Abstract
Objectives: To investigate the loss of enamel and dentin surface caused by the interaction between abrasives in toothpaste and toothbrush filament stiffness. Methods: The study followed a 2 (high-level or low-level abrasive; silica) × 3 (filament stiffness; soft, medium or hard) × 2 (cycling time; 3 or 5 days) factorial design. Polished bovine enamel and dentin specimens (n = 8 each per group) were subjected to 5 days of erosion/abrasion cycling: erosion (5 minutes, four times daily, 0.3% citric acid, pH 3.75); abrasion (15 seconds, twice daily, 45 strokes each, 150 g load, automated brushing machine); and fluoride treatment [15 seconds with abrasion and 45 seconds without abrasion; 275 p.p.m. fluoride (F−) as sodium fluoride (NaF) in abrasive slurry]. Enamel and dentin specimens were exposed to artificial saliva between erosion and abrasion/F− treatment (1 hour) and at all other times (overnight). Non-contact profilometry was used to determine surface loss (SL) after 3 and 5 days of cycling. Data were analysed using three-way analysis of variance (ANOVA) (factors: abrasive/filament stiffness/time), with separate analyses conducted for enamel and dentin. Results: For enamel, only ‘cycling time’ was found to affect SL, with 5 days of cycling resulting in a greater SL than 3 days of cycling. Overall, there was little SL for enamel (range: 0.76–1.85 μm). For dentin (SL range: 1.87–5.91 μm), significantly higher SL was found for 5 days of cycling versus 3 days of cycling, with particularly large differences for hard stiffness/high-level abrasive and medium stiffness/low-level abrasive. For high-level abrasive, after 5 days of cycling hard stiffness resulted in significantly higher SL than did medium stiffness, with no other significant differences according to stiffness. Overall, high-level abrasive resulted in significantly higher SL than did low-level abrasive, with strong effects for all combinations, except medium stiffness after 5 days. Conclusion: The interplay between abrasivity and filament stiffness appears to be more relevant for dentin than for enamel.
Key words: Abrasion, dentin, enamel, erosion, filament stiffness, toothbrushing
Introduction
Toothbrushing is the most common method to maintain good oral hygiene. The general consensus is that the use of toothpastes and toothbrushes in line with guidelines of governmental and professional bodies does not cause significant wear of enamel and dentin over the course of life1., 2.. However, both toothpastes and toothbrushes have been shown to play a crucial role in the manifestation of erosive tooth wear3., 4.. While a brief challenge with acid leads to no significant surface loss per se, it softens the hard tissue structure, making it more vulnerable to abrasion compared with hard tissue with no acid challenge5. Longitudinally, this can amount to clinically significant wear of the dentition, loss of form and function of the teeth and, ultimately, costly restorative procedures. Furthermore, dentin hypersensitivity has been considered an erosive tooth-wear phenomenon6, with more definitive evidence about their association emerging recently7.
There is a multitude of individual and often additive or synergistic, but rarely mitigating, factors that may potentially impact the severity of wear of the dental hard tissues. A significant body of literature exists regarding these factors, including: the type of abrasive and its concentration; slurry viscosity; brushing force, frequency and duration; type of toothbrush and its geometry and age; filament stiffness; and the condition of the substrate as modified by the severity, duration and timing of the acid challenge and remineralisation phase8., 9., 10., 11., 12., 13., 14., 15., 16.. Based on these data it has been postulated that the abrasivity of the toothpaste is the most important parameter that affects the abrasion process of the dental hard tissues, with the toothbrush acting as the carrier, thereby merely modifying the effects of the toothpaste abrasives1. This conclusion has been derived predominantly from a range of in vitro13., 15. studies with, however, conflicting results regarding the impact of filament stiffness on enamel and dentin13., 15..
While there is little doubt that toothpaste abrasivity, measured as either relative dentin abrasivity (RDA) or relative enamel abrasivity (REA)17, is positively correlated with wear of initially sound9 or eroded9., 15.dentin, and sound10 or eroded9., 13. enamel, respectively, the role of filament stiffness is somewhat unclear. Filament stiffness is controlled by filament diameter; so-called ‘hard toothbrushes’ have filaments with a larger diameter than do ‘soft toothbrushes’, with the most common ‘medium toothbrushes’ having filaments of diameter between those of ‘soft’ and ‘hard’ toothbrushes. It is often postulated that hard toothbrushes cause more wear than soft ones, which has led to recommendations of soft toothbrushes for patients diagnosed with erosive tooth wear18., 19.. Mechanistic laboratory studies, however, revealed the opposite, as soft toothbrushes were found to accelerate the wear process because of their greater ability to carry abrasive particles across the surface13., 15., 16.. Nonetheless, these studies were somewhat limited in their approach as acid challenges were mimicking intrinsic erosion13., 15. or abrasion periods were too long16, therefore probably exaggerating the effects. The present study aimed to address these shortcomings and resolve the interaction between toothbrush filaments and abrasives used in toothpastes using a clinically relevant erosion/abrasion cycling model11., 14.. The aim of this in vitro study was therefore to investigate the interaction between two distinct levels of abrasion commonly found in toothpastes and soft, medium and hard toothbrushes, on the development of erosive/abrasive lesions in enamel and dentin under pH cycling conditions.
Materials and Methods
Study design
This factorial design study investigated the interaction between abrasivity (two levels: high/low; silica) and toothbrush filament stiffness (three levels: soft/medium/hard) over different periods of time (3 or 5 days: cycling time). Polished bovine enamel and dentin specimens (n = 8 each per group) were subjected to 5 days of erosion/abrasion cycling: erosion (5 minutes, four times daily, 0.3% citric acid, pH 3.75); abrasion (15 seconds, twice daily, 45 strokes each, 150 g load, automated brushing machine); and fluoride treatment [15 seconds with abrasion and 45 seconds without abrasion; 275 p.p.m. fluoride (F–) as sodium fluoride (NaF) in abrasive slurry]. Enamel and dentin specimens were exposed to artificial saliva between erosion and abrasion/F– exposure (1 hour) and at all other times (overnight). Non-contact profilometry was used to determine surface loss (SL) after 3 and 5 days of cycling.
Specimen preparation
Enamel and dentin specimens (4 × 4 × 2 mm3), obtained from bovine incisors stored in 0.1% thymol solution, were prepared. Bovine teeth were obtained from Tri State Beef Co. (Cincinnati, OH, USA), from cattle of average age 3 years (range: 18 months to 5 years). The bottom and top of the enamel and dentin sides of the slabs were sequentially ground flat using silicon carbide grinding papers (Struers RotoPol 31/RotoForce 4 polishing unit; Struers, Cleveland, PA, USA). Enamel and dentin specimens were embedded, side by side with a small space in between, in acrylic resin (Varidur acrylic system, Buehler, Lake Bluff, IL, USA), utilising a custom-made silicone mold, leaving the enamel and dentin surfaces exposed. The embedded blocks were then serially ground and polished up to 4,000-grit grinding paper followed by a 1-μm diamond polishing suspension (Struers). Unplasticised polyvinyl chloride (uPVC) tapes were placed on the surface of the specimens, leaving an area of 1 mm × 4 mm exposed in the centre of the each enamel/dentin specimen. Specimens were selected based on the quality of enamel and dentin: those exhibiting surface scratches, cracks, hypomineralised areas or a non-uniform surface polish were excluded. Specimens were then randomised into six experimental groups with eight specimens per group.
Erosive and remineralising solutions
A solution of 0.3% (w/v) anhydrous citric acid (Sigma C1857, St. Louis, MO, USA) in deionised water (pH 3.75) was used as an erosive challenge in this study14. Artificial saliva [1.45 mM Ca2+, 5.4 mM PO43–, 0.1 M Tris buffer, 2.2 g/l of porcine gastric mucin, pH 7.0] was used as the remineralisation medium14.
Abrasive slurries, toothbrushes and brushing abrasion
Two aqueous abrasive slurries were prepared using precipitated silica abrasives: ‘high’ [15% (w/w) Zeodent 103; Huber Engineered Materials, Havre de Grace, MD, USA] and ‘low’ [5% (w/w) Zeodent 113]. Slurries also contained 275 p.p.m. F– as NaF (mimicking 1,100 p.p.m. F toothpaste after 1:3 dilution), 0.5% (w/w) carboxymethylcellulose (Blanose 7MF; Ashland, Covington, KY, USA) and 10% (w/w) glycerol. The RDA and REA, determined according to ISO 11609, of the abrasive slurries were: low, REA = 4.0/RDA = 69; and high, REA = 7.1/RDA = 208.
Table 1 provides information about the toothbrushes used in the study (Lactona, Bergen op Zoom, The Netherlands). Filament diameter was determined using a calibrated light reflection microscope (2100 HT; Wilson Instruments, Norwood, MA, USA).
Table 1.
Properties of the study toothbrushes
| Parameter | Soft | Medium | Hard |
|---|---|---|---|
| Filament diameter (μm) | 212.8 | 228.6 | 310.4 |
| Bristle length (mm) | 11 | 11 | 11 |
| Tufts | 43 | 43 | 43 |
| Bristles per tuft | 50 | 36 | 16 |
Specimens were positioned in an automated brushing machine and brushed for 15 seconds (45 strokes) with one of three test toothbrushes (load of 150 g) with their respective abrasive slurries.
Daily treatment regimen
The daily treatment regimen is presented in Table 2. The experiment was conducted at room temperature. Erosion was performed under static conditions, whereas the artificial saliva was stirred at 100 r.p.m. After each cycling procedure, specimens were rinsed with deionised water for 10 seconds.
Table 2.
Daily treatment schedule
| Treatment | Duration |
|---|---|
| Erosion (1/4) | 5 minutes |
| Remineralisation (1/6) | 60 minutes |
| Treatment/abrasion (1/2) | 1 minute* |
| Remineralisation (2/6) | 60 minutes |
| Erosion (2/4) | 5 minutes |
| Remineralisation (3/6) | 60 minutes |
| Erosion (3/4) | 5 minutes |
| Remineralisation (4/6) | 60 minutes |
| Erosion (4/4) | 5 minutes |
| Remineralisation (5/6) | 60 minutes |
| Treatment/abrasion (2/2) | 1 minute* |
| Remineralisation (6/6) | Overnight |
Brushing for 15 seconds (45 strokes) + exposure to slurry for 45 seconds.
Profilometry
After 3 and 5 days of cycling, enamel and dentin SL were measured by non-contact profilometry (Proscan 2000; Scantron, Taunton, Somerset, UK). The uPVC tapes were removed from the specimens and an area of 1 × 4 mm2 in the centre of the specimen (including both exposed and previously tape-covered areas) was scanned. Dedicated software (Proscan 2000; Scantron) was used to analyse SL using a three-point height tool.
Statistical analysis
The effects of cycling time, slurry abrasiveness and toothbrush filament hardness on surface loss were examined using analysis of variance (ANOVA). Separate analyses were performed for enamel and dentin. The ANOVA included main effect terms for each of the three factors, all interactions among the factors and a random effect to correlate the results from the two cycles within a sample. Fisher's Protected Least Significant Difference test was used to control the overall significance level of the tests. A 5% significance level was used.
Based on a previous study, the within-group standard deviation of the surface loss was expected to be 1.5 μm. With a sample size of eight specimens per abrasivity–hardness combination, the study had 80% power to detect differences of 2.3 μm between any two abrasivity–hardness combinations for each cycling time, assuming two-sided tests were conducted at a 5% significance level.
Results
Enamel
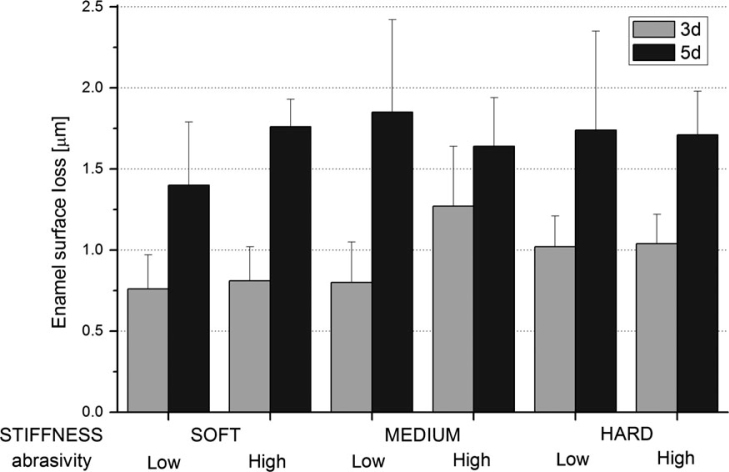
Figure 1 shows the enamel SL data (mean ± standard error) according to filament stiffness, abrasivity level and cycling time. The three-way interaction, abrasivity × stiffness × cycling time, was not significant (P = 0.48), and neither were any of the two-way interactions (P = 0.86–0.98). Neither abrasivity (P = 0.24) nor filament stiffness (P = 0.62) affected SL. Only cycling time significantly affected SL (P = 0.0003), with SL on day 5 (1.68 ± 0.16 μm) being proportionally higher than SL on day 3 (0.95 ± 0.10 μm).
Figure 1.
Enamel surface loss as a function of high-level or low-level abrasive and bristle stiffness during the pH-cycling period.
Dentin
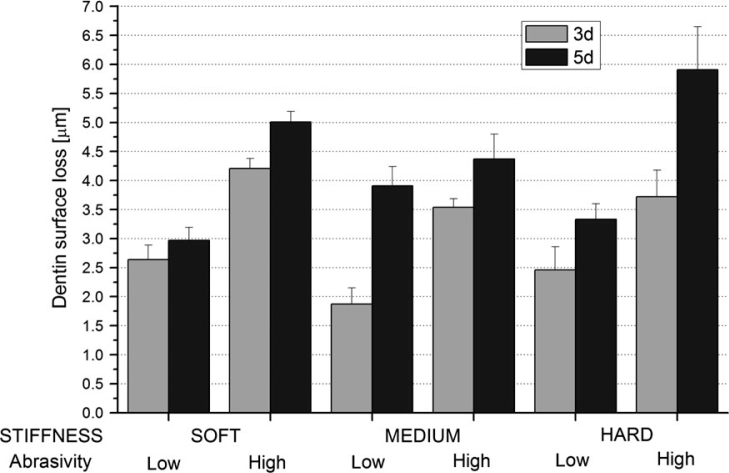
Figure 2 shows the dentin SL data (mean ± standard error) according to filament stiffness, abrasivity level and cycling time. The three-way interaction, abrasivity × stiffness × cycling time, was significant (P = 0.0464). However, the data did not show significant interaction between the two main factors (abrasivity and filament stiffness; P = 0.1948). Cycling time affected SL (P < 0.0001) but not proportionally (day 3: 3.07 ± 0.17 μm vs. day 5: 4.25 ± 0.21 μm), with particularly large differences for hard toothbrush/high abrasive (P < 0.0001) and medium toothbrush/low abrasive (P = 0.0001). Use of hard toothbrush and high abrasive resulted in significantly higher SL compared with use of medium toothbrush and high abrasive at day 5 (P = 0.0088), with no other significant toothbrush differences (P > 0.18). Overall, use of high abrasive resulted in significantly higher SL than did use of low abrasive (P < 0.0001), with strong effects for all combinations, except for medium toothbrush at day 5. SL was directionally, but disproportionately, correlated with RDA values: SL: 4.46 vs. 2.86 μm with ratio of 1.56:1; and RDA: 208 vs. 69 with ratio of 3.0:1).
Figure 2.
Dentin surface loss as a function of high-level or low-level abrasive and bristle stiffness during the pH-cycling period.
Discussion
The aim of the present study was to investigate SL of eroded enamel and dentin resulting from the interaction between toothpaste abrasivity and toothbrush filament stiffness using an established 5-day erosion/abrasion pH-cycling model11., 14.. The often recommended brushing time of 2 minutes per brushing equates to approximately 15 seconds per surface20 which was simulated in the present study by brushing each specimen for 15 seconds or 45 strokes per cycle. This equates to a total of 450 brushing strokes for the entire study duration of 5 days (90 strokes/days) and is considerably less than that used in previous in vitro (strokes/days: 45011; 16013., 15.; 450–1,50014) and in situ (daily brushing duration: 2 minutes9; 1 minutes10) studies on this topic. These levels of brushing abrasion are all justifiable as brushing duration varies not only between individuals but also between surfaces within individuals21. Not considering behavioural aspects, there is little increase in SL of sound enamel with increasing brushing duration; SL of previously eroded enamel, however, increases with the number of brushing strokes, although not in a linear manner22. This is a result of the gradual loss of affected surface enamel which behaves differently from the underlying bulk tissue. Similar results were obtained for dentin23, although wear of sound dentin can become significant during a lifetime of toothbrushing abuse.
Flat-trim manual toothbrushes bearing tufts of filaments with round-ended tips were chosen because they represent the most commonly used toothbrush. Likewise, the brushing load of 150 g was selected in line with recommendations of the International Standards Organization (ISO11609), for toothbrushing abrasivity tests. Slurries of the most commonly used abrasives (conventional and whitening precipitated silicas), rather than actual toothpastes, were used to minimise the influence of formulation parameters and excipients, which can modify the abrasion process (e.g. viscosity, pH, anti-tartar agents).
The high-abrasive slurry resulted in greater SL of eroded dentin than did the low-abrasive slurry. These results were expected and are in agreement with previous findings8., 9., 15.. From the present data, it can be assumed that low-abrasive toothpastes may only abrade the superficial layer of softened dentin, whereas their high-abrasive counterparts are likely to affect deeper parts of the challenged dentin structure. However, eroded dentin is susceptible to wear, even under mild erosive/abrasive conditions, and much more so than enamel. This may be related to the fact that dentin is a more vulnerable tissue than enamel with little tendency to remineralise once its structural backbone (collagen) has been affected by physical insult24.
The use of toothpastes with low RDA is part of the recommendations for patients with signs of erosive tooth wear18., 19.. While this is undoubtedly a ‘common sense’ recommendation, its implementation presents issues as manufacturers are not legally required to declare RDA (or REA) data for their products, leaving patients in the dark. Furthermore, RDA and REA are determined under conditions atypical of in vivo tooth wear and do not necessarily correlate linearly with clinical or laboratory observations. In the present study, the discrepancy found between RDA and SL data for dentin can be explained by various factors, such as the presence of fluoride in the abrasive slurries which would have allowed subsequent remineralisation, and the study design (continuous brushing abrasion vs. cycling to allow for relaxation and remineralisation of the tissue). Therefore, RDA data should be used solely as guidance.
Previous research has established that nylon toothbrushes alone have negligible effects on the dental hard tissues25 but may indirectly influence the abrasion process by modulating the action of toothpaste abrasives. This is related to the previous indication8 that different types of toothbrushes probably differ in their capacity to carry toothpaste abrasives across the surface, which may result in differences in abrasion of the dental substrate. Filament stiffness, density of the brush and area of the brush head covered by filaments were shown to modulate this process8.
In two previous studies of similar design15., 16., wear of eroded dentin increased with decreasing filament diameter. However, the data of the present study suggest that filament stiffness is likely to be a secondary factor in wear of eroded dentin, after abrasivity. Surprisingly, the data in the present study showed that toothbrushing, either alone or with abrasives, was not a significant factor in dentin SL. Only when combined with cycling time did the interaction became significant. Previous studies15., 16. employed stronger acid challenges, higher brushing loads and longer brushing durations than tested in the present study, which, taken together, may explain why previous studies were able to demonstrate filament effects. It is likely that the brushing abrasion was too mild in the present study, thus not allowing potential differences between different stiffnesses of filaments to be observed. However, it must be borne in mind that the present study was designed to mimic day-to-day life rather than to demonstrate the effect of a variable implicated in erosive tooth wear.
Enamel did not respond in the same manner as dentin. Neither abrasivity nor filament stiffness were implicated in the wear process. These findings are somewhat in agreement with previous investigations. Hooper et al.9 found no correlation between REA and SL; however, there was directionality between RDA and SL, indicating that surface-softened enamel may behave more like dentin than like enamel, although no such observation was made in the present study. In contrast, Wiegand et al.13 concluded that SL is positively correlated with REA and that filament stiffness does play a minor role in wear, which was confirmed by other investigators12. It is likely that differences in the brushing abrasion combined with the severity of the erosive challenge are responsible for the discrepancy between studies. Furthermore, when considering the findings from previous studies and those of the present study it must be borne in mind that enamel wears slowly in comparison with dentin (ratio of 1:2.5 in the present study), which suggests that in patients with gingival recession, non-carious cervical lesions are more likely to manifest than coronal wear.
As predicted, cycling time was positively correlated with SL of both enamel and dentin. While enamel SL was proportional (i.e. on day 5, SL was approximately five-thirds that of day 3), this was not the case for dentin. Structural differences between tissues may explain this finding. Enamel contains approximately 96% (w/w) mineral, which is also responsible for its structural backbone. Hence, SL is expected to be proportional over time. Dentin, however, contains 70% (w/w) mineral, which is embedded in a collagen matrix (20%; w/w). Erosion affects largely the mineral content of dentin, leaving behind collagen, which cannot be removed completely through abrasion (at least under the conditions of the present study). Depending on the severity of erosive challenges versus abrasive challenges, the SL measured may therefore not necessarily correlate to mineral loss. Several methodological considerations were brought forward recently to address differences between mineral loss and SL26; however, these considerations also highlighted the need for longitudinal clinical observations to provide better recommendations for in vitro research and model development.
‘Soft’, ‘medium’ and ‘hard’ toothbrushes from the same brand vary in the stiffness of their filaments and in the number of filaments per tuft but not in tuft diameter. However, the effective contact area of the filaments in each tuft with the tooth surface varies as filaments are packed more closely in soft toothbrushes than in hard toothbrushes. These differences affect how abrasive particles are carried across the surface – while wider filaments can drag more particles in the tip contact area than can narrower filaments, the larger number of narrower filaments can compensate for this difference. However, how effectively a filament can drag abrasive particles across the tooth surface depends on a variety of other factors and most importantly on the brush load, and consequently on the degree of filament deflection as well as the particle size27. The present study employed silica abrasives, which have similar particle size distribution but different surface geometry (J.M. Huber Corporation, personal communication). Future studies on a variety of other commonly used abrasives with different particle size distributions and/or hardness (e.g. calcium carbonate, aluminium oxide) may be able to provide more definite recommendations to patients at risk of erosive tooth wear.
Undoubtedly, laboratory studies have their limitations. Only a subset of commercially available toothbrushes and abrasives can be evaluated. Abrasives vary considerably between types as, for example, precipitated silica and calcium carbonate (chalk) have inherently different properties (e.g. particle size, hardness, concentration used). Likewise, a different brush design or filaments from different manufacturers may impact SL, especially when combined with varying abrasives. Moreover, as pointed out earlier, differences in brushing load or experimental design per se can lead researchers to different conclusions.
Conclusion
For enamel, neither abrasive nor filament stiffness affected the SL of softened enamel under the conditions of the present study. However, the SL of dentin was mainly affected by abrasivity, with some minor modulating effect of filament stiffness.
Acknowledgements
This study was supported by the Dental Erosion-Abrasion Research Program, of the Oral Health Research Institute, Indiana University School of Dentistry.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Revised and reviewed the paper: FL, GJE and ATH. Conceived and designed the experiments: FL, MA and ATH. Performed the experiments: MA. Analysed the data: FL, GJE and ATH. Wrote the paper: FL and MA.
References
- 1.Wiegand A, Schlueter N. The role of oral hygiene: does toothbrushing harm? Monogr Oral Sci. 2014;25:215–219. doi: 10.1159/000360379. [DOI] [PubMed] [Google Scholar]
- 2.Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53:177–186. doi: 10.1111/j.1875-595x.2003.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunter ML, Addy M, Pickles MJ, et al. The role of toothpastes and toothbrushes in the aetiology of tooth wear. Int Dent J. 2002;52:399–405. [Google Scholar]
- 4.Bartlett DW, Shah P. A critical review of non-carious cervical (wear) lesions and the role of abfraction, erosion, and abrasion. J Dent Res. 2006;85:306–312. doi: 10.1177/154405910608500405. [DOI] [PubMed] [Google Scholar]
- 5.Voronets J, Lussi A. Thickness of softened human enamel removed by toothbrush abrasion: an in vitro study. Clin Oral Invest. 2010;14:251–256. doi: 10.1007/s00784-009-0288-y. [DOI] [PubMed] [Google Scholar]
- 6.Addy M. Tooth brushing, tooth wear and dentine hypersensitivity – are they associated? Int Dent J. 2005;55:261–267. doi: 10.1111/j.1875-595x.2005.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 7.West NX, Sanz M, Lussi A, et al. Prevalence of dentine hypersensitivity and study of associated factors: a European population-based cross-sectional study. J Dent. 2013;41:841–851. doi: 10.1016/j.jdent.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Dyer D, Addy M, Newcombe RG. Studies in vitro of abrasion by different manual toothbrush heads and a standard toothpaste. J Clin Periodontol. 2000;27:99–103. doi: 10.1034/j.1600-051x.2000.027002099.x. [DOI] [PubMed] [Google Scholar]
- 9.Hooper S, West NX, Pickles MJ, et al. Investigation of erosion and abrasion on enamel and dentine: a model in situ using toothpastes of different abrasivity. J Clin Periodontol. 2003;30:802–808. doi: 10.1034/j.1600-051x.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.Joiner A, Pickles MJ, Tanner C, et al. An in situ model to study the toothpaste abrasion of enamel. J Clin Periodontol. 2004;31:434–438. doi: 10.1111/j.1600-051X.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Hara AT, Gonzalez-Cabezas C, Creeth J, et al. The effect of human saliva substitutes in an erosion-abrasion cycling model. Eur J Oral Sci. 2008;116:552–556. doi: 10.1111/j.1600-0722.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- 12.Voronets J, Jaeggi T, Buergin W, et al. Controlled toothbrush abrasion of softened human enamel. Caries Res. 2008;42:286–290. doi: 10.1159/000148160. [DOI] [PubMed] [Google Scholar]
- 13.Wiegand A, Schwerzmann M, Sener B, et al. Impact of toothpaste slurry abrasivity and toothbrush filament stiffness on abrasion of eroded enamel – an in vitro study. Acta Odontol Scand. 2008;66:231–235. doi: 10.1080/00016350802195041. [DOI] [PubMed] [Google Scholar]
- 14.Hara AT, Gonzalez-Cabezas C, Creeth J, et al. Interplay between fluoride and abrasivity of dentifrices on dental erosion-abrasion. J Dent. 2009;37:781–785. doi: 10.1016/j.jdent.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Wiegand A, Kuhn M, Sener B, et al. Abrasion of eroded dentin caused by toothpaste slurries of different abrasivity and toothbrushes of different filament diameter. J Dent. 2009;37:480–484. doi: 10.1016/j.jdent.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Bizhang M, Riemer K, Arnold WH, et al. Influence of bristle stiffness of manual toothbrushes on eroded and sound human dentin-an in vitro study. PLoS One. 2016;11:e0153250. doi: 10.1371/journal.pone.0153250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hefferren JJ. A laboratory method for assessment of dentifrice abrasivity. J Dent Res. 1976;55:563–573. doi: 10.1177/00220345760550040301. [DOI] [PubMed] [Google Scholar]
- 18.Imfeld T. Prevention of progression of dental erosion by professional and individual prophylactic measures. Eur J Oral Sci. 1996;104:215–220. doi: 10.1111/j.1600-0722.1996.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 19.Buzalaf MAR, Cardoso CAB, Magalhaes AC, et al. In: Dental Erosion and Its Clinical Management. 1st ed. Amaechi BT, editor. Springer; Cham: 2015. Prevention and Control of Dental Erosion: patient Self-Care; pp. 133–150. [Google Scholar]
- 20.Nassar HM, Lippert F, Eckert GJ, et al. Dentifrice fluoride and abrasivity interplay on artificial caries lesions. Caries Res. 2014;48:557–565. doi: 10.1159/000358401. [DOI] [PubMed] [Google Scholar]
- 21.Winterfeld T, Schlueter N, Harnacke D, et al. Toothbrushing and flossing behaviour in young adults-a video observation. Clin Oral Investig. 2015;19:851–858. doi: 10.1007/s00784-014-1306-2. [DOI] [PubMed] [Google Scholar]
- 22.Wiegand A, Köwing L, Attin T. Impact of brushing force on abrasion of acid-softened and sound enamel. Arch Oral Biol. 2007;52:1043–1047. doi: 10.1016/j.archoralbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand A, Lemmrich F, Attin T. Influence of rotating-oscillating, sonic and ultrasonic action of power toothbrushes on abrasion of sound and eroded dentine. J Periodontal Res. 2006;41:221–227. doi: 10.1111/j.1600-0765.2005.00850.x. [DOI] [PubMed] [Google Scholar]
- 24.Vanuspong W, Eisenburger M, Addy M. Cervical tooth wear and sensitivity: erosion, softening and rehardening of dentine; effects of pH, time and ultrasonication. J Clin Periodontol. 2002;29:351–357. doi: 10.1034/j.1600-051x.2002.290411.x. [DOI] [PubMed] [Google Scholar]
- 25.Manly RS, Brudevold F. Relative abrasiveness of natural and synthetic toothbrush bristles on cementum and dentin. J Am Dent Assoc. 1957;55:779–780. doi: 10.14219/jada.archive.1957.0262. [DOI] [PubMed] [Google Scholar]
- 26.Schlueter N, Jung K, Ganss C. Profilometric quantification of erosive tissue loss in dentine: a systematic evaluation of the method. Caries Res. 2016;50:443–454. doi: 10.1159/000448147. [DOI] [PubMed] [Google Scholar]
- 27.Lewis R, Dwyer-Joyce RS. Interactions between toothbrush and toothpaste particles during simulated abrasive cleaning. Proc Inst Mech Eng. 2006;220:755–765. [Google Scholar]