Abstract
Osteoradionecrosis (ORN) of the jaws is a pernicious complication of radiation therapy for head and neck tumours. This article aims to provide an update on data related to the definition, epidemiology, staging, and clinical and radiological findings of ORN of the jaws. Using certain keywords, an electronic search was conducted spanning the period from January 1922 to April 2014 to identify the available related investigations. Pooled data were then analysed. ORN is described as exposed irradiated bone that fails to heal over a period of 3 months without evidence of persisting or recurrent tumour. The prevalence of ORN varies in the literature. Several staging or scoring systems of ORN have been proposed. Clinical findings include ulceration or necrosis of the mucosa with exposure of necrotic bone. Radiological findings are not evident in the early stages of ORN. Furthermore ORN may not be apparent in imaging even when the disease is advanced. Taking into account the severity of ORN and the difficulties in diagnosing it early and accurately, the clinician should be aware of this complex entity in order to prevent its appearance or the development of more severe complications.
Key words: Clinical findings, epidemiology, jaw, osteoradionecrosis, radiation therapy
Introduction
Osteoradionecrosis (ORN) is a pernicious complication of radiotherapy in head and neck cancer. It was first described by Regaud1 in 1922 and is still a clinical challenge. According to the most recent literature, ORN of the jaws is defined as exposed irradiated bone that fails to heal over a period of 3 months without any evidence of persisting or recurrent tumour2., 3., 4.. The mechanism of pathogenesis is still under investigation. However, the most frequently reported reason is radiation arteritis. Radiation arteritis leads to the development of a hypocellular, hypovascular and hypoxic environment, which results in a pathological outcome5.
The purpose of this paper is to explore the current theories on the definition and staging systems of ORN of the jaws. In addition, the epidemiology and the clinical and radiological findings of ORN of the jaws, are critically reviewed based on the available literature. Finally, critical issues and considerations are discussed and pathways for future research are proposed.
Methods
An electronic search of three databases (PubMed, Scopus and the Cochrane Library) was performed using the keywords ‘osteoradionecrosis’, ‘radiotherapy’, ‘osteonecrosis’, ‘osteoradionecrosis’ and ‘mandible’, ‘osteoradionecrosis’ and ‘jaw’, ‘osteoradionecrosis’ and ‘radiotherapy’, ‘osteoradionecrosis’ and ‘staging’, ‘osteoradionecrosis’ and ‘clinical findings’, ‘osteoradionecrosis’ and ‘radiological findings’, to identify literature published in English and German between January 1922 and April 2014 (Table 1). Abstracts were obtained for all the titles identified during the electronic search. Two reviewers (A.C. and T.Z.) independently screened titles and abstracts to eliminate articles that were completely off-topic. No other exclusion criteria were utilised. Following exclusion of the articles irrelevant to the topic and the removal of duplicating papers, the remaining articles were carefully reviewed and their findings were critically analysed.
Table 1.
Example of electronic search strategy in PubMed
| Search strategy | Results |
|---|---|
| Osteoradionecrosis | 1,980 |
| Radiotherapy | 286,652 |
| Osteonecrosis | 15,087 |
| Osteoradionecrosis and mandible | 636 |
| Osteoradionecrosis and jaw | 738 |
| Osteoradionecrosis and radiotherapy | 1,332 |
| Osteoradionecrosis and staging | 76 |
| Osteoradionecrosis and clinical findings | 177 |
| Osteoradionecrosis and radiological findings | 30 |
Results
Definition
Several attempts have been made in order to define ORN. Ewing6 was the first to use the term ‘radiation osteitis’ to describe changes in bone after radiotherapy. In the following years, several terms were used to name these changes in bone, such as radiation osteitis, ORN and avascular bone necrosis7. In 1974, Guttenberg8 proposed the term ‘septic ORN of the mandible’ to describe the stage of necrosis when irradiated bone becomes superficially infected, ending up with a high risk of involvement of deeper structures.
In 1983, Marx5 defined ORN as ‘an area >1 cm of exposed bone in a field of irradiation that failed to show any evidence of healing for at least 6 months’. Marx also reported that superficial contamination and no interstitial infection was present. In 1987, Marx and Johnson3 suggested the definition of ORN as: ‘The exposure of nonviable bone which fails to heal without intervention’. Epstein et al.9 defined ORN as ‘an ulceration or necrosis of the mucous membrane, with exposure of necrotic bone for more than 3 months’.
Widmark et al.10 described ORN as ‘a non-healing mucosal or cutaneous ulcer with denuded bone, lasting for more than 3 months’. Both Widmark and Marx excluded from their definition conditions with necrotic bone for which the mucosa and skin were intact. Store and Boysen11 reported that exposed bone is sporadically present; radiological findings are evident in all cases of ORN. In 1997, Wong et al.12 defined ORN as ‘a slow-healing radiation-induced ischemic necrosis of variable extent occurring in the absence of local primary tumor necrosis, recurrence or metastatic disease’.
According to the literature published in the last 15 years, ORN of the jaws is defined as exposed irradiated bone that fails to heal over a period of 3 months without evidence of persisting or recurrent tumour2., 3., 4., 13., 14., 15.. At the time of diagnosis, the necrosis might involve the bone superficially or deeply. It might be a process that progresses slowly or an active progressive state that can lead to a pathological fracture16., 17., 18..
Epidemiology
The prevalence of ORN varies widely in the literature (Table 2)16., 17., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48. and ranges from 0.4% to 56%19., 20., 22., 24., 30., 31., 32., 33., 36., 49.. ORN usually affects patients over 55 years of age14., 36., 50., 51.. However, the most frequently reported prevalence rate is 5–15%7., 36., 52.. In up to 20% of patients with persistent ORN which does not respond to aggressive treatment, bone damage is reported to be caused by recurrent disease or a second primary tumour16., 53..
Table 2.
Incidence of osteoradionecrosis (ORN) in different studies
| Study | Year | Period | Total number of patients | Prevalence of ORN (%) | Mean irradiation dose |
|---|---|---|---|---|---|
| Watson and Scarborough20 | 1938 | 1930–1937 | 1,819 | 12.9 | |
| Martin and Sugarbaker21 | 1940 | 103 | 25 | ||
| MacComb22 | 1962 | 1952–1959 | 251 | 37.1 | |
| MacDougall et al.23 | 1963 | 364 | 5 | ||
| Grant and Fletcher24 | 1966 | 1954–1962 | 176 | 37.5 | 10,000* |
| Rahn and Drane25 | 1967 | 1960–1962 | 120 | 44.2 | |
| Rankow and Weissman26 | 1971 | 176 | 6.3 | 5,000* | |
| Wang27 | 1972 | 262 | 5.8 | ||
| Cheng and Wang28 | 1974 | 76 | 17.1 | 2,500* | |
| Marciani and Plezia29 | 1974 | 220 | 10.5 | ||
| Bedwinek et al.30 | 1976 | 1966–1971 | 381 | 14.2 | 7,000* |
| Murray et al.31 | 1980 | 1966–1975 | 653 | 21.1 | 5,000* |
| Morrish et al.19 | 1981 | 1971–1977 | 100 | 22 | 7,500* |
| Epstein et al.32 | 1987 | 1977–1984 | 1,000 | 2.7 | 6,000* |
| Withers et al.33 | 1995 | 1976–1985 | 676 | 4.7 | 65† |
| Turner et al.34 | 1996 | 1980–1987 | 333 | 5.9 | 55† |
| Thorn et al.16 | 2000 | 1992–1998 | 80 | 74 | 60† |
| Storey et al.35 | 2001 | 1965–1995 | 83 | 6 | 60† |
| Reuther et al.36 | 2003 | 1969–1999 | 830 | 8.2 | 60† |
| Oh et al.37 | 2004 | 1989–2004 | 81 | 4.9 | 52.6† |
| Studer et al.38 | 2004 | 1980–1998 | 268 | 12.5 | 60† |
| Ben-David et al.39 | 2007 | 1996–2005 | 176 | 0 | 65† |
| Jham et al.40 | 2008 | 2003–2005 | 207 | 5.5 | 58.9† |
| Katsura et al.41 | 2008 | 1996–2003 | 39 | 15 | 60.7† |
| Gomez et al.42 | 2009 | 2000–2006 | 35 | 5 | 60† |
| Lee et al.43 | 2009 | 1990–2000 | 198 | 6.6 | 60† |
| Stenson et al.44 | 2010 | 1994–2008 | 27 | 18.4 | 62† |
| Gomez et al.45 | 2011 | 2000–2007 | 168 | 1.2 | 67.9† |
| Monnier et al.17 | 2011 | 2000–2007 | 73 | 40 | 66† |
| Crombie et al.46 | 2012 | 2000–2007 | 54 | 36 | 69.7† |
| Niewald et al.47 | 2013 | 1993–2001 | 99 | 12 | 72 |
| Tsai et al.48 | 2013 | 2000–2008 | 402 | 7.5 | 70† |
Radiation dose given in rads.
Radiation dose given in Grays (Gy).
Staging
Several staging or scoring systems of ORN have been proposed (Table 3)2., 11., 32., 54., 55., 56., 57., 58., 59.. These systems are based on response to hyperbaric oxygen (HBO) therapy, degree of bone damage, clinical–radiological findings, duration of bone exposure and treatment required.
Table 3.
Classification systems of osteoradionecrosis (ORN)
| Study | Stages | Description of stages | Basis of stage | |
|---|---|---|---|---|
| Coffin55 | 2 | Minor | A series of small sequestra which separate spontaneously after varying periods of weeks or months. These areas cannot be demonstrated radiologically | Clinical and radiological findings |
| Major | Necrosis occurring to an extent that involves the entire thickness of the jaw, and a pathological fracture is inevitable. This form can be obviously seen radiologically | |||
| Marx2 | 3 | Stage I | Exposed alveolar bone without pathologic fracture, which responds to HBO therapy | Response to HBO therapy |
| Stage II | Disease does not respond to HBO therapy, and requires sequestrectomy and saucerisation | |||
| Stage III | Full-thickness bone damage or pathological fracture, usually requires complete resection and reconstruction with free tissue | |||
| Morton and Simpson56 | 3 | Minor | Ulceration with exposed bone and a history of bony spicules that healed spontaneously over a period of months | Clinical findings and response to treatment |
| Moderate | Exposed bone and small sequestra limited in nature and healing spontaneously with conservative treatment within 6–12 months | |||
| Major | Large areas of exposed bone, with formation of large sequestra, possible fracture and sinus formation. These cases often require radical treatment | |||
| Epstein et al.32 | 3 | Stage I Ia Ib |
Resolved, healed No pathological fracture Pathological fracture |
Disease progression |
| Stage II IIa IIb |
Chronic, persistent non-progressive No pathological fracture Pathological fracture |
|||
| Stage III IIIa IIIb |
Active, progressive No pathological fracture Pathological fracture |
|||
| Glanzmann and Gratz57 | 5 | Stage 1 | Bone exposure without signs of infection and persisting for at least 3 months | Duration of bone exposure and treatment necessity |
| Stage 2 | Bone exposure with signs of infection or sequester and without the signs of Grades 3–5 | |||
| Stage 3 | Bone necrosis treated with mandibular resection with a satisfactory result | |||
| Stage 4 | Bone necrosis with persisting problems despite mandibular resection | |||
| Stage 5 | Death from ORN | |||
| Clayman54 | 2 | Type I | Bone lysis occurs under intact gingiva or mucosa | Clinical findings |
| Type II | A more aggressive type in which soft tissues break down, exposing the bone to saliva, and causing secondary contamination | |||
| Store and Boysen11 | 4 | Stage 0 | Mucosal defects only | Combination of radiological and clinical parameters |
| Stage 1 | Radiological evidence of necrotic bone with intact mucosa | |||
| Stage 2 | Positive radiological findings with denuded bone intra-orally | |||
| Stage 3 | Clinically exposed radionecrotic bone, verified by imaging techniques, along with skin fistulae and infection | |||
| Schwartz and Kagan58 | 3 | Stage I | Minimal soft-tissue ulceration and limited exposed cortical bone. Patients are treated with conservative management | Imaging and clinical findings |
| Stage II IIa IIb |
Localised involvement of the mandibular cortex and underlying medullary bone Minimal soft tissue ulceration Presence of an oro-cutaneous fistula and mild soft-tissue necrosis |
|||
| Stage III | Full-thickness involvement of the bone, including the inferior border. Pathological fractures may also be present | |||
| Notani et al.59 | 3 | Stage I | ORN confined to alveolar bone | Clinical findings |
| Stage II | ORN limited to the alveolar bone and/or mandible above the level of the inferior alveolar canal | |||
| Stage III | ORN involving the mandible below the level of the inferior alveolar canal and/or skin fistula and/or pathological fracture | |||
HBO, hyperbaric oxygen.
Coffin55 divided cases of ORN into two groups: minor and major. Morton and Simpson56 subdivided ORN into three groups – ‘minor’, ‘moderate’ and ‘major’. In 1983, Marx2 proposed a three-stage system for ORN. According to his protocol, patients are categorised as Stage I if they exhibit exposed bone in a field of radiation that has failed to heal for at least 6 months and do not have a pathological fracture, cutaneous fistula or osteolysis to the inferior border. In Stage I, all patients receive 30 sessions of HBO at 2.4 atmospheres absolute for 90 minutes at depth. Patients who respond to HBO alone (Stage I responder) demonstrate a softening of the radiated tissues and spontaneous sequestration of exposed bone with formation of granulation tissue. Each Stage I responder undergoes an additional 10 HBO sessions and then the tissues are allowed to heal completely. Stage II patients are those who do not respond to the 30 sessions of HBO. This group is characterised by a large amount of non-viable bone that makes resorption and sequestration from HBO-induced angiogenesis alone impossible. Consequently, careful surgical debridement is required. Stage II patients undergo transoral resection with limited soft-tissue reflection. In particular, surgical treatment includes extraction of involved dentition and a non-continuity bone resection to clinically bleeding bone. Wound flaps are closed primarily and the patient is given 10 postsurgical sessions of HBO. Tissues that heal without complication are treated with a prosthesis when required, similarly to Stage I responders. Stage III patients are characterised by having a large quantity of non-viable bone and/or soft tissue unable to be managed by HBO-induced angiogenesis alone or HBO combined with local sequestrectomy. In addition to 30 presurgical HBO treatments, each Stage III patient requires a continuity resection, stabilisation and 10 postsurgical sessions of HBO, and are scheduled for later (usually 3 months) reconstruction (Stage III-R). Stage III patients are therefore those who fail to respond to Stage I and Stage II treatment and those who initially present with a pathological fracture, cutaneous fistula or osteolysis to the inferior border2., 60..
Epstein et al.32 suggested a new staging system for ORN with three tiers based on clinical findings. Glanzmann and Gratz57 proposed a system based on the duration of bone exposure and necessity of treatment. Clayman54 introduced a classification of ORN related to the integrity of the overlying mucosa. According to this classification, type I includes cases of ORN in which bone lysis occurs under intact gingiva or mucosa. Type II includes more aggressive cases of ORN in which soft tissues break down and the bone is exposed to saliva, causing secondary contamination. This is defined as radiation osteomyelitis. Type I cases heal with conservative therapy; type II cases do not. In 2000, Store and Boysen16 introduced a new classification of ORN that is based on the presence or absence of clinical and radiological signs.
The most recently designed staging systems are these of Schwartz and Kagan58 and Notani et al.59 The system of Schwartz and Kagan58 is based on clinical and radiological findings. Notani et al.59 divided the cases into three grades based on the extent of the ORN lesion. Grade I is defined as ORN confined to the alveolar bone. Grade II is defined as ORN limited to the alveolar bone and/or the mandible above the level of the mandibular alveolar canal. In Grade III the ORN extends to the mandible under the level of the mandibular alveolar canal and a skin fistula and/or a pathological fracture is present.
Clinical findings
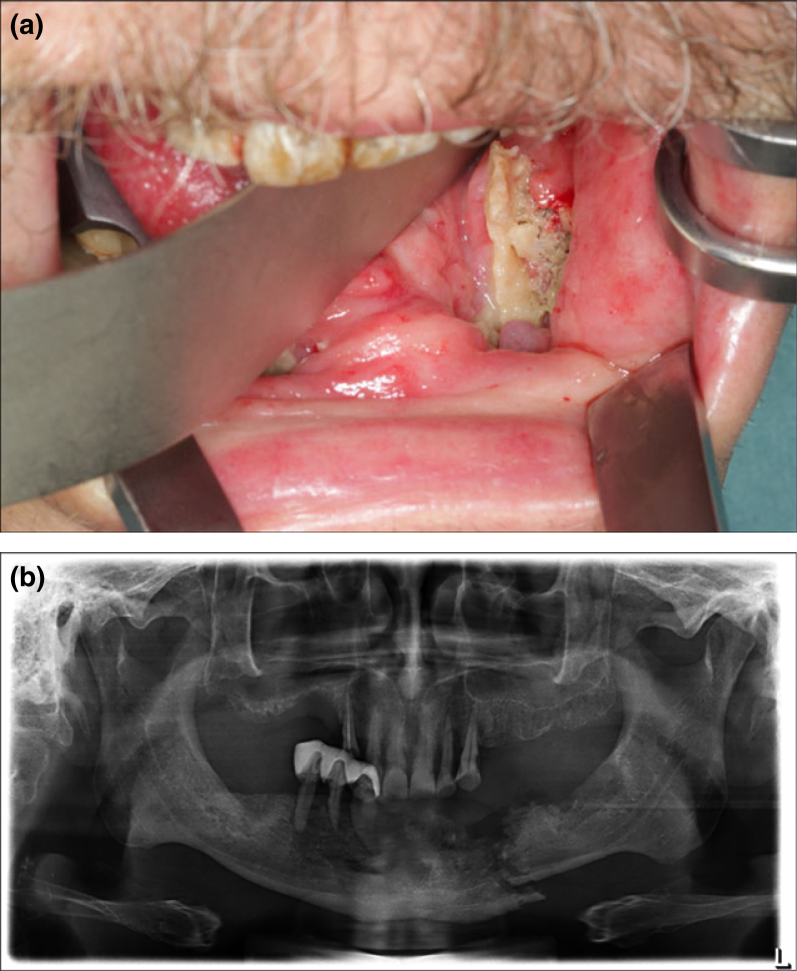
Clinical signs and symptoms of ORN include ulceration or necrosis of the mucosa with exposure of necrotic bone for longer than 3 months, pain, trismus and suppuration in the area (Figure 1a)32., 61., 62., 63., 64.. Neurological symptoms, such as pain, dysaesthesia or anaesthesia, as well as fetor oris, dysgeusia and food impaction in the area, are also usually present. Exposure of rough and irregular bone can result in physical irritation of adjacent tissues. Progression of ORN may lead to pathological fractures, intra-oral or extra-oral fistulae and local or systemic infection. Difficulties in mouth opening, mastication and speech frequently arise9., 65., 66., 67.. In patients treated with external beam radiation therapy (EBRT), osseous alterations usually appear in the body of the mandible (premolar and molar regions), whereas in those managed with brachytherapy, the lingual or buccal surfaces are affected68.
Figure 1.
A 69-year-old man presented with pain and a non-healing wound in the left lower jaw. The patient was an active smoker, suffered from pharynx cancer and had received radiotherapy (external beam radiotherapy with standard field sizes, conventional fractionation and mean dose 64 Gy) and chemotherapy. Clinically, exposed necrotic bone in the left lower jaw, inflammation, swelling and inferior alveolar nerve hypesthesia was present (a). The orthopantomogram revealed pathologic fracture of the left lower jaw (b). The patient was diagnosed with osteoradionecrosis of the lower jaw and was scheduled to be treated surgically.
The diagnosis of septic ORN appears to be easier. Marked pain is the primary symptom. A thorough clinical examination will reveal intra- or extra-oral draining fistulae, ulcerations of the mucous membrane, exposed devitalised bone, haemorrhage, cellulitis or pathological fractures. A biopsy is mandatory for final diagnosis in order to exclude metastatic cancer8.
Radiological findings
Radiographs, computed tomography (CT) scans, magnetic resonance imaging (MRI), Doppler ultrasound, nuclear medicine and near-infrared spectroscopy are often indicated in order to detect ORN18.
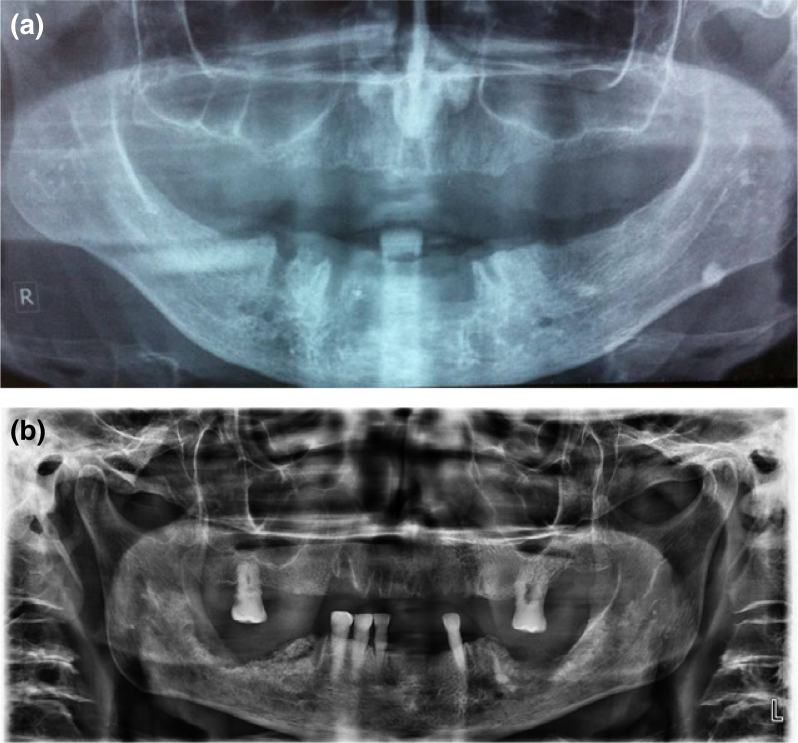
ORN is not usually detectable radiographically in early stages69. Imaging features are not correlated to the severity of ORN8., 23., 70., 71.. The described radiographic features range from normal appearance, to localised osteolytic areas, extensive osteolytic areas, sequestra and fracture (Figure 1b). Radioluncies indicating post extraction sockets will often remain visible for longer than 12 months (Figure 2a). The most definitive radiographic alterations in early disease are increased radiodensity, as well as a mixed radio-opaque/radiolucent lesion in which radiolucent areas represent bone destruction (Figure 2b)8.
Figure 2.
Orhtopantomograms of patients who have received radiotherapy (external beam radiotherapy with standard field sizes, conventional fractionation and mean dose 64 Gy) for oropharyngeal cancers. The patients underwent extractions of teeth of the lower jaw after completion of radiotherapy. The orthopantomogram revealed radiolucencies indicating post extraction sockets, which remained visible for longer than 12 months (a), as well as increased radiodensity with mixed radio-opaque/radiolucent lesions, where the radiolucent areas represent bone destruction (b).
Orthopantomogram (OPT) is the most frequently used imaging method for the diagnosis of ORN and is usually supplemented with other extra-oral or intra-oral radiographs. In an OPT, ORN is depicted as an undefined radiolucency, without sclerotic demarcation, which surrounds necrotic zone. Radiopaque areas can be identified when bone sequestra are formed. In order to be visible in an OPT, a substantial alteration in mineral content and extensive involvement of bone is required and this only occurs in later stages of ORN9. Ardran72 noted that a 30% loss of bone mineral content is necessary before any radiographic change can be seen.
CT73 shows osseous abnormalities, such as focal lytic areas, cortical interruptions and loss of the spongiosa trabeculation on the symptomatic side, frequently accompanied by soft-tissue thickening. Such a picture may cause difficulties in differential diagnosis between ORN and recurrent tumour68. In MRI with gadolinium administration, an abnormal marrow signal, cortical destruction and slight-to-mild irregular enhancement is demonstrated35., 74., 75., 76.. MRI has the advantage of excellent tissue contrast and high spatial resolution77.
Bone scintigraphy permits estimation of the extension and location of the lesion. It shows high sensitivity (up to 100%) but low specificity (about 60%) for the diagnosis of ORN77. Scintigraphy using 99mTc-marked diphosphonates (99mTc-MDP) allows highly sensitive depiction of mandibular lesions as a result of their altered phosphate metabolism. Pathophysiological changes in bone can be identified sooner when using 99mTc-MDP than when using conventional radiography because scan changes reflect osteoblastic activity and good blood flow78. Low spatial resolution and over-projection by soft tissues are the major disadvantages of the method, which can be overcome with the use of single photon emission computed tomography (SPECT)77. Finally, positron emission tomography (PET), which is a promising method for pretherapeutic assessment of the spread of squamous cell carcinomas, has been advocated as being efficient for differentiating between ORN and tumour recurrence79.
Discussion and Conclusion
Irradiation of bone is the main prerequisite for development of ORN. A differential diagnosis is required to exclude recurrence of tumour and bisphosphonate-related osteonecrosis of the jaws. In contrast to the findings of Store and Boysen11 mucosal breakdown or failure of healing is necessary for the diagnosis of ORN3., 9., 10..
The duration of bone exposure is still a matter of controversy. Some authors did not state the period of time that bone was exposed71. Other authors recommended a 2-month period of exposed bone before diagnosis73., 80., 81., or even 319., 82., 83. to 62., 5. months. There are also cases in which a late diagnosis is present. Berger and Symington84 reported two late presentations: one 45 years after radium implant therapy; and the other 38 years after external beam treatment.
A very short waiting period can lead to over-diagnosis as mucosal radionecrosis can occur without ORN. Moreover, any surgery and/or extraction usually takes up to 1 month to heal. On the other hand, monitoring a trauma for a longer period (such as 6 months) is contraindicated; intervention prior to this time is certainly needed. For the aforementioned reasons, in order to diagnose ORN, recent literature2., 3., 4., 13., 14., 15. indicates that bone exposure should be at least 3 months in duration.
The variability in prevalence of ORN can probably be attributed to differences in the study populations observation periods and existence of pretreatment dental assessment and dental management of cohorts. The literature reports numerous factors which are associated with the risk of ORN development and also affect its prevalence. Total radiation dose, brachytherapy, fractionation, poor oral hygiene, alcohol, tobacco use, dental extractions, tumour size and location, staging and chemotherapy have been highlighted7., 19., 36., 44., 47., 53., 85., 86., 87., 88., 89., 90..
The late onset of ORN can be attributed to the late occurrence of oropharyngeal cancers (OPCs) and their complications (mean age of patients over 60 years). This can be explained by the fact that oral tissues tend to undergo prolonged exposure to potential carcinogens with advancing age. In addition, aging cells may be more susceptible to DNA damage. ORN is predominant in the mandible (the ratio between ORN in the mandible and ORN in the maxilla is 24:1) 91. The reason for this could be that the mandible has a restricted localised blood supply, which is often completely within the radiation field, whereas the maxilla has many anastomoses located outside the area of irradiation. Furthermore, mandibular bone density is different from maxillary bone density, and the mandible absorbs a higher amount of radiation during radiotherapy.
The prevalence of ORN has decreased since the 1990s92. Recent studies have shown a decrease in the prevalence of ORN to levels lower than 5%. According to Clayman54, the application of megavoltage therapy resulted in a significant reduction of the overall prevalence of ORN from 11.8% before 1968 to 5.4% after this time. Wahl93 described similar results and noted a prevalence of ORN of 3% during the period 1997 to 2006. Lee et al.43 found that the frequency of ORN was 6.6% among 198 patients with either oral cavity or OPCs treated with radiation between 1990 and 2000. The overall reduction of ORN can be attributed to the advent of megavoltage radiotherapy, improved dental-preventive care and improved radiation techniques, including three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT)7., 36., 38..
Marx’s staging system2 is based on the use of, and response to, HBO. The advantages of this protocol include selection of patients who are able to respond to less aggressive treatments, use of minimal levels of HBO, resolution of the disease and preparation of patients’ tissues for reconstruction without further HBO2., 60..
Even though Marx’s staging system is the one still used by surgeons, it has been used less frequently from the mid-1990s to date. The reasons for this are that HBO is nowadays recommended for use as adjuvant therapy, its effectiveness has been questioned and most cases of ORN can be managed successfully without HBO according to the most recent literature58., 94., 95., 96.. The staging system of Epstein et al.32 is an improvement, but it is focussed on the presence or absence of a pathological fracture58.
The classification system of Notani et al.59 seems to be more accurate as it is based on: (i) the presence or absence of clinical and radiological signs, in contrast to other systems that are non-specific and are partly based on patients’ subjective interpretation; and (ii) pretreatment evaluation, not on treatment response or refractoriness. It is also simple and easily recalled97 (For review see Chronopoulos98
During the past 80 years, a number of theories about the origin of ORN have been proposed. Despite the controversy, the majority of authors agree that prerequisites for the diagnosis of ORN are: (i) previous irradiation of the affected bone; (ii) absence of recurrent tumour; (iii) presence of mucosal breakdown or failure of healing, resulting in bone exposure; and (iv) ‘necrosis’ of the overlying bone. The presence of pathological fracture, fistula formation or cellulitis is not necessary for the diagnosis. Mandibular ORN is predominant over maxillary ORN. ORN can lead to pain, fracture and sequestration of the bone, as well as to fistulae. Although the prevalence of ORN has decreased to levels lower than 5%, it still remains a pernicious complication of radiotherapy and a challenge to the clinician because of difficulty in its management. Owing to the severity of ORN and the difficulties in its early and accurate diagnosis and treatment, increased awareness of this complex entity among clinicians is desirable, with the aim to prevent its appearance or the development of severe complications. Further research is required in order to clarify its complex etiology, and guide new treatment strategies.
Acknowledgements
This review is included in the doctoral thesis of the first author, entitled ‘Clinical presentation and risk factors of osteoradionecrosis’.
Patient data were anonymised and de-identified before analysis. The clinical photograph and three radiographs were derived from the archive of the Department of Oral and Maxillofacial Surgery in Munich (LMU).
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. There was no funding received for this study.
References
- 1.Regaud C. Sur la sensibilite du tissu osseux normal vis-a-vis des rayons X et gamma et sur la mecanisme de l’osteoradionecrose. CR Soc Boil. 1922;87:629–932. [Google Scholar]
- 2.Marx RE. Osteoradionecrosis; a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64:379–390. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- 4.Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13:217–221. doi: 10.1097/01.moo.0000170527.59017.ff. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351–357. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 6.Ewing J. Radiation osteitis. Acta Radiol. 1926;6:399–412. [Google Scholar]
- 7.Jereczek-Fossa BA, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28:65–74. doi: 10.1053/ctrv.2002.0254. [DOI] [PubMed] [Google Scholar]
- 8.Guttenberg SA. Osteoradionecrosis of the jaw. Am J Surg. 1974;127:326–332. doi: 10.1016/0002-9610(74)90042-7. [DOI] [PubMed] [Google Scholar]
- 9.Epstein JB, Rea G, Wong FL, et al. Osteonecrosis-study of the relationship of dental extractions in patients receiving radiotherapy. Head Neck Surg. 1987;10:48–54. doi: 10.1002/hed.2890100108. [DOI] [PubMed] [Google Scholar]
- 10.Widmark G, Sagne S, Heikel P. Osteoradionecrosis of the jaws. Int J Oral Maxillofac Surg. 1989;18:302–306. doi: 10.1016/s0901-5027(89)80100-6. [DOI] [PubMed] [Google Scholar]
- 11.Store G, Boysen M. Mandibular osteoradionecrosis: clinical behavior and diagnostic aspects. Clin Otolaryngol. 2000;25:378–384. doi: 10.1046/j.1365-2273.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong JK, Wood RE, McLean M. Conservative management of osteoradionecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:16–21. doi: 10.1016/s1079-2104(97)90287-0. [DOI] [PubMed] [Google Scholar]
- 13.London SD, Park SS, Gampper TJ, et al. Hyperbaric oxygen for the management of radionecrosis of bone and cartilage. Laryngoscope. 1998;108:1291–1296. doi: 10.1097/00005537-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Pitak-Arnnop P, Sader R, Dhanuthai K, et al. Management of osteoradionecrosis of the jaws: an analysis of evidence. Eur J Surg Oncol. 2008;34:1123–1134. doi: 10.1016/j.ejso.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Khojastepour L, Bronoosh P, Zeinalzade M. Mandibular bone changes induced by head and neck radiotherapy. Indian J Dent Res. 2012;23:774–777. doi: 10.4103/0970-9290.111258. [DOI] [PubMed] [Google Scholar]
- 16.Thorn JJ, Hansen HS, Specht L, et al. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. [DOI] [PubMed] [Google Scholar]
- 17.Monnier Y, Broome M, Betz M, et al. Mandibular osteoradionecrosis in squamous cell carcinoma of the oral cavity and oropharynx: incidence and risk factors. Otolaryngol Head Neck Surg. 2011;144:726–732. doi: 10.1177/0194599810396290. [DOI] [PubMed] [Google Scholar]
- 18.Chrcanovic BR, Reher P, Sousa AA, et al. Osteoradionecrosis of the jaws–a current overview–part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg. 2010;14:3–16. doi: 10.1007/s10006-009-0198-9. [DOI] [PubMed] [Google Scholar]
- 19.Morrish RB, Chan E, Silverman S, et al. Osteoradionecrosis in patients irradiated for head and neck carcinoma. Cancer. 1981;47:1980–1983. doi: 10.1002/1097-0142(19810415)47:8<1980::aid-cncr2820470813>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Watson WL, Scarborough JE. Osteoradionecrosis in intraoral cancer. Am J Roentgenol. 1938;40:524–534. [Google Scholar]
- 21.Martin HE, Sugarbaker EL. Cancer of the floor of the mouth. Surg Gynecol Obstet. 1940;71:347–359. [Google Scholar]
- 22.MacComb WS. Necrosis in treatment of intraoral cancer by radiation therapy. Am J Roentgenol Radium Ther Nucl Med. 1962;87:431–440. [PubMed] [Google Scholar]
- 23.MacDougall JA, Evans AM, Lindsay RK. Osteoradionecrosis of the mandible and its treatments. Am J Surg. 1963;106:816–818. doi: 10.1016/0002-9610(63)90407-0. [DOI] [PubMed] [Google Scholar]
- 24.Grant BP, Fletcher GH. Analysis of complications following megavoltage therapy for squamous cell carcinomas of the tonsillar area. Am J Roentgenol Radium Ther Nucl Med. 1966;96:28–36. doi: 10.2214/ajr.96.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Rahn AO, Drane JB. Dental aspects of the problems, care and treatment of the irradiated oral cancer patient. J Am Dent Assoc. 1967;74:957–966. doi: 10.14219/jada.archive.1967.0154. [DOI] [PubMed] [Google Scholar]
- 26.Rankow RM, Weissman B. Osteoradionecrosis of the mandible. Ann Otol Rhinol Laryngol. 1971;80:603–611. doi: 10.1177/000348947108000426. [DOI] [PubMed] [Google Scholar]
- 27.Wang CC. Management and prognosis of squamous cell carcinoma of the tonsillar region. Radiology. 1972;104:667–671. doi: 10.1148/104.3.667. [DOI] [PubMed] [Google Scholar]
- 28.Cheng VST, Wang CC. Osteoradionecrosis of the mandible resulting from external megavoltage radiation therapy. Radiology. 1974;112:685–689. doi: 10.1148/112.3.685. [DOI] [PubMed] [Google Scholar]
- 29.Marciani RD, Plezia RA. Osteoradionecrosis of the mandible. J Oral Surg. 1974;32:435–440. [PubMed] [Google Scholar]
- 30.Bedwinek JM, Shukovsky LJ, Fletcher GH, et al. Osteoradionecrosis in patients treated with definite radiotherapy for squamous cell carcinoma of the oral cavity and naso- and oropharynx. Radiology. 1976;119:665–667. doi: 10.1148/119.3.665. [DOI] [PubMed] [Google Scholar]
- 31.Murray CG, Herson J, Daly TE, et al. Radiation necrosis of the mandible: a 10 year study. Part I. Factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:543–548. doi: 10.1016/0360-3016(80)90380-6. [DOI] [PubMed] [Google Scholar]
- 32.Epstein JB, Wong FLW, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg. 1987;45:104–110. doi: 10.1016/0278-2391(87)90399-5. [DOI] [PubMed] [Google Scholar]
- 33.Withers HR, Peters LJ, Taylor JM, et al. Late normal tissue sequelae from radiation therapy for carcinoma of the tonsil: patterns of fractionation study of radiobiology. Int J Radiat Oncol Biol Phys. 1995;33:563–568. doi: 10.1016/0360-3016(95)00229-R. [DOI] [PubMed] [Google Scholar]
- 34.Turner SL, Slevin NJ, Gupta NK, et al. Radical external beam radiotherapy for 333 squamous carcinomas of the oral cavity–evaluation of late morbidity and a watch policy for the clinically negative neck. Radiother Oncol. 1996;41:21–29. doi: 10.1016/s0167-8140(96)91785-5. [DOI] [PubMed] [Google Scholar]
- 35.Storey MR, Garden AS, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001;51:952–958. doi: 10.1016/s0360-3016(01)01724-2. [DOI] [PubMed] [Google Scholar]
- 36.Reuther T, Schuster T, Mende U, et al. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 37.Oh HK, Chambers MS, Garden AS, et al. Risk of osteoradionecrosis after extraction of impacted third molars in irradiated head and neck cancer patients. J Oral Maxillofac Surg. 2004;62:139–144. doi: 10.1016/j.joms.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Studer G, Gratz KW, Glanzmann C. Osteoradionecrosis of the mandibula in patients treated with different fractionations. Strahlenther Onkol. 2004;180:233–240. doi: 10.1007/s00066-004-1171-z. [DOI] [PubMed] [Google Scholar]
- 39.Ben-David MA, Diamante M, Radawski JD, et al. Lack of osteoradionecrosis of the mandible after intensity – modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved distributions. Int J Radiat Oncol Biol Phys. 2007;68:396–402. doi: 10.1016/j.ijrobp.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jham BC, Reis PM, Miranda EL, et al. Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin Oral Investig. 2008;12:19–24. doi: 10.1007/s00784-007-0149-5. [DOI] [PubMed] [Google Scholar]
- 41.Katsura K, Sasai K, Sato K, et al. Relationship between oral health status and development of osteoradionecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:731–738. doi: 10.1016/j.tripleo.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Gomez DR, Zhung JE, Gomez J, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2009;73:1096–1103. doi: 10.1016/j.ijrobp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Ij Lee, Koom WS, Lee CG, et al. Risk factors and dose-effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int J Radiat Oncol Biol Phys. 2009;75:1084–1091. doi: 10.1016/j.ijrobp.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 44.Stenson KM, Kunnavakkam R, Cohen EE, et al. Chemoradiation for patients with advanced oral cavity cancer. Laryngoscope. 2010;120:93–99. doi: 10.1002/lary.20716. [DOI] [PubMed] [Google Scholar]
- 45.Gomez DR, Estilo CL, Wolden SL, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensitymodulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:207–213. doi: 10.1016/j.ijrobp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Crombie AK, Farah C, Tripcony L, et al. Primary chemoradiotherapy for oral cavity squamous cell carcinoma. Oral Oncol. 2012;48:1014–1018. doi: 10.1016/j.oraloncology.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Niewald M, Fleckenstein J, Mang K, et al. Dental status, dental rehabilitation procedures, demographic and oncological data as potential risk factors for infected osteoradionecrosis of the lower jaw after radiotherapy for oral neoplasms: a retrospective evaluation. Radiat Oncol. 2013;8:227. doi: 10.1186/1748-717X-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85:415–420. doi: 10.1016/j.ijrobp.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Daly TE, Drane JB, MacComb WS. Management of problems of the teeth and jaw in patients undergoing irradiation. Am J Surg. 1972;124:539–542. doi: 10.1016/0002-9610(72)90082-7. [DOI] [PubMed] [Google Scholar]
- 50.Grötz KA, Riesenbeck D, Brahm R, et al. Chronic radiation effects on dental hard tissue (radiation caries). Classification and therapeutic strategies. Strahlenther Onkol. 2001;177:96–104. doi: 10.1007/pl00002390. [DOI] [PubMed] [Google Scholar]
- 51.Almazrooa SA, Woo SB. Bisphosphonate and non-bisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140:864–875. doi: 10.14219/jada.archive.2009.0280. [DOI] [PubMed] [Google Scholar]
- 52.Mendenhall WM. Mandibular osteoradionecrosis. J Clin Oncol. 2004;22:4867–4868. doi: 10.1200/JCO.2004.09.959. [DOI] [PubMed] [Google Scholar]
- 53.Hao SP, Chen HC, Wei FC, et al. Systematic management of osteoradionecrosis in the head and neck cancer. Laryngoscope. 1999;109:1324–1327. doi: 10.1097/00005537-199908000-00027. [DOI] [PubMed] [Google Scholar]
- 54.Clayman L. Management of dental extractions in irradiated jaws: a protocol without hyperbaric oxygen therapy. J Oral Maxillofac Surg. 1997;55:275–281. doi: 10.1016/s0278-2391(97)90542-5. [DOI] [PubMed] [Google Scholar]
- 55.Coffin F. The incidence and management of osteoradionecrosis of the jaws following head and neck radiotherapy. Br J Radiol. 1983;56:851–885. doi: 10.1259/0007-1285-56-671-851. [DOI] [PubMed] [Google Scholar]
- 56.Morton ME, Simpson W. The management of osteoradionecrosis of the jaws. Br J Oral Maxillofac Surg. 1986;24:332–341. doi: 10.1016/0266-4356(86)90018-5. [DOI] [PubMed] [Google Scholar]
- 57.Glanzmann C, Gratz KW. Radionecrosis of the mandibula: a retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36:94–100. doi: 10.1016/0167-8140(95)01583-3. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz HC, Kagan AR. Osteoradionecrosis of the mandible: scientific basis for clinical staging. Am J Clin Oncol. 2002;25:168–171. doi: 10.1097/00000421-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Notani K, Yamazaki Y, Kitada H, et al. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck. 2003;25:181–186. doi: 10.1002/hed.10171. [DOI] [PubMed] [Google Scholar]
- 60.Peleg M, Lopez EA. The treatment of osteoradionecrosis of the mandible: the case for hyperbaric oxygen and bone graft reconstruction. J Oral Maxillofac Surg. 2006;64:956–960. doi: 10.1016/j.joms.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 61.Baker SR. Management of osteoradionecrosis of the mandible with myocutaneous flaps. J Surg Oncol. 1983;24:282–289. doi: 10.1002/jso.2930240409. [DOI] [PubMed] [Google Scholar]
- 62.Nakatsuka T, Harii K, Yamada A, et al. Surgical treatment of mandibular osteoradionecrosis: versatility of the scapular osteocutaneous flap. Scand J Plast Reconstr Hand Surg. 1996;30:291–298. doi: 10.3109/02844319609056407. [DOI] [PubMed] [Google Scholar]
- 63.Shaha AR, Cordeiro PG, Hidalgo DA, et al. Resection and immediate microvascular reconstruction in the management of osteoradionecrosis of the mandible. Head Neck. 1997;19:406–411. doi: 10.1002/(sici)1097-0347(199708)19:5<406::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Oh HK, Chambers MS, Martin JW, et al. Osteoradionecrosis of the mandible: treatment outcomes and factors influencing the progress of osteoradionecrosis. J Oral Maxillofac Surg. 2009;67:1378–1386. doi: 10.1016/j.joms.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Jacobson AS, Buchbinder D, Hu K, et al. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46:795–801. doi: 10.1016/j.oraloncology.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Mücke T, Konen M, Wagenpfeil S, et al. Low-dose preoperative chemoradiation therapy compared with surgery alone with or without postoperative radiotherapy in patients with head and neck carcinoma. Ann Surg Oncol. 2011;18:2739–2747. doi: 10.1245/s10434-011-1643-1. [DOI] [PubMed] [Google Scholar]
- 67.Mücke T, Koschinski J, Wagenpfeil S, et al. Functional outcome after different oncological interventions in head and neck cancer patients. J Cancer Res Clin Oncol. 2011;138:371–376. doi: 10.1007/s00432-011-1106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermans R, Fossion E, Ioannides C, et al. CT findings in osteoradionecrosis of the mandible. Skeletal Radiol. 1996;25:31–36. doi: 10.1007/s002560050028. [DOI] [PubMed] [Google Scholar]
- 69.Miles DA. Imaging inflammatory disorders of the jaw: simple osteitis to generalised osteomyelitis. Oral Maxillofac Surg Clin North Am. 1992;1:207–221. [Google Scholar]
- 70.Niebel HH, Neeman EW. Dental aspects of osteoradionecrosis. Oral Surg Oral Med Oral Pathol. 1957;10:1011–1024. doi: 10.1016/0030-4220(57)90048-8. [DOI] [PubMed] [Google Scholar]
- 71.Epstein JB, Wong FLW, Dickens A, et al. Bone and gallium scans in postradiotherapy osteonecrosis of the jaw. Head Neck. 1992;14:288–292. doi: 10.1002/hed.2880140406. [DOI] [PubMed] [Google Scholar]
- 72.Ardran G. Bone destruction not demonstrable by radiography. Br J Radiol. 1951;24:107–109. doi: 10.1259/0007-1285-24-278-107. [DOI] [PubMed] [Google Scholar]
- 73.Tobias JS, Thomas PRM. Arnold; London: 1996. Current Radiation Oncology; pp. 144–177. [Google Scholar]
- 74.Fujita M, Harada K, Masaki N, et al. MR imaging of osteoradionecrosis of the mandible following radiotherapy for head and neck cancers. Nihon Igaku Hoshasen Gakkai Zasshi. 1991;51:892–900. [PubMed] [Google Scholar]
- 75.Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes in the central nervous system and head and neck. Radiographics. 1996;16:1055–1072. doi: 10.1148/radiographics.16.5.8888390. [DOI] [PubMed] [Google Scholar]
- 76.Yoshioka H, Nakano T, Kandatsu S, et al. MR imaging of radiation osteitis in the sacro-iliac joints. Magn Reson Imaging. 2000;18:125–128. doi: 10.1016/s0730-725x(99)00129-0. [DOI] [PubMed] [Google Scholar]
- 77.Bachmann G, Rossler R, Klett R, et al. The role of magnetic resonance imaging and scintigraphy in the diagnosis of pathologic changes of the mandible after radiation therapy. Int J Oral Maxillofac Surg. 1996;25:189–195. doi: 10.1016/s0901-5027(96)80027-0. [DOI] [PubMed] [Google Scholar]
- 78.Alexander JM. Radionuclide bone scanning in the diagnosis of lesions of the maxillofacial region. J Oral Surg. 1976;34:249–256. [PubMed] [Google Scholar]
- 79.Minn H, Aitasalo K, Happonen RP. Detection of cancer recurrence in irradiated mandible using positron emission tomography. Eur Arch Otorhinolaryngol. 1993;250:312–315. doi: 10.1007/BF00186234. [DOI] [PubMed] [Google Scholar]
- 80.Beumer J, 3rd, Curtis T, Harrison RE. Radiation therapy of the oral cavity: sequelae and management, part 2. Head Neck Surg. 1979;1:392–408. doi: 10.1002/hed.2890010504. [DOI] [PubMed] [Google Scholar]
- 81.Hutchinson IL, Colpe M, Delpy DT, et al. The investigation of osteoradionecrosis of the mandible by near infrared spectroscopy. Br J Oral Maxillofac Surg. 1990;28:150–154. doi: 10.1016/0266-4356(90)90077-x. [DOI] [PubMed] [Google Scholar]
- 82.Beumer J, 3rd, Harrison R, Sanders B, et al. Pre-radiation dental extractions and the incidence of bone necrosis. Head Neck Surg. 1983;5:514–521. doi: 10.1002/hed.2890050611. [DOI] [PubMed] [Google Scholar]
- 83.Harris M. The conservative management of osteoradionecrosis of the mandible with ultrasound therapy. Br J Oral Maxillofac Surg. 1992;30:313–318. doi: 10.1016/0266-4356(92)90181-h. [DOI] [PubMed] [Google Scholar]
- 84.Berger RP, Symington JM. Long-term clinical manifestation of osteoradionecrosis of the mandible: report of two cases. J Oral Maxillofac Surg. 1990;48:82–84. doi: 10.1016/0278-2391(90)90187-7. [DOI] [PubMed] [Google Scholar]
- 85.Kluth EV, Jain PR, Stuchell RN, et al. A study of factors contributing the development of osteoradionecrosis of the jaws. J Prosthet Dent. 1988;59:194–201. doi: 10.1016/0022-3913(88)90015-7. [DOI] [PubMed] [Google Scholar]
- 86.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol. 2000;18:1458–1464. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 87.Denis F, Garaud P, Bardet E, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys. 2003;55:93–98. doi: 10.1016/s0360-3016(02)03819-1. [DOI] [PubMed] [Google Scholar]
- 88.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 89.Budach V, Stuschke M, Budach W, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J Clin Oncol. 2005;23:1125–1135. doi: 10.1200/JCO.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 90.Semrau R, Mueller RP, Stuetzer H, et al. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:1308–1316. doi: 10.1016/j.ijrobp.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 91.Perrier M, Moeller P. Osteoradionecrosis. A review of the literature. Schweiz Monatsschr Zahnmed. 1994;104:271–277. [PubMed] [Google Scholar]
- 92.Berger A, Bensadoun RJ. Normal tissue tolerance to external beam radiation therapy: the mandible. Cancer Radiother. 2010;14:295–300. doi: 10.1016/j.canrad.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Wahl MJ. Osteoradionecrosis prevention myths. Int J Radiat Oncol Biol Phys. 2006;64:661–669. doi: 10.1016/j.ijrobp.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 94.Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw—a randomized, placebo-controled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893–4900. doi: 10.1200/JCO.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 95.Bessereau J, Annane D. Treatment of osteoradionecrosis of the jaw: the case against the use of hyperbaric oxygen. J Oral Maxillofac Surg. 2010;68:1907–1910. doi: 10.1016/j.joms.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Pitak-Arnnop P, Hemprich A, Dhanuthai K, et al. A systematic review in 2008 did not show value of hyperbaric oxygen therapy for osteoradionecrosis. J Oral Maxillofac Surg. 2010;68:2644–2645. doi: 10.1016/j.joms.2010.06.195. [DOI] [PubMed] [Google Scholar]
- 97.Shaw RJ, Dhanda J. Hyperbaric oxygen in the management of late radiation injury to the head and neck. Part I: Treatment. Br J Oral Maxillofac Surg. 2011;49:2–8. doi: 10.1016/j.bjoms.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 98.Chronopoulos A. (2015). Clinical presentation and risk factors of osteoradionecrosis (Doctoral dissertation). Available from: https://edoc.ub.uni-muenchen.de/18089/ and https://edoc.ub.uni-muenchen.de/18089/1/Chronopoulos_Aristeidis.pdf (Persistent Identifier URN: urn:nbn:de:bvb:19-180892). Accessed 15 April 2015.