Abstract
Objectives: To evaluate the effects of systemic antibiotics as adjuncts to nonsurgical periodontal treatment (NSPT), as opposed to using NSPT alone, on periodontal clinical parameters of diabetic patients with periodontitis. Materials and methods: Randomised controlled trials with a follow-up of 3 months or more, assessing the effects of NSPT in combination with antibiotics, in diabetic patients with periodontitis were included. Trials published up to August 2016 were identified from MEDLINE, EMBASE and LILACS databases. Meta-analyses were conducted to determine changes in clinical attachment level (CAL), probing pocket depth (PPD), bleeding on probing (BOP) and gingival index (GI). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in this review. Results: Of the 164 papers potentially admissible to this systematic review, 15 articles on 11 randomised clinical trials were considered as eligible. The results of the meta-analyses presented a modest additional benefit of 0.14 mm (95% confidence interval: 0.08–0.20) in reducing PPD but no further benefit in CAL gain. Conclusion: When the data for all antibiotic protocols were considered together for the treatment of periodontitis patients with DM, a significant, albeit small, reduction of PPD and no improvement in CAL gain was observed. When the antibiotic protocols were analysed separately, the combination of amoxicillin plus metronidazole yielded the best results for PPD.
Key words: Diabetes mellitus, periodontal diseases, anti-infective agents, root planing, systematic review
Introduction
Diabetes mellitus (DM) is a complex, chronic disease that requires continuous treatment and multifactorial strategies to control glycaemic levels. There are two principal types of DM: type 1 and type 2. Type 1 DM is characterised by insulin deficiency caused by autoimmune destruction of pancreatic β-cells, and type 2 DM is a result of resistance to insulin action1.
DM is considered to be a risk factor for periodontitis2. It has been demonstrated that diabetic patients have a greater prevalence and severity of periodontal disease than nondiabetic patients3., 4., 5., 6., 7., 8., 9.. Moreover, periodontal infection may lead to poorer glycaemic control in patients with diabetes10 and periodontal treatment can help with glycaemic control11, indicating a bidirectional relationship between DM and periodontitis10.
As a result of the severity of periodontal disease in diabetic patients, some clinical studies have evaluated the adjunctive effect of antibiotics in nonsurgical periodontal treatment for these patients12., 13., 14., 15.. A recent systematic review (SR)16 has shown that local antimicrobials are effective in reducing probing pocket depth (PPD) and increasing clinical attachment level (CAL) in diabetic patients. Two recent SRs have addressed the effect of systemic antimicrobials in diabetic patients with periodontitis17., 18.. However, both included studies that used doxycycline at subantimicrobial doses. Furthermore, different antibiotics may have different efficacy against periodontal infection. When analysed together, antimicrobials’ effectiveness may be underestimated. Thus, this SR aimed to evaluate the adjunctive effects of systemic antibiotics used in nonsurgical periodontal treatment (NSPT), compared with NSPT alone, on the periodontal clinical parameters of diabetic patients with periodontitis. Moreover, this review aimed to analyse the individual effect of different antibiotics, in order to identify which one provides an additional effect on periodontal therapy.
Methodology
The following focussed question was addressed: ‘In periodontitis patients with diabetes, is the use of systemic antimicrobials adjunct to NSPT more effective than NSPT alone in reducing PPD and improving CAL?’ We have registered the protocol of this SR at the National Institute for Health Research PROSPERO, International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO, registration number CRD42016032831). Guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)19, the Cochrane Handbook of Systematic Reviews of Interverstions20 and Check Review checklist20 were used to structure the review text.
Eligibility criteria
We included randomised trials of 3 months or longer with follow-up that evaluated the effects of systemic antibiotics adjunctive to NSPT, compared with NSPT alone, in DM (type 1 and/or type 2) patients with periodontitis. We excluded studies with pregnant women, patients with gestational diabetes or patients who had received systemic antimicrobials 3 months before the study. In addition, trials that used local antimicrobials, antibiotics in sub-antimicrobial doses or that presented inadequate information about the antibacterial agent or the therapy protocol, were not included in this review. The primary outcomes were change in CAL and change in PPD.
Information source and search strategy
We searched MEDLINE via PubMed, EMBASE and LILACS databases to identify relevant publications up to August 2016. MeSH terms and keywords were combined with Boolean operators and used to search the databases. There was no restriction regarding language or publication year. Search strategies are presented in Data S1. In addition, reference lists of the selected studies were hand-searched, and unpublished studies were searched at OpenGray21.
Study selection
Initially, titles and abstracts of the studies were screened independently by two reviewers (E.S.R. and M.L.S.). After this phase, the same reviewers conducted a full-text screening of those trials apparently meeting the inclusion criteria, as well as any papers without available abstracts. In both phases, a third reviewer (C.M.P.) resolved any disagreement between the two reviewers. Data extraction and validity assessment were performed on the publications that met the inclusion criteria. Moreover, the reasons for excluding publications were recorded.
Data collection
Two reviewers (E.S.R. and M.L.S.) performed the data extraction independently using extraction forms22. A third reviewer (C.M.P.) solved any disagreements in the data extraction. Also, if needed, the authors of the included trials were contacted to elucidate questions or missing data.
The reviewers collected the following data from the eligible studies: (i) citation; (ii) country of the study; (iii) participants’ characteristics; (iv) definition of periodontitis and diabetes; (v) follow-up duration; (vi) intervention characteristics (active principle, concentration and dose interval); (vii) sample size; (viii) outcome variables; and (ix) financial support and conflict of interest.
Risk of bias in individual studies
Risk of bias was assessed using the Cochrane Collaboration’s Tool for Assessing Risk of Bias20. Two reviewers (E.S.R. and M.L.S.) independently performed the quality assessment, and any disagreement was solved by a third investigator (C.M.P.). The following domains were classified as adequate (+), inadequate (−) or unclear (?): sequence generation; allocation concealment; blinding of patients, personnel and examiners; incomplete outcome data; selective reporting; and other biases. Each trial was rated as being at low, unclear or high risk of bias.
Summary measures and synthesis of results
We used a software package (Review Manager software, version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) to conduct a random-effects meta-analyses for CAL gain, PPD reduction, bleeding on probing (BOP) change and gingival index (GI) change. Weighted mean differences (WMDs) between groups were calculated. Cochran’s Q statistic and I2 were used to assess heterogeneity among trials.
Results
Study selection
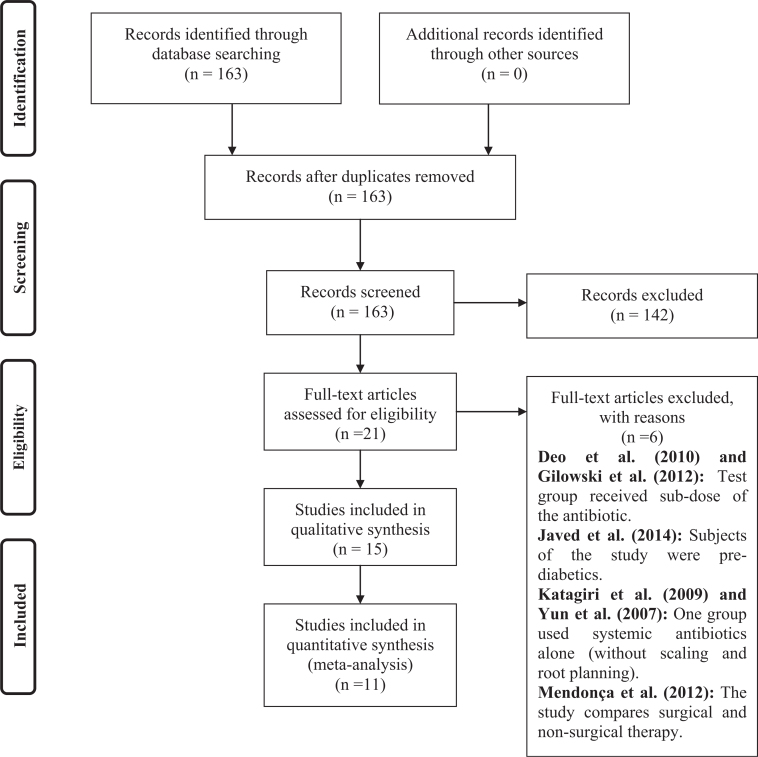
The search in the databases resulted in the identification of 164 publications #bib143 of which were excluded after reviewing titles and abstracts. The complete texts of 21 publications were analysed13., 15., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., and of these, six32., 33., 35., 36., 37., 40. were excluded (Figure 1).
Figure 1.
Flow-chart.
Included studies
Fifteen articles, regarding 11 RCTs, were included in this review (Tables 1 and 2). Three RCTs13., 15., 25. had their data reported in more than one article each (i.e. according to the follow-up period or type analysis). Consequently, the articles were included under one study name. Table 1 presents the characteristics of the included trials. Overall 541 patients with chronic periodontitis and diabetes were included in the trials, and 496 (91.68%) completed the follow-up period. Six studies15., 19., 23., 26., 28., 31. excluded smokers, two13., 24. included smokers and three25., 27., 30. did not report participants’ smoking status.
Table 1.
Characteristics of the studies
| Studyref.no. (country) | Study design | Follow-up | Sample size (baseline) | Peridontitis definition/clinical examination | Diabetes definition | Funding |
|---|---|---|---|---|---|---|
| Al-Nowaiser et al. (2014)23 (Saudi Arabia) | Parallel RCT | 6 months |
N = 76 (47 male and 29 female subjects) Age mean: 42 ± 6.41 years |
Severe chronic periodontitis. Presence of at least 16 teeth, and a minimum of 8 sites with probing depth >5 mm and CAL >5 mm Florida probe® |
Type 2, diagnosed for ≥1 year and good physical condition with no additional serious medical conditions | No |
| Al-Zahrani et al. (2009)24 (Saudi Arabia) | Parallel RCT | 3 months |
N = 45 (17 male and 26 female subjects; gender distribution is reported at the end of the study only) Age Mean: 52.21 ± 8.35 years |
Moderate to severe chronic periodontitis; ≥20 remaining teeth; CAL ≥3 mm at ≥30% of sites Probe not reported |
Type 2 diabetes No diabetes criteria reported |
No |
| Botero et al. (2013)25/Hincapié et al. (2014)34 (Colombia) | Parallel RCT | 9 months | N = 105 (74 male and 31 female subjects) | Moderate periodontitis. Two or more interproximal sites with CAL ≥4 mm, not on the same tooth, or two or more interproximal sites with probing depth ≥5 mm, not on the same tooth UNC-15 probe |
Diabetes types 1 or 2 Patients >18 years of age, confirmed diagnosis of type 1 and 2 diabetes with ≥2 years duration |
Partially supported by a grant from Colgate-Palmolive (020-2009) and the Universidad de Antioquia. Azithromycin was provided by Tecnoquımicas (Cali, Colombia). Placebo tablets were provided by the Faculty of Pharmaceutical Chemistry (Universidad de Antioquia, Medellin, Colombia) |
| Gaikwad et al. (2013)26 (India) | Parallel RCT | 4 months |
N = 50 (34 male and 16 female subjects) Age Range: 30–70 years (mean not mentioned) |
No periodontitis definition reported UNC-15 probe |
Diabetes type 2 No diabetes criteria reported |
No |
| Grossi et al. (1997)27 (USA) | Parallel RCT | 6 months |
N = 113 (81 male and 32 female subjects) Age Range: 25–65 years (mean not mentioned) |
Moderate to severe periodontitis. No criteria definition reported Constant force probe |
Diabetes type 2 World Health Organization, Expert Committee on Diabetes (1980) |
Eastman Kodak, Rochester, NY and National Institute of Dental Research |
| Llambés et al. (200513 #bib200838 #bib2012)39 (Spain) | Parallel RCT | 3 months |
N = 60 (30 male and 30 female subjects) Age Mean: 35.3 ± 9 years |
Moderate to severe periodontitis, minimum of 14 natural teeth with at least five areas with probing depth of ≥5 mm and CAL ≥3 mm Probe not reported |
Diabetes type 1 Diabetic patients for more than 1 year. Diabetic control was measured by HbA1c |
No |
| O’Connel et al. (2008)28 (Brazil) | Parallel RCT | 3 months |
N = 35 (14 male and 16 female subjects; gender distribution is reported at the end of the study only) Age Mean: 52.9 years |
At least one site with probing depth of ≥5 mm and two teeth with CAL ≥6 mm Florida probe® |
Diabetes type 2 Diabetic patients for more than 5 years and HbA1c >8% |
The State of São Paulo Research Foundation, São Paulo, SP, Brazil (grant 04/09844-8 to Dr Taba) and the National Council for Scientific and Technological Development, Brasilia, DF, Brazil (grant 470638/2006 to Drs Novaes and Taba) |
| Rodrigues et al. (2003)29 (Brazil) | Parallel RCT | 3 months |
N = 30 (gender distribution not mentioned) Age Mean and range not mentioned |
Chronic periodontal disease. At least one site with probing depth ≥5 mm and two teeth with CAL ≥6 mm Florida probe® and customised acrylic stent |
Diabetes type 2 Diagnosis in the past 5 years |
Capes (Edducacional Support Center, São Paulo, Brazil) and Fapesp (São Paulo State Research Foundation, Brazil) |
| Singh et al. (2008)30 (India) | Parallel RCT | 3 months |
N = 45 (gender distribution not mentioned) Age Mean and range not mentioned |
No periodontitis definition reported. Willian’s probe and customised acrylic Stent |
Diabetes type 2 No diabetes criteria reported |
No |
| Miranda et al. (2014)41/Tamashiro et al. (2016)15 (Brazil) | Parallel RCT | 24 months |
N = 58 (30 male and 26 female subjects; gender distribution are reported at the end of the study only) Age Mean: 54.0 ± 8.2 (test) and 53.7 ± 8.0 (control) |
Generalised chronic periodontitis. At least 15 teeth, more than 30% of the sites with probing depth and CAL ≥4 mm and a minimum of six teeth with at least one site with probing depth and CAL ≥5 mm and BOP at baseline. UNC-15 probe | Diabetes type 2 Diabetic patients for more than 5 years, diabetes treatment with diet and insulin supplementation or oral hypoglycaemic agents and HbA1c ≥6.5% and ≤11% |
São Paulo State Research Foundation (São Paulo, Brazil) |
| Tsalikis et al. (2014)31 (Greece) | Parallel RCT | 6 months |
N = 70 (38 male and 28 female subjects; gender distribution are reported at the end of the study only) Age Mean: 62.9 ± 10 (test) and 57.94 ± 8.22 (control) |
Moderate or advanced periodontitis. Six pockets with probing depth >5 mm and CAL >3 mm with radiographic bone loss >10% in more than 30% of teeth. Florida probe® |
Diabetes type 2 Diagnosed at least 1 year before baseline examination. At least two consecutive values of HbA1c <7.5% |
Procter and Gamble Hellas through the Koulourides 2011 Award for Dental Research in Greece |
BOP, bleeding on probing; HbA1c, glycated haemoglobin; RCT, randomised controlled trial.
Table 2.
Participants, interventions, outcomes and results
| Studyref. no. | Participants | Interventions | Outcomes measures of interest for the review |
|---|---|---|---|
| Al-Nowaiser et al. (2014)23 | Test group: N baseline = 38 N end of trial = 35 |
Test group: 6–8 sessions of SRP. After 45 days, re-evaluation and subgingival debridement + antimicrobial dose of systemic DOXY 100 mg once a day for 14 days with a loading dose of 200 mg on the first day | Test Overall CAL gain: 0.74 ± 0.17 mm Overall probing depth reduction: 1.5 ± 0.38 mm |
| Control group: N baseline = 38 N end of trial = 33 |
Control group: 6–8 sessions of SRP. After 45 days, re-evaluation and subgingival debridement | Control Overall CAL gain: 0.96 ± 0.22 mm Overall probing depth reduction: 1.4 ± 0.28 mm |
|
| Al-Zahrani et al. (2009)24 | Test group: N baseline = 15 N end of trial = 14 |
Test group: 1–4 sessions of SRP + DOXY 200 mg on the first day and DOXY 100 mg once daily for 13 days |
Test Overall CAL gain: 0.49 ± 0.64 mm Overall probing depth reduction: 0.44 ± 0.38 mm Reduction of sites with probing depth ≥5: 0.1 |
| Control group: N baseline = 15 N end of trial = 15 Note: PDT group = 15 |
Control group: 1–4 sessions of SRP only |
Control Overall CAL gain: 0.56 ± 1.14 mm Overall probing depth reduction: 0.6 ± 0.67 mm Reduction of sites with probing depth ≥5: 0.06 |
|
| Botero et al. (2013)25/Hincapié et al. (2014)34 | Test group: N baseline = 33 N end of trial = 28 |
Test group: subgingival scaling in a single session + systemic AZT 500 mg/day for 3 days | Test Overall CAL gain: 0.2 ± 0.75 mm Overall probing depth reduction: 0.6 ± 0.51 mm |
| Control group: N baseline = 37 N end of trial = 31 |
Control group: subgingival scaling in a single session + placebo | Control Overall CAL gain: 0.3 ± 1.08 mm Overall probing depth reduction: 0.4 ± 0.62 mm |
|
| Gaikwad et al. (2013)26 | Test group: N baseline = 25 N end of trial = not reported |
Test group: full-mouth SRP + systemic DOXY 100 mg once a day for 15 days | Test Overall CAL gain: 0.93 ± 0.45 mm Overall probing depth reduction: 0.69 ± 0.11 mm |
| Control group: N baseline = 25 N end of trial = not reported |
Control group: full-mouth SRP only | Control Overall CAL gain: 0.47 ± 0.52 mm Overall probing depth reduction: 0.52 ± 0.34 mm |
|
| Grossi et al. (1997)27 | Test group: N baseline = not reported N end of trial = not reported |
Test group: SRP + water irrigation + systemic DOXY 100 mg | Test Overall CAL gain: 0.54 ± 0.3 mm Overall probing depth reduction: 0.72 ± 0.2 mm |
| Control group: N baseline = not reported N end of trial = not reported |
Control group: SRP + water irrigation + placebo SRP in two sessions (half of the mouth in each session) and irrigation with water. Doxycycline 100 mg or placebo per day for 2 weeks starting in the first session of SRP |
Control Overall CAL gain: 0.4 ± 0.2 mm Overall probing depth reduction: 0.56 ± 0.1 mm |
|
| Llambés et al. (200513 #bib200838 #bib201239) | Test group: N baseline = 30 N end of trial = 30 |
Test group: SRP in one or two sessions plus chlorhexidine 0.2% rinses (20 ml for 30 s, twice daily) for 12 weeks plus systemic DOXY 100 mg (twice daily for the first day and then one capsule/day thereafter) for 15 days | Test Overall CAL gain: 0.45 ± 0.55 mm Overall probing depth reduction: 0.74 ± 0.46 mm |
| Control group: N baseline = 30 N end of trial = 30 |
Control group: SRP in one or two sessions plus chlorhexidine 0.2% rinses (20 ml for 30 s, twice daily) | Control Overall CAL gain: 0.42 ± 0.37 mm Overall probing depth reduction: 0.65 ± 0.33 mm |
|
| O’Connel et al. (2008)28 | Test group: N baseline: not reported N end of trial: 15 |
Test group: full-mouth SRP + systemic DOXY 100 mg | Test Overall CAL gain: 0.9 ± 1.6 mm Overall probing depth reduction: 1.1 ± 0.4 mm Reduction of sites with probing depth 4–5 mm: 21.8 Reduction of sites with probing depth ≥6 mm: 5.0 |
| Control group: N baseline: not reported N end of trial: 15 |
Control group: full-mouth SRP + placebo 2–4 sessions of SRP within 24 to 36 hours plus systemic DOXY 100 mg or placebo for 14 days after an initial dose of 200 mg (the antibiotic or placebo therapy started the day before SRP was performed) |
Control Overall CAL gain: 0.5 ± 1.35 mm Overall probing depth reduction: 0.8 ± 0.7 mm Reduction of sites with probing depth 4–5 mm: 18.7 Reduction of sites with probing depth ≥6 mm: 8.9 |
|
| Rodrigues et al. (2003)29 | Test group: N baseline: 15 N end of trial: not reported |
Test group: full-mouth SRP in two sessions within 24–6 hours. One day before the first session, AMOX/clavulanic acid 875 mg was systemically administered twice daily for 2 weeks |
Test Overall CAL gain: 0.0 ± 1.2 mm Overall probing depth reduction: 0.8 ± 0.6 mm |
| Control group: N baseline: 15 N end of trial: not reported |
Control group: full-mouth SRP in two sessions within 24–36 hours only | Control Overall CAL gain: 0.0 ± 1.35 mm Overall probing depth reduction: 0.9 ± 0.7 mm |
|
| Singh et al. (2008)30 | Test group: N baseline: 15 N end of trial: not mentioned |
Test group: full-mouth SRP plus systemic DOXY 100 mg daily for 14 days (200 mg in the first day) | Test Overall CAL gain: 0.34 ± 0.61 mm Overall probing depth reduction: 0.38 ± 0.47 mm |
| Control group: N baseline: 15 N end of trial: not mentioned |
Control group: full-mouth SRP only | Control Overall CAL gain: 0.3 ± 0.45 mm Overall probing depth reduction: 0.34 ± 0.35 mm |
|
| Miranda et al. (2014)41/Tamashiro et al. (2016)15 | Test group: N baseline: 29 N end of trial: 16 |
Test group: SRP + systemic MTZ 400 mg and AMOX 500 mg | Test Overall CAL gain: 0.9 ± 1.08 mm Moderate sites (probing depth 4–6 mm) CAL gain: 1.42 ± 0.10 mm Deep sites (probing depth ≥7 mm) CAL gain: 3.35 ± 0.26 mm Overall probing depth reduction: 1.1 ± 0.43 mm Moderate sites (probing depth 4–6 mm) probing depth reduction: 1.89 ± 0.10 mm Deep sites (probing depth ≥7 mm) probing depth reduction: 4.32 ± 0.24 mm Reduction of sites with probing depth ≥5 mm: 29.14 ± 1.68 Reduction of sites with probing depth ≥6 mm: 14.85 ± 1.12 |
| Control group: N baseline: 29 N end of trial: 17 |
Control group: SRP + placebo After the first session of SRP, the antibiotics (MTZ 400 mg and AMOX 500 mg) or placebo were administered three times daily for 14 days |
Control Overall CAL gain: 0.5 ± 0.85 mm Moderate sites (probing depth 4–6 mm) CAL gain: 0.88 ± 0.10 mm Deep sites (probing depth ≥7 mm) CAL gain: 2.39 ± 0.25 mm Overall probing depth reduction: 0.7 ± 0.6 mm Moderate sites (probing depth 4–6 mm) probing depth reduction: 1.19 ± 0.10 mm Deep sites (probing depth ≥7 mm) probing depth reduction: 2.82 ± 0.24 mm Reduction of sites with probing depth ≥5 mm: 18.69 ± 1.74 Reduction of sites with probing depth ≥6 mm: 10.49 ± 1.16 |
|
| Tsalikis et al. (2014)31 | Test group: N baseline: 35 N end of trial: 31 |
Test group: SRP + systemic DOXY 100 mg |
Test Overall CAL gain: 0.71 ± 0.78 mm Overall probing depth reduction: 0.84 ± 0.74 mm Reduction of sites with probing depth ≥5 mm: 178 |
| Control group: N baseline: 35 N end of trial: 35 |
Control group: SRP + placebo SRP in two sessions plus systemic DOXY 100 mg (200 mg as loading dose and 100 mg for 20 days) or placebo |
Control Overall CAL gain: 0.9 ± 1.1 mm Overall probing depth reduction: 0.76 ± 0.66 mm Reduction of sites with probing depth ≥5 mm: 198 |
AMOX, amoxicillin; AZT, azithromycin; CAL, clinical attachment level; DOXY, doxycycline; HbA1c, glycated haemoglobin; MTZ, metronidazole; PDT, photodynamic therapy; SRP, scaling and root planing.
Methodological quality of included studies
Three trials15., 25., 31. were judged to have low risk of bias, seven13., 23., 24., 26., 27., 29., 30. to have high risk of bias and one28 to have unclear risk of bias (Table 3).
Table 3.
Summary of risk of bias (low/high/? unclear) in selected studies
| Systemic antimicrobials | Random sequence generation | Allocation concealment | Masking patient | Masking operator | Masking examiner | Attrition bias | Selective reporting | Sample size calculation | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Al-Nowaiser et al. (2014)23 | + | ? | – | ? | ? | ? | + | ? | High |
| AL-Zahrani et al. (2009)24 | + | ? | – | ? | + | + | + | + | High |
| Botero et al. (2013)25 | + | + | + | + | + | + | + | + | Low |
| Gaikwad et al. (2013)26 | ? | ? | – | ? | ? | ? | + | ? | High |
| Grossi et al. (1997)27 | ? | ? | + | – | + | ? | + | ? | High |
| Llambés et al. (2005)13 | – | ? | – | ? | ? | – | + | ? | High |
| Tamashiro et al. (2014)15 | + | + | + | + | + | + | + | + | Low |
| O’Connell et al. (2008)28 | ? | ? | + | + | + | ? | + | ? | ? |
| Rodrigues et al. (2003)29 | ? | ? | – | – | – | ? | + | ? | High |
| Singh et al. (2008)30 | ? | ? | – | ? | ? | ? | + | ? | High |
| Tsalikis et al. (2014)31 | + | + | + | + | + | + | + | + | High |
+, adequate; –, inadequate; ?, unclear.
Results of individual studies
Eleven trials assessed the use of systemic antibiotics as adjunct to scaling and root planing (SRP) (doxycycline13., 23., 24., 26., 27., 28., 30., 31., azithromycin25, amoxicillin + metronidazole15, and amoxicillin + clavulanic acid29). Of these studies, three15., 26., 27. presented a significant PPD reduction/CAL gain associated with the use of systemic antibiotics when compared with the placebo group (Table 2).
Synthesis of results
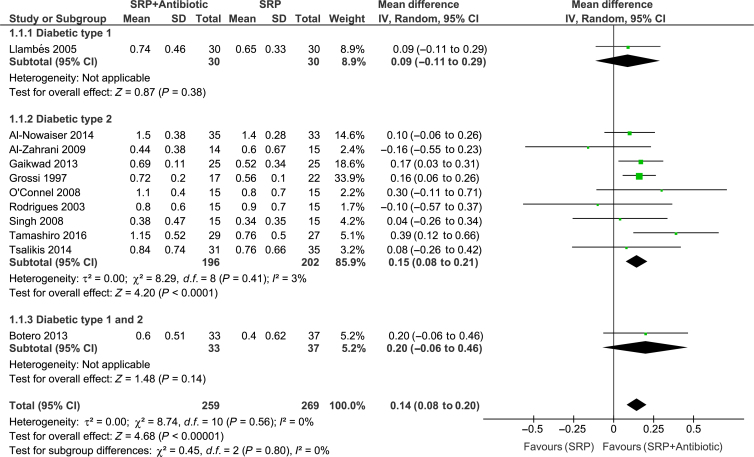
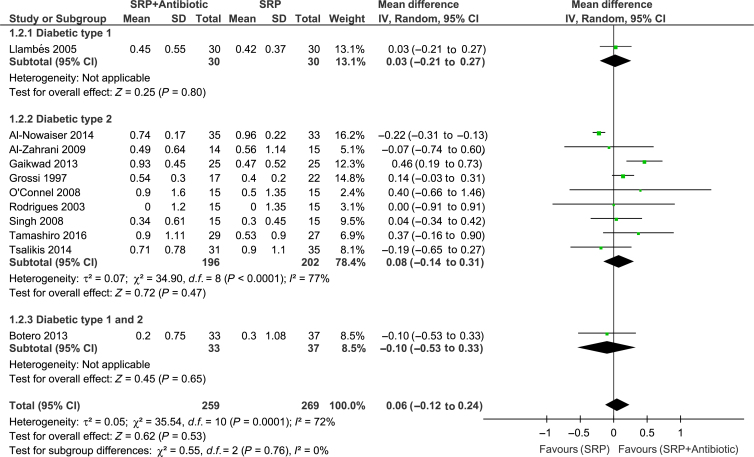
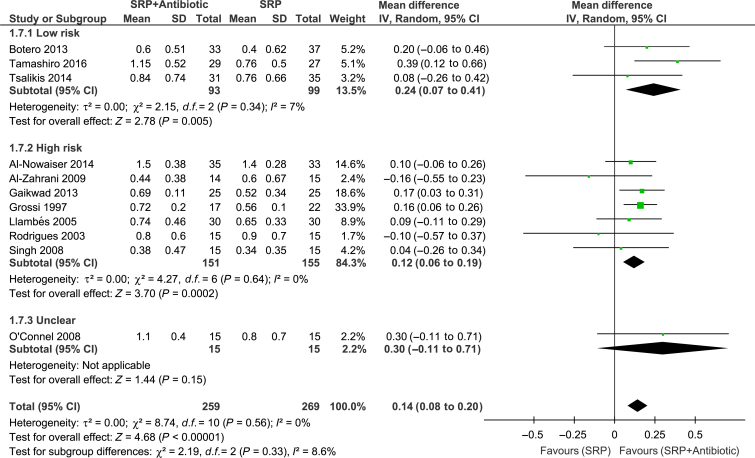
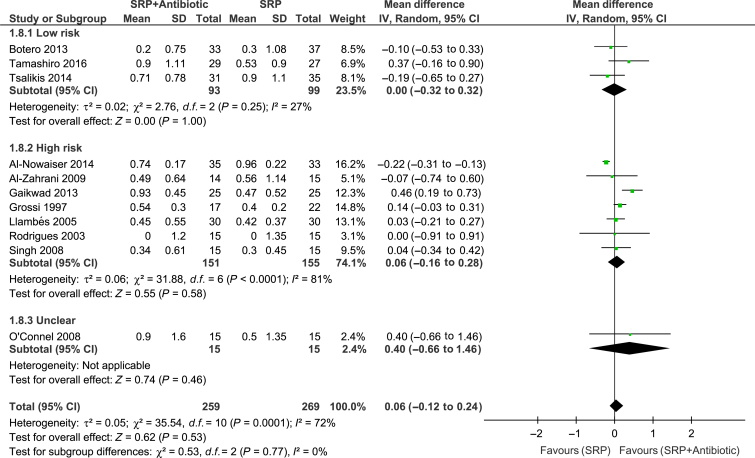
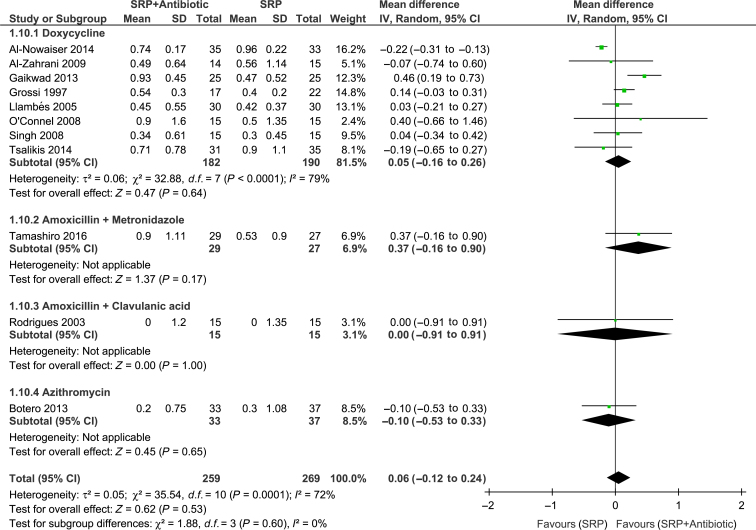
Meta-analyses of the studies were conducted with data from 11 trials. Significant differences between groups for overall PPD reduction were observed [WMD = 0.14; 95% confidence interval (95% CI): 0.08–0.20; P < 0.00001, I2 = 0%] (Figure 2). Subgroup analysis revealed a significant effect of systemic antibiotics for PPD reduction only in subjects with type 2 diabetes (WMD = 0.15; 95% CI: 0.08–0.21; P < 0.0001, I2 = 3%) (Figure 2). However, there was no significant difference between groups in CAL gain (Figure 3). Moreover, studies considered to have low (WMD = 0.27; 95% CI: 0.07–0.41; P = 0.005, I2 = 7%) and high (WMD = 0.12; 95% CI: 0.06–0.19; P = 0.002, I2 = 0%) risk of bias showed a significant PPD reduction favouring the test group (Figure 4). The risk of bias of the trials did not influence CAL gain (Figure 5). Regarding antibiotic type, meta-analyses showed that only doxycycline and the combination of amoxicillin + metronidazole resulted in significant PPD reduction (WMD = 0.13; 95% CI: 0.07–0.20; P < 0.0001, I2 = 0%; WMD = 0.39; 95% CI: 0.12–0.66; P = 0.004, respectively (Figure 6). Furthermore, none of the antimicrobials provided CAL gain (Figure 7).
Figure 2.
Forest-plot probing pocket depth (PPD) reduction. 95% CI #bib95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Figure 3.
Forest-plot of clinical attachment level (CAL) gain. 95% CI #bib95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Figure 4.
Forest-plot of probing pocket depth (PPD) reduction and risk of bias. 95% CI #bib95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Figure 5.
Forest-plot of clinical attachment level (CAL) gain and risk of bias. 95% CI #bib95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Figure 6.
Forest-plot of probing pocket depth (PPD) reduction according to antibiotic type. 95% CI, 95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Figure 7.
Forest-plot of clinical attachment level (CAL) gain according to antibiotic type. 95% CI, 95% confidence interval; SD, standard deviation; SRP, scaling and root planing.
Adverse effects
One trial25 mentioned that one participant in the placebo group reported gastrointestinal discomfort with the last tablet. One study15 reported that diarrhoea (three subjects in the control group and seven in the test group), headache (one patient in the control group and four in the test group), metallic taste (two patients in the control group and four in the test group) and nausea/vomiting (two participants in the control group and five in the test group) were adverse effects informed by the participants. Another study31 reported that one female patient in the control group reported dizziness and difficulty in swallowing. Only one trial26 reported that there were no adverse events. The other seven papers13., 23., 24., 27., 28., 29., 30. included in this meta-analysis did not mention adverse effects or complications in the paper.
Discussion
Main results
Overall, meta-analysis showed a modest additional benefit of 0.14 mm in PPD reduction in subjects treated with SRP + antimicrobial in comparison with SRP + placebo/alone. Conversely, no further benefit was found in CAL gain. Only three15., 26., 27. of the 11 investigations showed significant CAL gain and PPD reduction when adjunctive systemic antimicrobials were used. Two of these studies investigated the effect of doxycycline 100 mg26., 27. and one study assessed the effects of the association of metronidazole 400 mg + amoxicillin 500 mg15.
Doxycyline was the antimicrobial most commonly studied13., 23., 24., 26., 27., 28., 30., 31., 38., 39.. However, subgroup analysis showed a modest additional benefit of 0.13 mm in PPD reduction and no further benefit in CAL gain compared with SRP alone. These findings are similar to those found in the use of doxycycline as adjunct to SRP in healthy subjects42. Thus, the findings of the present SR do not support the use of doxycycline in combination with SRP for the treatment of periodontitis in diabetic subjects. Furthermore, also based on one study, subanalyses revealed no additional benefits regarding the use of azithromycin or amoxicillin + clavulanic acid as adjuncts to SRP. Only one trial15 included in the present review assessed the effect of amoxicillin + metronidazole as adjuncts to SRP, which is the combination with more evidence of efficacy43. Subgroup analysis revealed an additional benefit of 0.39 mm in PPD reduction when compared with SRP alone. Noteworthy, this trial presented low risk of bias and was the study with the longest follow-up period (2 years). Thus, the results favouring the antibiotic therapy observed by these authors, such as the significant reduction of sites with ≥5 mm of CAL gain and PPD reduction must be highlighted. A larger number of well-conducted clinical trials, which assess the effects of amoxicillin plus metronidazole in combination with SRP in the treatment of chronic periodontitis in diabetic patients, should be conducted to corroborate these findings.
Quality assessment and limitations
According to Cochrane Collaboration’s tool20, the risk of bias analyses showed that among the 11 trials, only three15., 25., 31. (27.27%) were considered to have low risk of bias. In SRs, qualitative assessments of the studies represent a pivotal tool for evaluating methodological weakness that may influence the results of the trials. In the present SR, most of the studies13., 24., 26., 28., 29. included chose reduction of glycated haemoglobin as the primary outcome, and were conducted with short-term follow-up periods. Although the follow-up of 3 and 4 months may be sufficient to evaluate this outcome, the short follow-up might not have been sufficient to detect improvement in clinical parameters (PPD reduction and CAL gain). Furthermore, although smoking has a negative influence in the periodontal therapy42., 43., 44., some of the trials24., 25., 27., 30. included in the present SR did not exclude smokers or report participant’s smoking status.
High heterogeneity (>70%) was observed in pooled estimates of CAL gain (Figure 3), whereas no heterogeneity was found in the assessment of PPD (Figure 2). This could be a result of the different definitions of periodontitis used in the studies, different baseline periodontal status (mainly initial PPD) and different treatment protocols (including the various agents and concentrations). Furthermore, risk of bias of the studies (high/unclear) may have influenced the pooled estimates (Figures 4 and 5). However, despite these differences and the limitations of most of the studies included in this review, the outcomes of these trials could be considered as in agreement in terms of PPD reduction, in view of the lack of significant heterogeneity detected (I2 = 0%). Still, the findings of the present review should be interpreted with caution.
Comparison with the literature
The meta-analyses of this review demonstrated a limited advantage in PPD reduction and no further advantage in CAL gain in subjects treated with SRP plus systemic antimicrobials in comparison with SRP alone or placebo. These main findings are in agreement with the other two SRs17., 18.. One of the SRs18 excluded one trial26 that was included in our review. The reason was that the final number of participants in the groups was not reported in the article. Moreover, the same authors excluded two studies28., 29. from the global meta-analyses because the CAL was measured using customised acrylic stents. In our review, we chose to include both because we analysed CAL change from baseline. In addition, another difference from our study is that Grellmann et al.18 opted to include a study32 with subantimicrobial dose doxycycline, even though the literature shows that the administration in the long-term administration of this kind of therapy does not present antibacterial effects44. The main differences between our SR and the one conducted by Santos et al.17 are the inclusion of a study with subantimicrobial dose doxycycline32 and studies with at least 6 months of follow-up. Thus, the number of trials included in their meta-analyses was restricted to four.
Suggestion for future studies
Future trials on the use of systemic antibiotics in DM subjects with chronic periodontitis should present: (i) at least 12 months of follow-up; (ii) well-defined inclusion criteria on diabetes status; and (iii) exclusion of smokers or randomisation stratified according to smoking status.
In conclusion, when the data for all antibiotic protocols were considered together for the treatment of periodontitis patients with DM, a significant, albeit small, PPD reduction and no improvement in CAL gain were observed. When the antibiotic protocols were analysed separately, the combination of amoxicillin + metronidazole yielded the best results for PPD reduction.
Acknowledgements
No funding. No conflict of interest.
References
- 1.Cameron F. Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–S5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 2.Oppermann RV, Weidlich P, Musskopf ML. Periodontal disease and systemic complications. Braz Oral Res. 2012;26(Suppl 1):39–47. doi: 10.1590/s1806-83242012000700007. [DOI] [PubMed] [Google Scholar]
- 3.Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 4.Taylor GW, Burt BA, Becker MP, et al. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol. 1998;3:30–39. doi: 10.1902/annals.1998.3.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay D, Marlow NM, Fernandes JK, et al. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol. 2010;37:501–509. doi: 10.1111/j.1600-051X.2010.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cianciola LJ, Park BH, Bruck E, et al. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes) J Am Dent Assoc. 1982;104:653–660. doi: 10.14219/jada.archive.1982.0240. [DOI] [PubMed] [Google Scholar]
- 7.Lalla E, Cheng B, Lal S, et al. Diabetes mellitus promotes periodontal destruction in children. J Clin Periodontol. 2007;34:294–298. doi: 10.1111/j.1600-051X.2007.01054.x. [DOI] [PubMed] [Google Scholar]
- 8.Lalla E, Cheng B, Lal S, et al. Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care. 2006;29:295–299. doi: 10.2337/diacare.29.02.06.dc05-1355. [DOI] [PubMed] [Google Scholar]
- 9.Hodge PJ, Robertson D, Paterson K, et al. Periodontitis in non-smoking type 1 diabetic adults: a cross-sectional study. J Clin Periodontol. 2012;39:20–29. doi: 10.1111/j.1600-051X.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 10.Borgnakke WS, Ylöstalo PV, Taylor GW, et al. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84(4 Suppl):S135–S152. doi: 10.1902/jop.2013.1340013. [DOI] [PubMed] [Google Scholar]
- 11.Botero JE, Rodríguez C, Agudelo-Suarez AA. Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Aust Dent J. 2016;61:134–148. doi: 10.1111/adj.12413. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj P, Pradeep AR, Agarwal E, et al. Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well-controlled type 2 diabetes: a randomized controlled clinical trial. J Investig Clin Dent. 2012;3:276–283. doi: 10.1111/j.2041-1626.2012.00168.x. [DOI] [PubMed] [Google Scholar]
- 13.Llambes F, Silvestre F-J, Hernandez-Mijares A, et al. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol. 2005;32:915–920. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 14.Martorelli de Lima AF, Cury CC, Palioto DB, et al. Therapy with adjunctive doxycycline local delivery in patients with type 1 diabetes mellitus and periodontitis. J Clin Periodontol. 2004;31:648–653. doi: 10.1111/j.0303-6979.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamashiro NS, Duarte PM, Miranda TS, et al. Amoxicillin plus metronidazole therapy for patients with periodontitis and type 2 diabetes: a 2-year randomized controlled trial. J Dent Res. 2016;95:829–836. doi: 10.1177/0022034516639274. [DOI] [PubMed] [Google Scholar]
- 16.Rovai ES, Luisa M, Souto S, et al. Efficacy of local antimicrobials in the nonsurgical treatment of periodontitis patients with diabetes: a systematic review. J Periodontol. 2016;29:1–17. doi: 10.1902/jop.2016.160214. [DOI] [PubMed] [Google Scholar]
- 17.Santos CM, Lira-Junior R, Fischer RG, et al. Systemic antibiotics in periodontal treatment of diabetic patients: a systematic review. PLoS One. 2015;10:e0145262. doi: 10.1371/journal.pone.0145262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grellmann AP, Sfreddo CS, Maier J, et al. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: a meta-analysis. J Clin Periodontol. 2016;43:250–260. doi: 10.1111/jcpe.12514. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. John Wiley & Sons; Hoboken, NJ: 2011. Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1. Available at: www.cochrane-handbook.org. Accessed December 01, 2015. [Google Scholar]
- 21.OpenGrey. System for information on grey literature in Europe. Available at: http://www.opengrey.eu. Accessed February 05, 2016.
- 22.Chambrone L, Faggion CM, Jr, Pannuti CM, et al. Evidence-based periodontal plastic surgery: an assessment of quality of systematic reviews in the treatment of recession-type defects. J Clin Periodontol. 2010;37:1110–1118. doi: 10.1111/j.1600-051X.2010.01634.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Nowaiser AM, Al-Zoman H, Baskaradoss JK, et al. Evaluation of adjunctive systemic doxycycline with non-surgical periodontal therapy within type 2 diabetic patients. Saudi Med J. 2014;35:1203–1209. [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Zahrani MS, Bamshmous SO, Alhassani AA, et al. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol. 2009;80:1568–1573. doi: 10.1902/jop.2009.090206. [DOI] [PubMed] [Google Scholar]
- 25.Botero JE, Yepes FL, Ochoa SP, et al. Effects of periodontal non-surgical therapy plus azithromycin on glycemic control in patients with diabetes: a randomized clinical trial. J Periodontal Res. 2013;48:706–712. doi: 10.1111/jre.12058. [DOI] [PubMed] [Google Scholar]
- 26.Gaikwad SP, Gurav AN, Shete AR, et al. Effect of scaling and root planning combined with systemic doxycycline therapy on glycemic control in diabetes mellitus subjects with chronic generalized periodontitis: a clinical study. J Periodontal Implant Sci. 2013;43:79–86. doi: 10.5051/jpis.2013.43.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 28.O’Connell PA, Taba M, Nomizo A, et al. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol. 2008;79:774–783. doi: 10.1902/jop.2008.070250. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues DC, Taba MJ, Novaes AB, et al. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74:1361–1367. doi: 10.1902/jop.2003.74.9.1361. Erratum in: J Periodontol 2004;75:780. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Kumar V, Kumar S, et al. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries. 2008;28:38–44. doi: 10.4103/0973-3930.43097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsalikis L, Sakellari D, Dagalis P, et al. Effects of doxycycline on clinical, microbiological and immunological parameters in well-controlled diabetes type-2 patients with periodontal disease: a randomized, controlled clinical trial. J Clin Periodontol. 2014;41:972–980. doi: 10.1111/jcpe.12287. [DOI] [PubMed] [Google Scholar]
- 32.Deo V, Gupta S, Bhongade ML, et al. Evaluation of subantimicrobial dose doxycycline as an adjunct to scaling and root planing in chronic periodontitis patients with diabetes: a randomized, placebo-controlled clinical trial. J Contemp Dent Pract. 2010;11:9–16. [PubMed] [Google Scholar]
- 33.Gilowski L, Kondzielnik P, Wiench R, et al. Efficacy of short-term adjunctive subantimicrobial dose doxycycline in diabetic patients–randomized study. Oral Dis. 2012;18:763–770. doi: 10.1111/j.1601-0825.2012.01943.x. [DOI] [PubMed] [Google Scholar]
- 34.Hincapié JP, Castrillón CA, Yepes FL, et al. Microbiological effects of periodontal therapy plus azithromycin in patients with diabetes: results from a randomized clinical trial. Acta Odontol Latinoam. 2014;27:89–95. doi: 10.1590/S1852-48342014000200008. [DOI] [PubMed] [Google Scholar]
- 35.Javed F, Ahmed HB, Mehmood A, et al. Effect of nonsurgical periodontal therapy (with or without oral doxycycline delivery) on glycemic status and clinical periodontal parameters in patients with prediabetes: a short-term longitudinal randomized case-control study. Clin Oral Investig. 2014;18:1963–1968. doi: 10.1007/s00784-014-1185-6. [DOI] [PubMed] [Google Scholar]
- 36.Katagiri S, Nitta H, Nagasawa T, et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract. 2009;83:308–315. doi: 10.1016/j.diabres.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Yun F, Firkova EI, Jun-Qi L, et al. Effect of non-surgical periodontal therapy on patients with type 2 diabetes mellitus. Folia Med (Plovdiv) 2007;49:32–36. [PubMed] [Google Scholar]
- 38.Llambés F, Silvestre FJ, Hernández-Mijares A, et al. The effect of periodontal treatment on metabolic control of type 1 diabetes mellitus. Clin Oral Investig. 2008;12:337–343. doi: 10.1007/s00784-008-0201-0. [DOI] [PubMed] [Google Scholar]
- 39.Llambes F, Silvestre FJ, Hernandez-Mijares A, et al. Effect of periodontal disease and non surgical periodontal treatment on C-reactive protein. Evaluation of type 1 diabetic patients. Med Oral Patol Oral Cir Bucal. 2012;17:e562–e568. doi: 10.4317/medoral.17793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendonça AC, Santos VR, Ribeiro FV, et al. Surgical and non-surgical therapy with systemic antimicrobials for residual pockets in type 2 diabetics with chronic periodontitis: a pilot study. J Clin Periodontol. 2012;39:368–376. doi: 10.1111/j.1600-051X.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 41.Miranda TS, Feres M, Perez-Chaparro PJ, et al. Metronidazole and amoxicillin as adjuncts to scaling and root planing for the treatment of type 2 diabetic subjects with periodontitis: 1-year outcomes of a randomized placebo-controlled clinical trial. J Clin Periodontol. 2014;41:890–899. doi: 10.1111/jcpe.12282. [DOI] [PubMed] [Google Scholar]
- 42.Smiley CJ, Tracy SL, Abt E, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–524.e5. doi: 10.1016/j.adaj.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Herrera D, Matesanz P, Bascones-Martínez A, et al. Local and systemic antimicrobial therapy in periodontics. J Evid Based Dent Pract. 2012;12(3 Suppl):50–60. doi: 10.1016/S1532-3382(12)70013-1. [DOI] [PubMed] [Google Scholar]
- 44.Caton J, Ryan ME. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD) Pharmacol Res. 2011;63:114–120. doi: 10.1016/j.phrs.2010.12.003. [DOI] [PubMed] [Google Scholar]