to their aerobic exercise prescription would recover faster than participants who did not. We also hypothesized that those with greater initial symptom load and greater initial exercise intolerance would be less likely to adhere to the prescribed exercise program.
METHODS
Study Design
This study is a secondary analysis of the intervention arm of a 1:1 parallel, multi-center randomized clinical trial (RCT) that recruited from July 2018 to April 2020 (Clinicaltrials.gov: NCT02959216) in Buffalo, NY (University at Buffalo, UB), Philadelphia, PA (Children’s Hospital of Philadelphia, CHOP) and Boston, MA (Boston Children’s Hospital, BCH) (5). A common Institutional Review Board (SmartIRB) reviewed and approved the study. Participants were randomly assigned to either an individualized aerobic exercise program or a placebo-like stretching program designed to not elevate heart rate (HR) (5). Only participants randomized to the aerobic exercise arm are included in this secondary analysis. Participants returned to the office every week for up to 4 weeks from injury. Each week, until recovered, participants received a new training target HR based on reassessment of exercise tolerance on the Buffalo Concussion Treadmill Test (BCTT). If a participant did not recover by 4 weeks, a more comprehensive form of treatment was initiated. Additionally, all participants completed the Post-Concussion Symptom Inventory (PCSI) (15) at each visit and daily during the intervention using ecological momentary assessment via the LifeData mobile application platform called ReCoUPS (Recovering Concussion Update on Progression of Symptoms)(16). Participants were prompted to complete PCSI reports from 8:00 PM-12:00 AM to capture symptoms experienced that day.
Participants
Male and female adolescents (aged 13–18 years) presenting within 10 days of injury and diagnosed with SRC by an experienced sports medicine physician using standardized criteria were invited to participate (1). Participants were excluded if they had any of the following: 1) 3-point or less difference between current and pre-injury symptoms as measured by the PCSI (15); 2) moderate or severe TBI as indicated by a score < 13 on GCS, lesion on CT/MRI, and/or focal neurologic signs consistent with intracerebral lesion; 3) injury involving loss of consciousness for ≥ 30 minutes or post-traumatic amnesia for ≥ 24 hours; 4) inability to exercise on a treadmill because of lower-extremity orthopedic injury, clinically severe vestibular or visual dysfunction, or increased cardiac risk; 5) pre-existing comorbidities that prevented participation in active testing and/or rehabilitation; 6) history of more than 3 diagnosed concussions; 7) currently on medications that affect autonomic function, such as ADHD medication or mood stabilizers; 8) active substance abuse/dependence; 9) unwillingness to participate in research; and 10) limited English proficiency.
Exercise Tolerance Assessment and Prescription
Exercise tolerance was assessed weekly at each study on the BCTT, which has high inter-rater reliability and has been proven safe (6). The HR at exercise cessation due to concussion symptom exacerbation was recorded as the heart rate threshold (HRt). Participants were prescribed to perform at least 20 minutes of aerobic exercise of their choice (walking, jogging, stationary cycling) daily for six days out of seven, at 90% of the HRt (which was called their “target HR”). Participants were given a Polar OH1 monitor (Polar Electro Oy, Kempele, Finland) and instructed to record HR data during their home exercise sessions only. Participants were instructed to stop exercise if their symptoms increased by 2 or more points on a 1–10 point visual analog scale (VAS) when compared with their pre-exercise value. Exercise intolerance was defined as the inability to complete 15 minutes on the BCTT due to concussion symptom exacerbation (6). Moderate exercise intolerance was defined as a HRt below 135 bpm while mild exercise intolerance was defined as a HRt of 135 bpm or more. Previous research has shown that a HRt ≤135 bpm on the BCTT within 10 days of SRC is associated with greater risk for PPCS (13).
Recovery from SRC
Recovery was defined a-priori as 1) resolution of concussion symptoms to their pre-injury level (which was defined a-priori as 2 or fewer new, no more than mild symptoms [i.e., severity 2/6 or less] on the current PCSI, captured during the initial visit, when compared with the patient’s report of his/her symptoms on the initial visit PCSI that were present prior to the concussion); 2) physical examination within normal limits; and 3) ability to exercise to at least 80% of age-appropriate maximum HR without exacerbation of concussion-like symptoms on the BCTT. The exact date of recovery was identified as the first date the participant reached and stayed below the defined recovery symptom threshold on daily symptom reports when later confirmed by a normal physical examination and successful completion of the BCTT at the next office visit (5, 6). If the participant did not recover by the end of the study intervention (4 weeks from injury), the date of recovery was determined by medical record review.
Definition and Quantification of Adherence
Adherence was defined as participants completing at least two-thirds of their aerobic exercise prescription in any given week (8,9).The following formula was used to quantify adherence:
The numerator represents the volume of exercise performed while the denominator represents the volume of exercise prescribed to the participant. Volume actual was determined by multiplying weekly mean HR recorded during home exercise by weekly mean exercise time in minutes by the number of days per week out of 6 completed (i.e. 3 out of 6 days completed = 3/6 = 0.5). Volume prescribed was determined by multiplying weekly target HR by 20 minutes by days prescribed (everyday excluding day of office visit = 6/6 =1). Using this formula, participants who had an adherence rate of 66.67% or more were considered to be adherent whereas participants with a rate below 66.67% were considered to be non-adherent. Some participants had adherences rates of greater than 100% and were considered to be adherent.
Statistical Analysis
No a-priori sample size estimation was done for this secondary analysis. Demographics of participants included in the analysis were compared with those excluded from the analysis to assess baseline and outcome differences. Participants were grouped as adherent (≥66.67%) or non-adherent (<66.67%). The main dependent variable, duration of recovery in days, was not normally distributed and a log transformation greatly improved normality of the data; hence, the log of recovery time in days was used in all regressions (see Supplemental Figure 1, Supplemental Digital Content, SDC 1, Distribution of recovery without log transformation; and Supplemental Figure 2, Supplemental Digital Content, SDC 2, Distribution of recovery with log transformation). Adherent participants were compared with non-adherent participants to assess baseline and outcome differences. Continuous variables were compared using a non-parametric Mann-Whitney U test and binary/categorical variables were compared using a Chi-squared test or Fisher’s Exact test, depending on sample size. Percent adherence was calculated and its distribution was plotted. Since initial symptom severity (11) and degree of exercise intolerance (12–14) are thought to affect concussion recovery times, their association with adherence was compared separately. All regressions were controlled for sex (17). A p-value less than 0.05 was considered significant and all analyses were performed using SPSS Version 28 (IBM Corp, Armonk, NY) (18).
RESULTS
Out of the 118 participants included in the original RCT (5), 57 were randomized to a placebo-like stretching program and are not a part of this secondary analysis. From the 62 adolescents randomized to the aerobic exercise intervention, 1 participant withdrew consent and 1 participant was lost-to-follow-up immediately after consent and randomization. Both participants were removed from analysis. Nine participants did not have any recorded HR data from their home exercise prescriptions and were not included. Therefore, 51 participants are included in the analysis. Possible reasons for missing HR monitor data are discussed in the limitations section. Participants without any HR data (n=9) did not differ significantly in age (15.77 ± 1.6 years, p=0.940), sex (78% male, p=0.329), concussion history (44% positive, p=0.669), height (170.1 ± 7.2 cm, p=0.506), weight (60.7 ± 10.5 kg, p=0.790), initial symptom severity (31.0 ± 18.3, p=0.785), days from injury to first visit (5.7 ± 2 days, p=0.308), or incidence of PPCS (11%, p=0.544) versus included participants. Out of the 51 included participants, 31 qualified as adherent and 20 as non-adherent. Figure 1 presents the study inclusion flow diagram.
Figure 1.
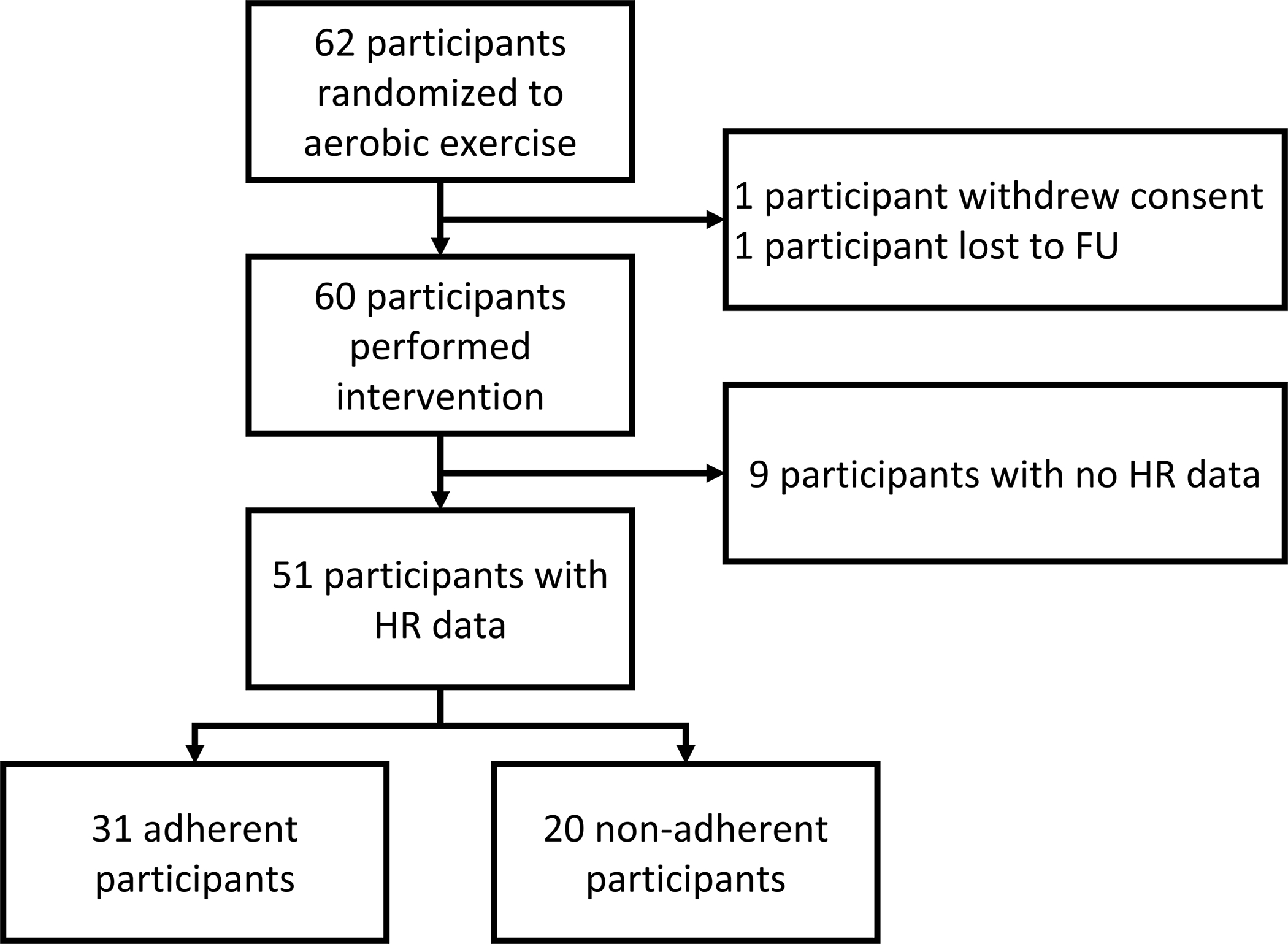
Consort flow diagram of study inclusion.
Sample demographics are presented in Table 1 Thirty-one participants were seen at community sports medicine practices (UB) and 20 participants were seen at hospital-based practices (CHOP=17, BCH=3). There was no difference in mean adherence between the two clinical settings (p=0.463). Adherent participants reported more symptoms at the initial visit and were more exercise intolerant (i.e., had a lower HRt). Adherent participants recovered significantly faster than those who were not adherent (median recovery time 12 [Interquartile range [IQR] 9, 22] days versus 21.5 [IQR 13, 29.8] days, p = 0.016).
Table 1.
Sample Demographics, physical characteristics, sport of injury and BCTT results
| Total Sample | Adherent Participants | Non-Adherent Participants | p-value | |
|---|---|---|---|---|
| n | 51 | 31 | 20 | |
| Age in years | 15.77 ± 1.6 | 15.51 ± 1.5 | 16.18 ± 1.7 | 0.137 |
| Sex | 61% Male | 57% Male | 63% Male | 0.620 |
| Previous Concussion, n (%) | 0.595 | |||
| 0 | 29 (56.9%) | 16 (52%) | 13 (65%) | |
| 1 | 15 (29.4%) | 10 (32%) | 5 (25%) | |
| 2 | 5 (9.8%) | 3 (10%) | 2 (10%) | |
| 3 | 2 (3.9%) | 2 (7%) | 0 (0%) | |
| Height in cm | 166.90 ± 13.9 | 165.44 ± 15.0 | 169.04 ± 12.2 | 0.434 |
| Weight in kg | 62.12 ± 15.1 | 61.58 ± 15.4 | 62.92 ± 15.2 | 0.539 |
| Sport of injury, n (%) | 0.350 | |||
| Basketball | 1 (2.0%) | 1 (3.2%) | 0 (0%) | |
| Cheerleading | 3 (5.9%) | 3 (9.7%) | 0 (0%) | |
| Dodgeball | 1 (2.0%) | 0 (0%) | 1 (5.0%) | |
| Field Hockey | 2 (3.9%) | 2 (6.5%) | 0 (0%) | |
| Football | 14 (27.5%) | 7 (22.6%) | 7 (35.0%) | |
| Gymnastics | 1 (2.0%) | 1 (3.2%) | 0 (0%) | |
| Ice Hockey | 8 (15.7%) | 3 (9.7%) | 5 (25.0%) | |
| Lacrosse | 5 (9.8%) | 4 (12.9%) | 1 (5.0%) | |
| Rugby | 1 (2.0%) | 0 (0%) | 1 (5.0%) | |
| Soccer | 13 (25.5%) | 8 (25.8%) | 5 (25.0%) | |
| Volleyball | 1 (2.0%) | 1 (3.2%) | 0 (0%) | |
| Wrestling | 1 (2.0%) | 1 (3.2%) | 0 (0%) | |
| Sport setting, n (%) | 0.500 | |||
| Organized sport | 37 (72.5%) | 24 (77.4%) | 13 (65.0%) | |
| Organized sport practice | 11 (21.6%) | 5 (16.1%) | 6 (30.0%) | |
| Recreational/Gym class | 3 (5.9%) | 2 (6.5%) | 1 (5.0%) | |
| Initial Symptom Severity (PCSI, max=126) | 36.24 ± 20.9 | 41.62 ± 16.9 | 28.89 ± 24.0 | 0.020 |
| Exercise Tolerance | < 0.001 | |||
| Exercise Tolerant | 4 (8%) | 0 (0%) | 4 (20%) | |
| Mild Exercise Intolerance (HRt ≥ 135 bpm) | 17 (33%) | 7 (23%) | 10 (50%) | |
| Moderate Exercise Intolerance (HRt < 135 bpm) | 30 (59%) | 24 (77%) | 6 (30%) | |
| Injury to Visit in days | 6.48 ± 2.2 | 6.24 ± 2.4 | 6.84 ± 1.8 | 0.272 |
| Mean Recovery Time in days | 22.76 ± 23.4 | 19.44 ± 19.0 | 40.38 ± 37.9 | 0.016 |
| Median Recovery Time in days | 14 (11, 28) | 12 (9, 22) | 21.5 (13, 29.8) | |
| Incidence of PPCS, n (%) | 10 (19.6%) | 4 (12.9%) | 6 (30.0%) | 0.163 |
p-value column entries are comparisons between adherent and non-adherent participants; bold values represent p < 0.05; Mann-Whitney U tests were used for continuous data; Chi-squared tests for binary/ordinal data; and Fisher’s Exact test for group sizes below 5. BCTT= Buffalo Concussion Treadmill Test.
Figure 2 presents mean adherence during weeks 1 and 2 of the intervention period. Only 9 participants were still in the intervention by week 3 so the distribution for week 3 is not presented. Due to the reduced sample size over time, only the level of adherence during week 1 was used in our regression analysis. One participant performed substantially more aerobic exercise (approximately 3-fold) than was prescribed during the first week and is not shown in Figure 2. This participant (17 y/o female) had moderate exercise intolerance (HRt = 103 bpm) and reported a high symptom severity score of 41 on the PCSI at 3 days post-injury. She exercised for a mean of 69 minutes per day at a mean HR of 104 bpm. She satisfied recovery criteria by day 9 after injury (6 days after first study visit). No adverse events or near misses were recorded during assessment or intervention, including in those who performed more exercise than they were prescribed (i.e., adherence > 100%).
Figure 2.
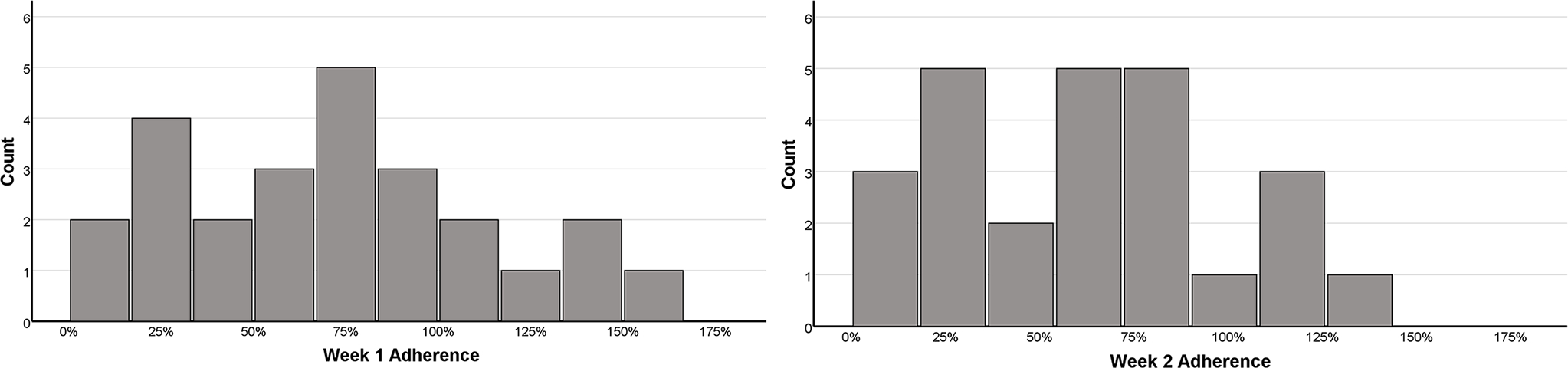
Mean distribution of adherence during week 1 (n=51) and week 2 (n=25).
Figure 3 presents the relationship between week 1 adherence and the log of recovery time. On multivariate linear regression, higher adherence was associated with faster recovery (β = −0.002 [−0.003, 0.0], p=0.046) when controlling for participant sex (β = −0.052 [−0.213, 0.109], p=0.518).
Figure 3.
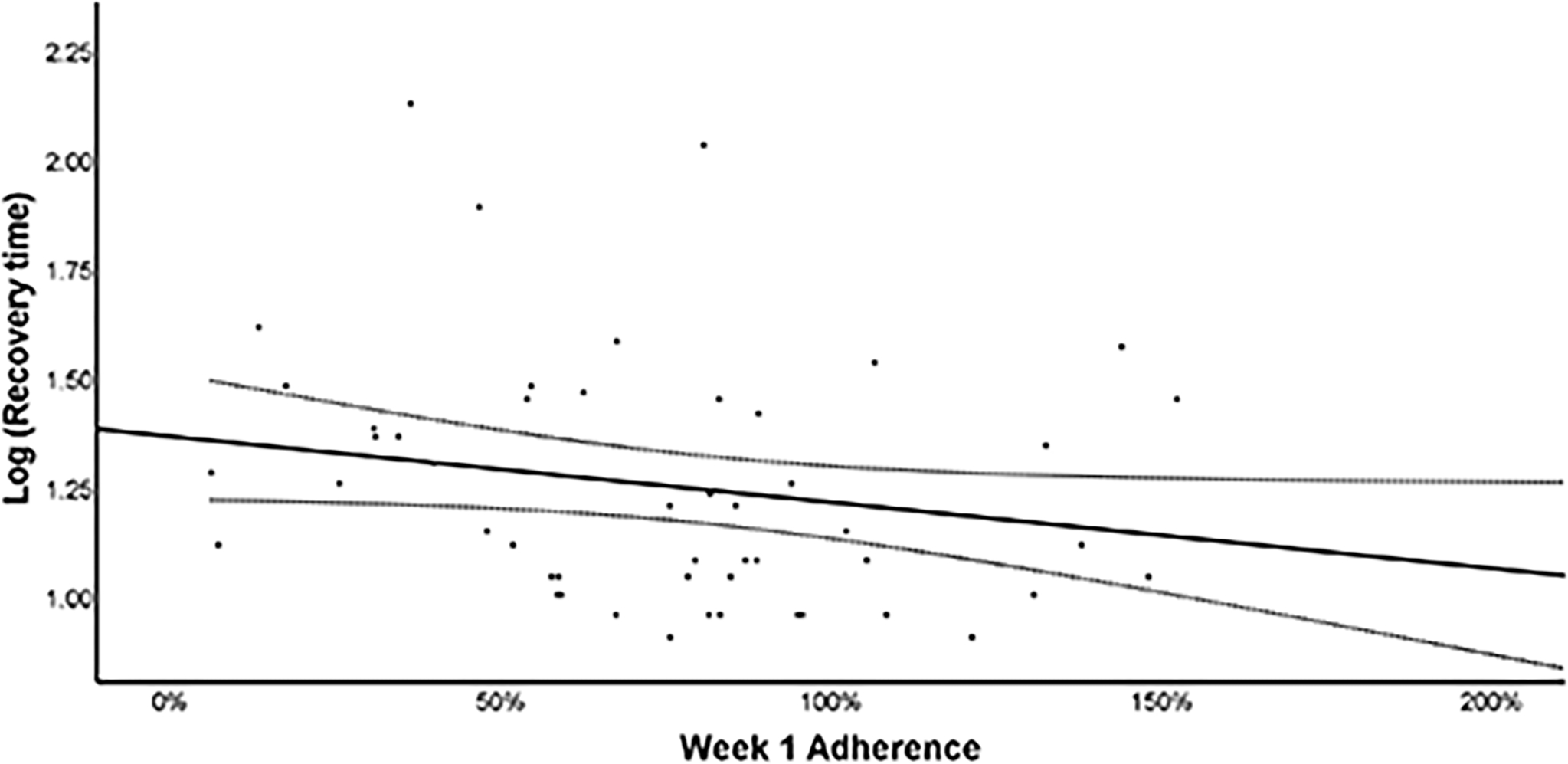
Scatterplot of log of adherence at week 1 and recovery time with line of best fit and 95% Confidence Intervals.
Figure 4 presents the relationship between week 1 adherence and the level of initial exercise intolerance. On multivariate linear regression, a lower HRt was associated with greater adherence (β = −0.886 [−1.385, −0.387], p<0.001) when controlling for participant sex (β = −24.990 [−52.484, 2.504], p=0.074).
Figure 4.
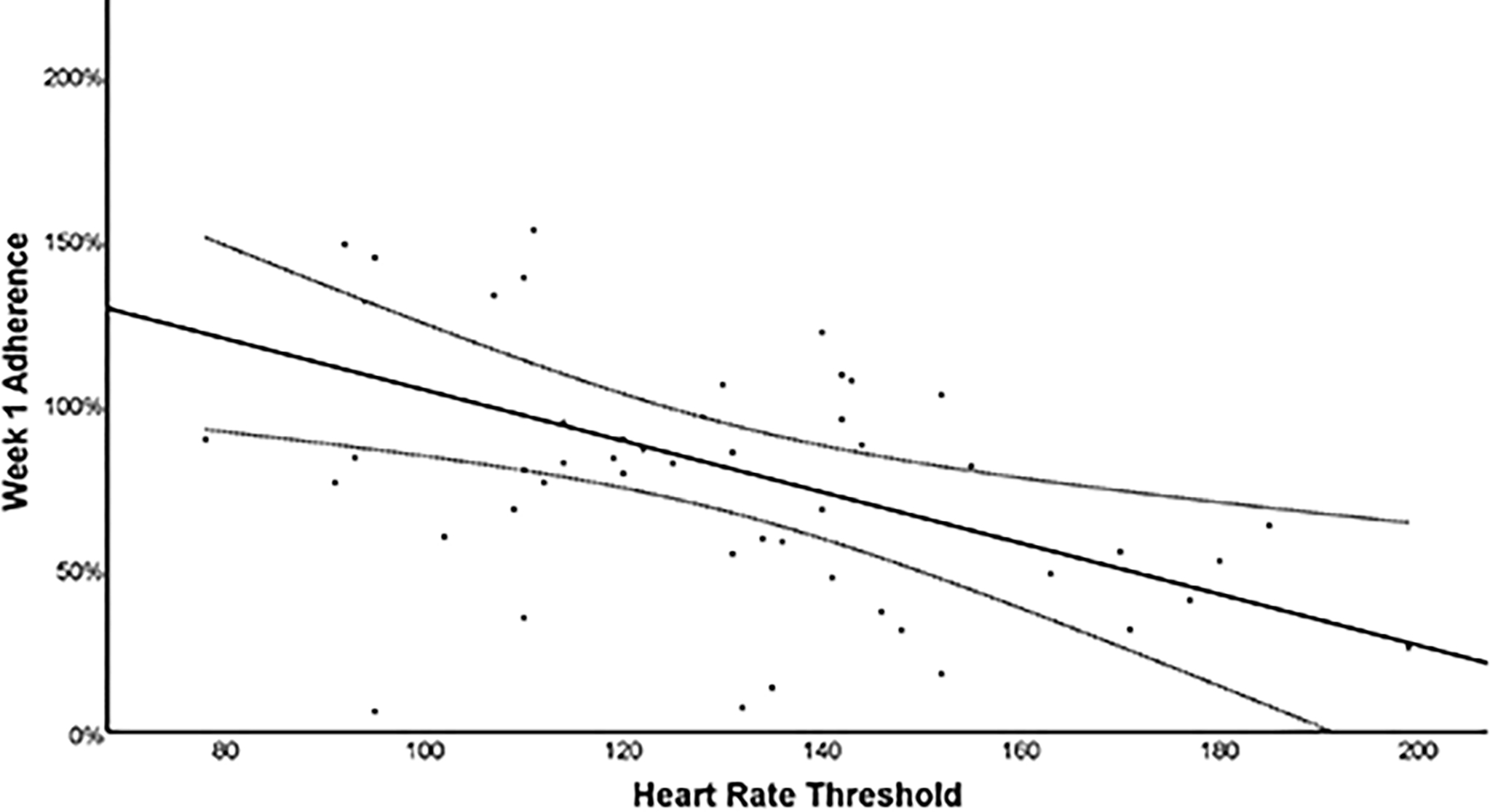
Scatterplot of adherence at week 1 and initial level of exercise intolerance (HRt on initial BCTT) with line of best fit and 95% Confidence Intervals.
Figure 5 presents the relationship between week 1 adherence and initial symptom severity. On multivariate linear regression, no significant association was found between adherence and initial symptom severity (β = 0.545 [−0.232, 1.323], p=0.164) when controlling for participant sex (β = −11.901 [−44.469, 20.667], p=0.465).
Figure 5.
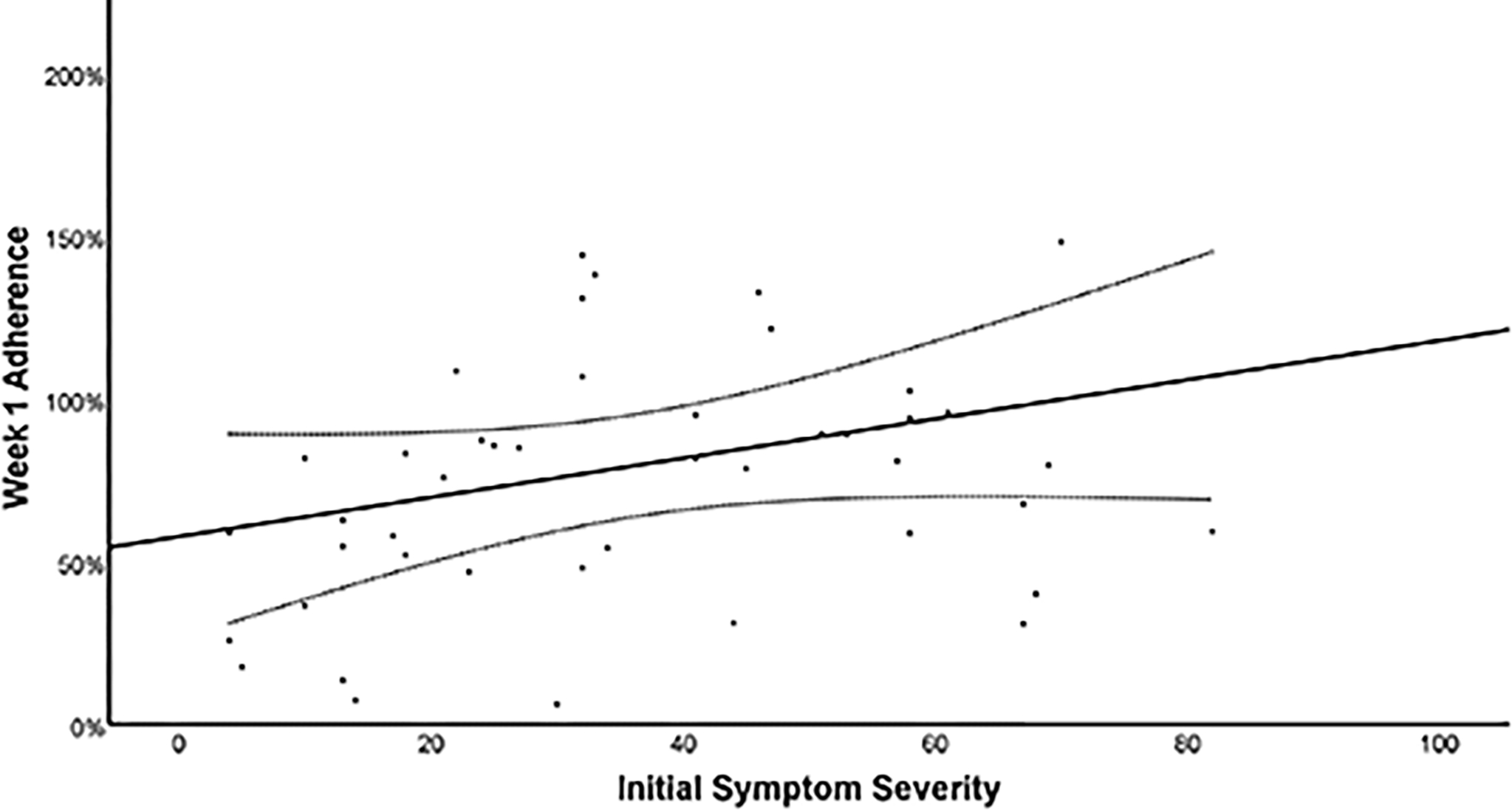
Scatterplot of adherence at week 1 and initial symptom severity (PCSI) with line of best fit and 95% Confidence Intervals.
DISCUSSION
Two recent RCTs demonstrated that individualized sub-symptom threshold aerobic exercise prescribed to adolescents within 10 days of SRC safely and significantly improved recovery (5,6). The most recent of these also demonstrated a significant reduction in the rate of persisting symptoms (PPCS) in a variety of concussion practice settings (5). The current study, a secondary analysis of data from the most recent RCT (5), confirmed that adolescents who best adhered to the exercise prescription within the first week after evaluation recovered significantly faster from SRC than those who did not. Interestingly, adolescents who were more symptomatic and who had worse initial exercise tolerance were more adherent than those with fewer symptoms and with better exercise tolerance. This was unexpected and may indicate a stronger motivation for more symptomatic patients to engage in a potentially effective intervention. Differences in motivation for participants playing organized sport versus recreational sport cannot explain this observation since almost all participants (94%) sustained their concussion during interscholastic games or practices. There were no adverse events or near misses during the study, even though exercise began within days of injury in sometimes highly symptomatic adolescents. Furthermore, there were no adverse events or near misses in those who exercised beyond their prescribed exercise volume. Please note that no participants exercised above their prescribed intensity (heart rate). This speaks to the value and safety of individualized aerobic exercise treatment based upon a systematic determination of each adolescent’s degree of exercise intolerance early after sustaining SRC (19). Others have suggested that greater aerobic exercise volumes (> 100 min/week) are more effective for ameliorating post-concussion symptoms than lower volumes (<100 min/week) (20). Our data support the approach that clinicians may consider encouraging patients to exercise as much as they can, even if it exceeds the volume (not intensity) that their individual prescription specifies, provided that symptoms during exercise remain stable and that the patient stops the exercise bout once the mild symptom exacerbation point is exceeded.
Adolescent adherence rates to exercise prescriptions vary greatly, ranging from 10% to 88% (9).Using the definition of completing at least two-thirds of the prescribed volume of aerobic exercise, 31 out of 51 (61%) of our participants were adherent. Compared with prior studies (8,9), our participants exhibited a moderate level of adherence to the exercise program. Physician encouragement is known to influence patient adherence to medical interventions (21). However, it should be noted that treating physicians in this study were blinded to study arm assignment and so the effect of physician encouragement on participant adherence was minimal. Other factors associated with adherence to exercise prescriptions include, but are not limited to: perceived benefit, self-confidence, accessibility, support, availability, reminders, symptom relief, enjoyment, competition, and goal setting (8).The RCT study design did not account for these factors; therefore, future studies should investigate how to improve adherence to exercise prescriptions after concussion, including the role of the treating physician.
This study provides evidence regarding the amount of exercise required to elicit a meaningful clinical effect. While most participants followed the exercise prescription closely, a number of participants exercised considerably more than their prescription dictated. All of the participants who exercised more than required recovered rapidly without adverse effects. One participant who exercised at a volume three–fold beyond her prescription recovered significantly faster than would have been expected by her high initial symptom burden and clinically meaningful degree of early exercise intolerance. Future research should examine early sub-symptom threshold aerobic exercise programs of varying intensities and volumes for concussion treatment.
A limitation of this study is that some participants did not have any HR data during the home-based exercise intervention, typically due to malfunctioning HR monitors despite attempts to troubleshoot during the weekly visits (including replacing the monitor with a new one). We excluded these participants from analysis because we could not objectively verify their level of adherence. The original RCT was powered to evaluate the effect of early subthreshold aerobic exercise on concussion recovery, not adherence to the prescriptions. Our study did not evaluate other potential influences upon adherence such as timing within season (e.g., motivation to return to an important game), socio-economic status, and level of competition. Future research should investigate additional factors that influence adherence to exercise prescriptions in adolescents after concussion. This study would have benefitted from a larger sample size to more accurately evaluate adherence beyond the first week of treatment. Additionally, our findings cannot be generalized to the non-athlete population or to young children or adults, which warrants future investigations.
Individualized aerobic exercise that mildly but does not significantly exacerbate concussion symptoms has been proven to be a safe and effective treatment for adolescents who suffer SRC (5,6). The effectiveness of any prescription, however, depends not only upon the benefit of the prescription itself but also on the degree to which the patient adheres to the prescription. We found that the majority of concussed adolescent athletes adhered to their aerobic exercise prescription. While we are not able to conclude that better adherence was the proximate cause for improved outcome with early aerobic exercise (22), the data show that those who better adhered to this proven effective treatment for SRC recovered faster than those who were not as well adherent. Interestingly, adolescents appeared to benefit even more when they had greater initial symptom burden and when they were more exercise intolerant. Thus early, controlled aerobic exercise was appealing to not only those with fewer symptoms, who may have felt well enough to engage in exercise, but also to those with a greater post-concussion symptom burden. Understanding factors that influence adherence will help clinicians and researchers optimize this non-pharmacological treatment for SRC.
Supplementary Material
SDC 1: Supplementary Figure_1.tif – Distribution of recovery without log transformation
SDC 2: Supplementary Figure_2.tif – Distribution of recovery with log transformation
Conflict of Interest and Funding Source:
Funded by the American Medical Society for Sports Medicine (AMSSM) Collaborative Research Network (awarded to JJL). The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors do not declare any relevant conflicts of interest.
REFERENCES
- 1.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–47. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. [DOI] [PubMed] [Google Scholar]
- 3.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014–25. [DOI] [PubMed] [Google Scholar]
- 4.Russell K, Selci E, Black B, Ellis MJ. Health-related quality of life following adolescent sports-related concussion or fracture: a prospective cohort study. J Neurosurg Pediatr. 2019;23(4):455–64. [DOI] [PubMed] [Google Scholar]
- 5.Leddy JJ, Master CL, Mannix R, et al. Early targeted heart rate aerobic exercise versus placebo stretching for sport-related concussion in adolescents: a randomised controlled trial. Lancet Child Adolesc Health. 2021;5(11):792–9. [DOI] [PubMed] [Google Scholar]
- 6.Leddy JJ, Haider MN, Ellis MJ, et al. Early Subthreshold Aerobic Exercise for Sport-Related Concussion: A Randomized Clinical Trial. JAMA Pediatr. 2019;173(4):319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACSM’s Guidelines for Exercise Testing and Prescription 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 8.Holt CJ, McKay CD, Truong LK, Le CY, Gross DP, Whittaker JL. Sticking to it: a scoping review of adherence to exercise therapy interventions in children and adolescents with musculoskeletal conditions. J Orthop Sports Phys Ther. 2020;50(9):503–15. [DOI] [PubMed] [Google Scholar]
- 9.Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. J Athl Train. 2013;48(5):627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meehan WP 3rd, Mannix R, Monuteaux MC, Stein CJ, Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology. 2014;83(24):2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haider MN, Leddy JJ, Wilber CG, et al. The predictive capacity of the Buffalo Concussion Treadmill Test after sport-related concussion in adolescents. Front Neurol. 2019;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leddy JJ, Hinds AL, Miecznikowski J, et al. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin J Sport Med. 2018;28(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr R, Bogg T, Fyffe A, Lam LT, Browne GJ. Graded exercise testing predicts recovery trajectory of concussion in children and adolescents. Clin J Sport Med. 2021;31(1):23–30. [DOI] [PubMed] [Google Scholar]
- 15.Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol. 2014;29(4):348–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiebe DJ, Nance ML, Houseknecht E, et al. Ecologic momentary assessment to accomplish real-time capture of symptom progression and the physical and cognitive activities of patients daily following concussion. JAMA Pediatr. 2016;170(11):1108–10. [DOI] [PubMed] [Google Scholar]
- 17.Ono KE. Sex-Based differences as a predictor of recovery trajectories in young athletes after a sports-related concussion: response. Am J Sports Med. 2016;44(6):NP31. [DOI] [PubMed] [Google Scholar]
- 18.Stehlik-Barry K, Babinec AJ. Data analysis with IBM SPSS statistics. Packt Publishing Ltd; 2017. [Google Scholar]
- 19.Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion. Curr Sports Med Rep. 2018;17(8):262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell DR, Hunt DL, Aaron SE, Meehan WP 3rd, Tan CO. Influence of aerobic exercise volume on postconcussion symptoms. Am J Sports Med. 2021;49(7):1912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47(8):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stovitz SD, Verhagen E, Shrier I. Distinguishing between causal and non-causal associations: implications for sports medicine clinicians. Br J Sports Med. 2019;53(7):398–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: Supplementary Figure_1.tif – Distribution of recovery without log transformation
SDC 2: Supplementary Figure_2.tif – Distribution of recovery with log transformation


