Introduction
A sea-change occurred in the field of spine surgery in 2005 following the landmark publication of Patchell et al1 regarding the efficacy of spine surgery for restoration of ambulatory function in patients with spinal metastatic disease. In the succeeding 15 years, enthusiasm grew for surgical interventions as a standard treatment option for patients with spinal metastases.2 Several investigations touted that surgery not only preserved ambulatory ability, but also improved survival.3–6 There was a concern that many of these investigations were confounded by selection bias and controversy remains regarding the utility of spine surgery in subsets of patients with spinal metastases based on baseline neurologic status.7 In order to address this, we planned an analysis that accounted for confounding by indication and compared patients treated operatively and non-operatively for spinal metastases within the Prospective Observational study of Spinal metastasis Treatment (POST).2,8 We hypothesized that patients treated surgically would have superior 1-year survival to those managed non-operatively.
Methods
This study was conducted among patients enrolled in the POST study (2017–2019).2 Enrollment details, inclusion criteria and study protocol have been published previously.2 The study was approved through institutional review before commencement and patients consented before participation. The investigation was registered with clinicaltrials.gov (NCT03224650). Eligible patients were adults presenting for initial treatment of spinal metastases at participating centers and received operative, or non- operative, management.2 Patients were treated based on shared decision-making and as directed by treating clinicians. Overall, the POST investigation was powered to detect differences in survival at 1-year based on the New England Spinal Metastasis Score (NESMS) at presentation.2,8 Enrollment was structured to create a comparative balance between operative and non-operative cohorts with a 2:3 ratio. The date of enrollment was considered time-zero and patients were followed to one of two time-points: death or 365 days following enrollment.2 In cases where patients initially managed non-operatively subsequently received surgery, we extended surveillance to 365 days following the date of surgery. Sixty-four percent of eligible participants consented to be enrolled, with 80 individuals receiving surgical intervention as the initial treatment strategy and an additional 7 crossovers from non-operative to surgical management. Data for this analysis was finalized on July 31, 2021.
Per study protocol, the primary outcome for the analysis presented here was survival at 1-year following treatment initiation.2 The primary predictor was treatment, categorized as operative, or non-operative management. Crossovers were handled via statistical cloning.9 Unadjusted comparisons between the operative and non-operative cohorts were made using chi-square tests for categorical variables and the Wilcoxon rank-sum test for non-parametric, continuous data. Survival was assessed using Kaplan-Meier curves. Per protocol, we developed a propensity score around the likelihood for surgical intervention using age, biologic sex, co-morbidities, primary tumor, neurologic symptoms and NESMS at presentation based on our conceptual model.2 Inclusion of the NESMS in the propensity score is supported by prior work validating the association between the NESMS and 1-year survival in this cohort.8 The propensity score was used in final adjusted models for survival at 1-year, presented using odds ratios (OR) and 95% confidence intervals (CI). Calibration was evaluated using observed to expected plots and Hosmer-Lemeshow testing.10
Results
We considered 87 instances of surgical intervention and 122 cases of non-operative treatment. The average age of both cohorts approximated 60.5 years. Lung cancer was the most common primary tumor (20%), followed by breast (16%) and prostate (14%). The thoracic spine was the most common site of surgical intervention (70%). The majority of surgeries consisted of fusion-based procedures (79%), including 26 corpectomies. Combined chemotherapy and radiation was the most common non-operative modality (80%).
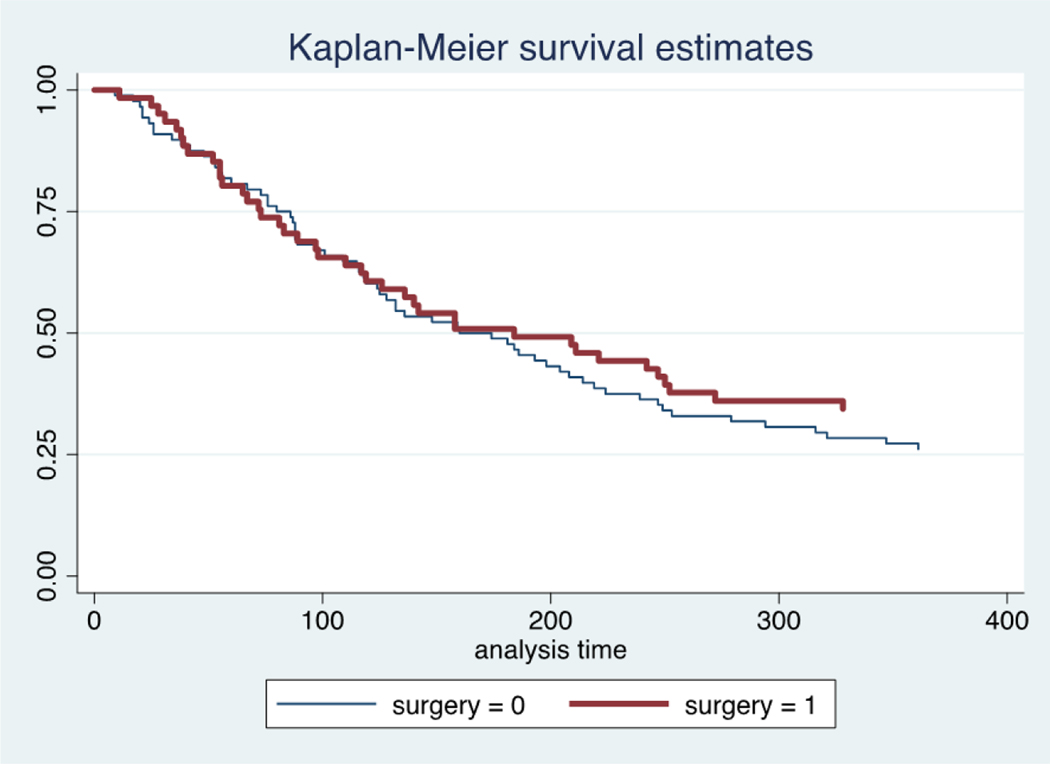
There was reasonable balance across socio-demographic and clinical characteristics between the operative and non-operative cohorts (Table 1). There was no significant difference in primary tumor between groups, with lung cancer the most common in non-operative (20%) and operative (18%) cohorts (p=0.12). A relatively normal distribution was also appreciated across all prognostic scoring utilities. Overall, 50% of the cohort died by 1-year following presentation (105/209). In the operative group, the mortality rate was 46% at 1-year, as compared to 54% in the non-operative cohort (Figure 1). This represented a 25% reduction in the odds of mortality (OR 0.75; 95% CI 0.43, 1.30) but was not significantly different (p=0.3). Following propensity score adjustment, accounting for confounding by indication in the decision for surgery, surgical intervention offered a 28% reduction in the odds of mortality (OR 0.72; 95% CI 0.40, 1.29) but still did not demonstrate statistical significance (p=0.27). There was no evidence of statistical lack of fit (p=0.39) with good calibration on observed to expected plots (Appendix 1).
Table 1.
Demograhic and clinical characteristics of the operative and non-operative cohorts*
| Characteristic | Non-Operative | Operative | p-value |
|---|---|---|---|
|
| |||
| Age (mean, SD) | 60.2(11.9) | 60.8(11.9) | .24 |
| Biologic Sex | - | - | .11 |
| Male Sex | 62(51) | 54(62) | - |
| Female Sex | 60 (49) | 33(38) | - |
| White | 102(84) | 76(87) | .45 |
| Body Mass Index (mean. SD) | 26.9(6.1) | 27.7(6.1) | .22 |
| Number of Co-morbidities (mean. SD) | 2.5 (0.9) | 2.3 (0.8) | .04 |
| Serum Albumin | - | - | .86 |
| Albumin <3.5g/dL | 35 (29) | 24 (28) | - |
| Albumin ≥3.5g/dL | 87(71) | 63(72) | - |
| Ambulatory Status at Presentation | - | - | .39 |
| Independent Ambulator | 76 (62) | 49(56) | - |
| Ambulatory with Assistance/Non-ambulatory | 46(38) | 38 (44) | - |
| Performance Status | - | - | .50 |
| Poor | 11(9) | 11(13) | - |
| Moderate | 40(33) | 32(37) | - |
| Good | 71 (58) | 44(51) | - |
| Neurologic Status at Presentation | - | - | .008 |
| Neurologic Intact | 97 (80) | 52(60) | - |
| Neurologic Deficits | 24 (20) | 34(39) | - |
| Bone Metastases | 72(59) | 42(48) | .12 |
| Visceral Metastases | 66(54) | 44(51) | .62 |
| Type of Lesion | - | - | .05 |
| Blastic | 29(24) | 13(15) | - |
| Mixed (lytic/blastic) | 32 (26) | 15(18) | - |
| Lytic | 61(50) | 57(67) | - |
| New England Spinal Metastases Score | - | - | .69 |
| 0 | 16(13) | 14(16) | - |
| 1 | 29(24) | 23(26) | - |
| 2 | 52 (43) | 30(34) | - |
| 3 | 25(20) | 20 (23) | - |
| Tokuhashi Score (mean/SD) | 8.5 (2.9) | 8.3 (2.9) | .53 |
| Tomita Score (mean/SD) | 5.9 (2.5) | 5.9 (2.8) | .94 |
| Spinal Instability Neoplastic (SINS) Score (mean/SD) | 9.7 (3.0) | 11 (3.2) | .004 |
All values are presented as raw number and percentage (rounded to the nearest whole number) except where noted.
Fig. 1.
Kaplan-Meier survival curves for patients treated operatively (surgery=1) and non-operatively (surgery=0) over the course of the first year following presentation.
Discussion
This is the first investigation we are aware of that prospectively compares operative and non-operative treatment in patients with spinal metastases while accounting for selection and indication bias in the decision for treatment. This work is advantaged by its prospective nature as well as broad and representative variation in clinical parameters across both operative and non-operative cohorts, including ambulatory capacity and neurologic status. Given the relatively large size of the sample, we were able to account for confounders using propensity score adjustment and cloning for treatment crossovers.9,10
We believe that our findings add to a growing body of evidence that indicates surgical intervention is not uniformly beneficial across all individuals with spinal metastases. Although the benefits of surgery for patients with neurologic deficits, acute loss of ambulatory function and spinal instability are incontrovertible1,3,7, robust evidence is lacking for those without neurologic compromise or impaired ambulatory function7,8. We demonstrated an 8-percentage point difference in 1-year survival that, based on power estimates, would require a sample of over 1200 patients in total to demonstrate significance given high near-term mortality. While it is interesting that propensity adjustment slightly increased the advantage for surgery, the estimated 25–28% reduction in the odds of mortality should be balanced against the risks associated with these high-intensity interventions and relatively low survival rates, irrespective of treatment strategy.7,8 This may be especially important in instances where the metastatic process is largely asymptomatic, or if patients do not manifest neurologic deficits or impaired ambulatory ability.
Supplementary Material
Acknowledgement:
Contributors to the POST Study group also include: Drs. Michael Groff, Yi Lu, John Chi, Hasan Zaidi, Mai Anh Huynh, Alexander Spektor, Ayal Aizer, Karen Marcus, Tracy Balboni, John Shin, Joseph Schwab, Daniel Tobert and Larissa Lee. The authors thank Lauren Barton, Justin Blucher and Lananh Nguyen for their contributions to the data collection used in this investigation. All statistical testing was performed using STATA v 15.1 (STATA Corp., College Station, TX).
Funding Statement
This research was supported in part by a grant from the Orthopaedic Research and Education Foundation (OREF). The OREF was not involved in the conduct of the study or the preparation of the manuscript. The findings and views expressed here are those of the authors and should not be viewed as reflective of the opinions of the OREF.
This research was funded in part by National Institutes of Health (NIH-NIAMS) grant K23-AR071464 to Dr. Schoenfeld. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH or the Federal government.
Footnotes
Declaration of Competing Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Moiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366: 643–648. [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld AJ, Blucher JA, Barton LB, Schwab JH, Balboni TA, Chi JH, Shin JH, Kang JD, Harris MB, Ferrone ML. Design of the Prospective Observational study of Spinal metastasis Treatment (POST). Spine J 2020;20: 572–579. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Coppes MH, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Wang M, Crockard HA. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg 2016;30: 337–44. [DOI] [PubMed] [Google Scholar]
- 4.Depreitere B, Turner I, Vandoren C, Choi D. Cost-Utility Analysis of Surgery and Radiotherapy for Symptomatic Spinal Metastases in a Belgian Specialist Center. World Neurosurg. 2019;125: e537–e543. [DOI] [PubMed] [Google Scholar]
- 5.Turner I, Kennedy J, Morris S, Crockard A, Choi D. Surgery and Radiotherapy for Symptomatic Spinal Metastases Is More Cost Effective Than Radiotherapy Alone: A Cost Utility Analysis in a U.K. Spinal Center. World Neurosurg 2018;109: e389–e397. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki S, Kakutani K, Sakai Y, Ejima Y, Maeno K, Takada T, Yurube T, Terashima Y, Ito M, Kakiuchi Y, Takeoka Y, Hara H, Kawamoto T, Sakashita A, Okada T, Kiyota N, Kizawa Y, Sasaki R, Akisue T, Minami H, Kuroda R, Nishida K. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: prospective cohort study. Int Orthop 2017;41: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 7.Schoenfeld AJ, Bensen GP, Blucher JA, Ferrone ML, Balboni TA, Schwab JH, Harris MB, Katz JN, Losina E. The cost-effectiveness of surgical intervention for spinal metastases: A model-based evaluation. J Bone Joint Surg Am 2021. Jul 21. doi: 10.2106/JBJS.21.00023. Online ahead of print. PMID: 34288901 [DOI] [PMC free article] [PubMed]
- 8.Schoenfeld AJ, Ferrone ML, Schwab JH, Blucher JA, Barton LB, Tobert DG, Chi JH, Shin JH, Kang JD, Harris MB. Prospective validation of a clinical prediction score for survival in patients with spinal metastases: the New England Spinal Metastasis Score. Spine J 2021;21: 28–36. [DOI] [PubMed] [Google Scholar]
- 9.Maringe C, Majano SB, Exarchakou A, Smith M, Rachet B, Belot A, Leyrat C. Reflection on modern methods: Trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int J Epidemiol 2020;49: 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long JS, Freese J. Regression models for categorical dependent variables using STATA. 2nd Edition. College Station, TX: STATA Press, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.