Abstract
Background: The oral cavity is a potential reservoir for respiratory pathogens. This longitudinal study investigated the association between upper respiratory tract infection (URI) and oral health among children. Methods: A total of 288 children aged 4 years were recruited. Their dental caries and oral hygiene status were clinically determined, using the dmft (decayed, missing and filled teeth) index and the Silness-Löe plaque index. Questionnaires were completed by parents to collect information on the child’s socio-demographic background and URI episodes and symptoms in the following 12 months. Standard or zero-inflated negative binomial regressions were used to analyse the association between URI and both oral health indicators (dmft and plaque score). Results: Some 138 (47.9%) children had URI in 12 months, including 63 (21.9%) and 75 (26.0%) children with 1–2 episodes and ≥3 episodes, respectively. The reported URI episodes fell into two peaks, coinciding with the two influenza peaks in Hong Kong. Significantly a higher dmft was found among children without URI compared with children who had ≥3 URI episodes (1.32 vs. 0.49; P = 0.043). The number of URI episodes was inversely associated with dmft (IRR = 0.851; 95% CI: 0.766–0.945; P = 0.003). There was no significant association between the plaque score and URI (P > 0.05). Conclusions: The children’s caries experience was associated with reduced episodes of URI. Whether this inverse association is attributed to the immune response induced by dental caries is yet to be investigated.
Key words: Oral health, upper respiratory tract infection, dental caries, dental plaque, children
Introduction
The oral cavity is considered to be a potential reservoir of respiratory pathogens because of the anatomical continuity between the oral cavity and the respiratory tract1., 2., 3.. Systematic reviews reported fair evidence supporting an association between pneumonia and oral health; the odds ratio ranged from 1.2 to 9.6 across various oral health indicators4. Also, there was weak evidence suggesting an association between chronic obstructive pulmonary disease (COPD) and oral health (periodontal disease) among adults, especially among elderly people4. In addition, randomised controlled trials have shown that improving oral hygiene and regular professional dental care could reduce lower respiratory tract infection in high-risk elderly staying in nursing homes or hospitals5., 6., 7..
The reported studies focused on investigating the association between oral health and lower respiratory tract infection (infection below the level of the larynx; including bronchiolitis, bronchitis and pneumonia)4., 5.. The relationship between upper respiratory tract infection (URI; i.e. infections involving nose, paranasal sinuses, pharynx and larynx)8 and oral health remains unclear. Scannapieco and others included influenza in their study of the relationship between respiratory diseases and oral condition but no association was found in their sample9. Furthermore, studies investigating the association between oral health and respiratory diseases were predominantly conducted in adults and elders, especially in hospitals or nursing homes. Such an association among children living in the community remains unclear4., 5..
According to the U.S. National Institutes of Health estimates, dental caries (tooth decay) is one of the most salient oral health problems and the most prevalent chronic infectious condition in childhood10. On the other hand, respiratory infection is the first leading cause of death in children younger than 5 years, accounting for over 20% of the 10.6 million yearly deaths worldwide11. Exploring the relationship between dental caries and URI would be of great importance for controlling these two diseases and safeguarding children’s health. Dental plaque is the microbial biofilm attached to tooth surfaces. It harbours bacteria that contribute to the occurrence of dental caries12 and might also serve as a reservoir for respiratory pathogens13., 14.. Recent studies showed that dental plaque was colonised with Haemophilus influenzae13, which is a common pathogen for URI15, and Staphylococcus aureus and Pseudomonas aeruginosa14, which are common pathogens for lower respiratory tract infections16.
This prospective study aimed to investigate the relationship between oral health and URI among children. Two oral health indicators were used, namely the number of decayed/missing/filled primary teeth (dmft) and the dental plaque index, which are recognized measures for dental caries and oral hygiene status, respectively.
Methods
Subject recruitment
This study was conducted among preschool children in Hong Kong. The study protocol was reviewed by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Ethical approval was granted (#UW 11-483). The study was conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki.
Previous studies showed that dental caries in children does not follow a normal distribution17., 18.. The sample size was therefore calculated by using G*Power (version 3.1.9.2; Franz Faul, Universitat Kiel, Germany) based on power analysis under minimum asymptotic relative efficiency (ARE) of the non-parametric Mann–Whitney U test relative to the t-test19. Aiming to achieve an 80% power and a 0.05 significance level, a total sample size of 216 was needed to detect an effect size (based on standardised mean difference) of 0.5.
The participants were recruited from five kindergartens located in various districts of Hong Kong with diverse socioeconomic status. To be eligible to join this study, a child should fulfil the following criteria: (i) be 4 years of age, (ii) physically fit without any systemic disease, (iii) not under any regular medication, and (iv) be able to cooperate in the oral health examination. Of the 379 eligible children enrolled in the selected kindergartens, 288 participated. The response rate was 76.0%. A child entered the study only after written parental informed consent was obtained.
Baseline data collection on oral health
At baseline, the parents completed a pre-tested questionnaire about the child’s demographic background (age and gender) and their family’s socioeconomic status (using mother’s education level as the indicator). All children were then clinically examined by the same examiner, who was trained and calibrated against an experienced oral epidemiologist. Duplicate examinations were performed on 10% randomly selected subjects to assess the intra-examiner reliability. At least 10 other children were examined between the first and duplicate examinations for each selected child.
The children’s tooth status (dental caries) and oral hygiene status were assessed by visual and tactile inspections. No radiographs were taken. A disposable mouth mirror illuminated by an intraoral LED light and a community periodontal index (CPI) probe were used. Dental caries was registered at the cavitation level according to criteria recommended by the World Health Organisation (WHO)20. Caries was considered as present when there was a cavity, detectable softened floor or wall, or undermined enamel or a surface with a temporary filling. A tooth was scored as missing due to caries only if other reasons for missing teeth were excluded. A tooth was recorded as filled when one or more permanent restorations were present. If a tooth had both a carious lesion and a filling, it was counted as a decayed tooth. The children’s oral hygiene status was evaluated using the Silness-Löe plaque index21. Four surfaces (buccal/labial, lingual/palatal, mesial and distal) of six index teeth (# 55, 52, 64, 75, 72 and 84) were examined according to the following scoring system: 0, no plaque; 1, plaque detectable with probe but not visible; 2, visible plaque; 3, surface covered with abundant plaque.
Data collection on URI in 12 months
A questionnaire was completed by parents to collect the information on URI experienced by the child in the next 12 months after the baseline examination. The questionnaire had been validated in our previous study22. URI episodes, related symptoms (including fever, cough, chill, headache, score throat, runny nose and muscle pain), length of each infection episode and the days of absence from kindergarten in each month were recalled by the parents. The children’s URI were then recorded using three definitions, including (i) number of self-reported URI episodes, (ii) number of influenza-like-illness episodes, defined as fever ≥37.8 °C plus cough or sore throat, and (iii) the number of febrile acute respiratory infections, defined as fever ≥38 °C with any respiratory symptom such as cough, runny nose or sore throat23.
Statistical analysis
Mann–Whitney U tests and ANOVA post hoc tests were used to compare the mean dmft and mean plaque score between groups with different episodes of URI, respectively. Standard or zero-inflated negative binomial regression models, as appropriate, were employed to identify the association between the oral health parameters (dmft and plaque scores) and the three URI measures with adjustment for potential confounders (gender and mother’s education level). The incidence rate ratio (IRR) and its 95% confidence interval (CI) were reported. Linear regression and a Poisson regression were not used since the data did not follow a normal distribution or Poisson distribution. To monitor the possible influence of parents’ recall bias on child’s URI, the same multivariate analysis was conducted using the data from the last 1 month, the last 3 months and the last 12 months, separately. Analyses were performed by SPSS version 24 (SPSS Inc., Chicago, IL, USA) and Stata version 11 (Stata Corp., College Station, TX, USA; for zero-inflated negative binomial regression).
Results
Of the 288 children who participated in this study 155 (53.8%) were boys and 133 (46.2%) were girls. The majority (over 60%) of parents had completed secondary education, whereas around one-third received tertiary education (Table 1). A high intra-examiner reproducibility of the dental examination was achieved (κ value = 0.95 for caries examination, weighted κ value = 0.86 for oral hygiene evaluation).
Table 1.
Socio-demographic characteristics of participants
| n | % | |
|---|---|---|
| Gender of child | ||
| Male | 155 | 53.8 |
| Female | 133 | 46.2 |
| Age of child | ||
| 4 years | 288 | 100 |
| Mother’s education | ||
| Primary education or below | 9 | 3.1 |
| Secondary education | 179 | 62.6 |
| Tertiary education | 98 | 34.3 |
| Father’s education | ||
| Primary education or below | 9 | 3.2 |
| Secondary education | 179 | 62.8 |
| Tertiary education | 97 | 34.0 |
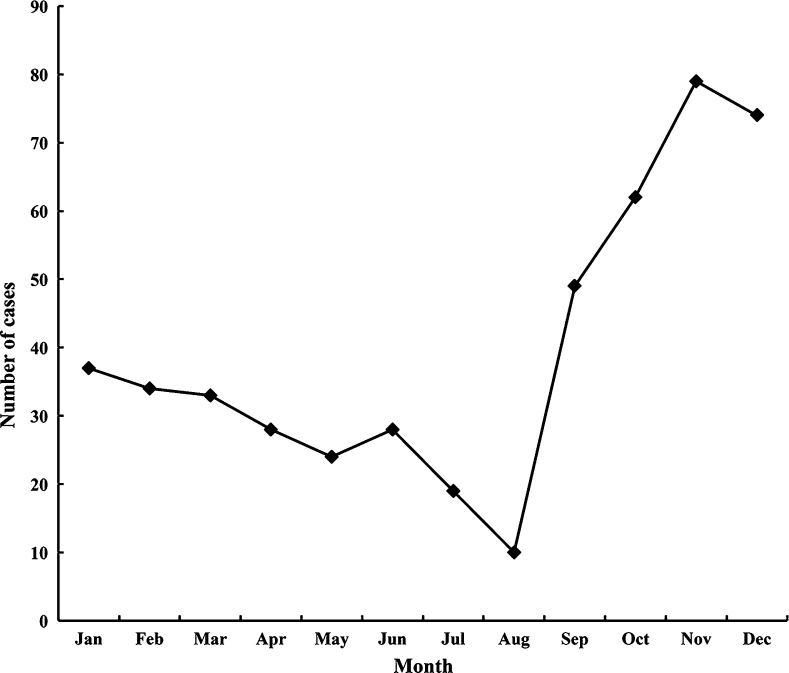
A total of 138 (47.9%) children had URI in the 12 months, including 63 (21.9%) and 75 (26.0%) children with 1–2 episodes and three or more episodes, respectively (Table 2). A significantly higher mean dmft score was found among children without URI compared with children who had three or more URI episodes (1.32 vs. 0.49; P = 0.043). There was no significant difference in the mean plaque score among children with a different number of URI episodes (Table 2). Figure 1 illustrates the URI episodes in each month. The number of reported URI episodes was high in January and February, and then decreased until June, forming a small peak, followed by another peak in November and December. This trend was consistent with the two influenza peaks in Hong Kong24.
Table 2.
Upper respiratory tract infection episodes and oral conditions of children
| Upper respiratory tract infection episodes | n (%) | Dental caries | Oral hygiene status | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) dmft | Comparison with Group 2 (P-value) | Comparison with Group 3 (P-value) | Mean (SD) plaque score | Comparison with Group 2 (P-value) | Comparison with Group 3 (P-value) | ||
| Group 1: 0 | 150 (52.1) | 1.32 (2.91) | 0.362 | 0.043* | 1.94 (0.18) | 1.00 | 1.00 |
| Group 2: 1–2 | 63 (21.9) | 0.71 (1.58) | – | 0.328 | 1.93 (0.14) | – | 1.00 |
| Group 3: ≥3 | 75 (26.0) | 0.49 (1.36) | 0.328 | – | 1.93 (0.18) | 1.00 | – |
Non-parametric tests (Mann–Whitney U tests) were used for comparing mean dmft. Parametric tests (ANOVA post hoc tests) were used for comparing mean plaque score.
Significant difference.
Figure 1.
Distribution of upper respiratory tract infection cases by month.
The multivariate analysis showed that (i) the dmft score was significantly negatively associated with the number of URI episodes over 12 months, after adjusting for potential confounders including gender and mother’s education level (IRR = 0.851; 95% CI: 0.766–0.945; P = 0.003; Table 3); (ii) there was no significant association between the plaque score and the number of URI episodes (IRR = 1.066; 95% CI: 0.370–3.073; P = 0.906; Table 3); and (iii) there was no significant association between oral health parameters and influenza-like-illness or febrile acute respiratory infection (all P > 0.05; Tables 4 and 5).
Table 3.
Multivariate analysis for factors associated with the number of upper respiratory tract infection episodes
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | |
| Gender | ||||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 0.843 (0.578–1.230) | 0.376 | 0.790 (0.543–1.149) | 0.218 |
| Mother’s education | ||||
| Primary education or below | 1 (reference) | 1 (reference) | ||
| Secondary education | 0.765 (0.267–2.192) | 0.619 | 0.547 (0.187–1.602) | 0.271 |
| Tertiary education | 0.813 (0.279–2.370) | 0.705 | 0.544 (0.180–1.641) | 0.280 |
| Mean plaque index (continuous) | 1.066 (0.370–3.073) | 0.906 | – | – |
| Mean dmft (continuous) | – | – | 0.851 (0.766–0.945) | 0.003* |
The dependent variable was the number of upper respiratory tract infection episodes. Negative binomial regression was used for the analysis to account for the over-dispersion in data. IRR refers to the incidence-rate ratio.
Significant association after adjusting for other factors.
Table 4.
Multivariate analysis for factors associated with the number of influenza-like illness
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | |
| Gender | ||||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 1.085 (0.733–1.606) | 0.682 | 1.055 (0.714–1.558) | 0.788 |
| Mother’s education | ||||
| Primary education or below | 1 (reference) | 1 (reference) | ||
| Secondary education | 1.341 (0.394–4.565) | 0.639 | 1.383 (0.409–4.670) | 0.602 |
| Tertiary education | 1.779 (0.514–6.163) | 0.363 | 1.728 (0.501–5.960) | 0.387 |
| Mean plaque index (continuous) | 0.768 (0.257–2.299) | 0.637 | – | – |
| Mean dmft (continuous) | – | – | 0.925 (0.839–1.019) | 0.113 |
The dependent variable was the number of influenza-like illness, defined as fever ≥37.8 °C plus cough or sore throat. Negative binomial regression was used for the analysis to account for the over-dispersion in data. IRR refers to incidence-rate ratio.
Table 5.
Multivariate analysis for factors associated with number of febrile acute respiratory tract infections
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | |
| Gender | ||||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 1.073 (0.726–1.588) | 0.723 | 1.024 (0.6965–1.505) | 0.906 |
| Mother’s education | ||||
| Primary education or below | 1 (reference) | 1 (reference) | ||
| Secondary education | 1.625 (0.439–6.013) | 0.467 | 1.646 (0.455–5.958) | 0.448 |
| Tertiary education | 2.120 (0.567–7.932) | 0.264 | 2.025 (0.550–7.454) | 0.288 |
| Mean plaque index (continuous) | 0.742 (0.244–2.254) | 0.598 | – | – |
| Mean dmft (continuous) | – | – | 0.919 (0.833–1.014) | 0.068 |
The dependent variable was the number of febrile acute respiratory infections, defined as fever ≥38.0 °C with any respiratory symptom such as cough, runny nose or sore throat. Zero-inflated negative binomial regression with an intercept-only logit model was used for the analysis to account for the excess zeros in data. IRR refers to incidence-rate ratio.
When using the 3-month recall data on URI, the negative association between the dmft score and the number of URI episodes remained statistically significant (P = 0.04). When using the 1-month recall data, the negative association between dmft score and URI episodes became marginally insignificant (P = 0.08). No statistically significant association between oral health and influenza-like-illness or febrile acute respiratory infection was found when using data from the last 3 months or last 1 month (all P > 0.10).
Discussion
To the best of our knowledge, this longitudinal study is the first one to reveal an association between dental caries and URI among children. The association appeared to be negative; the higher the caries rate (dmft), the lower the chance of URI. Such an association remained significant after adjustment for potential confounders. Analysis using the last 3 months and the last 1 month confirmed this inverse association between dental caries and URI.
The target population of this study was preschool children. In Hong Kong, children attending kindergarten are usually aged 3–5. The youngest children (3-year-olds) attending year 1 in the kindergarten are still settling in a new environment and are less cooperative during an oral examination. The oldest ones (5-year-olds) attending year 3 in the kindergarten will leave in less than 12 months, creating a problem in following up to record their incidence of URI. Therefore, 4-year-old children were recruited in this longitudinal study. Although the kindergartens were not randomly selected, they are located in districts with different socio-economic backgrounds.
A questionnaire was used for parents to recall the episodes and symptoms of URI of their child. Although this data collection method has been widely applied in previous studies25., 26., 27., the laboratory test is considered the gold standard for diagnosing URI. Due to practicality issues, we did not perform laboratory tests to confirm the URI. This was the main limitation of this study. Nevertheless, the validity of our data on children’s URI is indirectly supported by the following facts: (i) Children in our study were in their first year in kindergarten and their parents usually pay more attention to their health status and social adaption27; (ii) The seasonal pattern of URI reported by parents in our study coincided with the influenza circulation pattern in Hong Kong24, and (iii) The short recall (last 1 month and last 3 months) data point to the same findings as revealed by the 12 month results. The consistent results from the analysis of data with a shorter recall period (last month and the last 3 months) for the incidence of URI help to address the concern regarding the reliability of the 12-month recall data.
In this study, dental caries was recorded at the cavitation level, following the WHO criteria and method. Although recording white-spot lesions will include reversible/early caries, such lesions are difficult to detect reliably in field settings. Although taking radiographs may improve the detection of caries in interproximal tooth surfaces, this imposes an additional risk for the participating children due to radiation exposure and was not practical in an epidemiological study. These limitations in the data collection for dental caries should be noted.
Common confounders, namely gender and socioeconomic status (using mother’s education level as an indicator), were controlled for in the multivariate analysis. Age was not entered in the regression models, since all subjects were the same age (4 years old). In addition, children with systemic disease, or under regular medication were excluded from this study, to rule out the confounding effect of the general health condition. Some other potential confounders, such as detailed dietary intake and nutritional status, have not been controlled for in this study, since characterising these factors requires intensive and costly assessment and was thus excluded for practical constraints.
Where lower respiratory tract infection was concerned, previous studies supported a positive association between oral health and respiratory incidents, such as pneumonia and COPD among adults and elderly people4., 5.. In our present study, upper respiratory tract infection (URI) was investigated and an inverse association was found between dental caries and URI. The mechanisms of this inverse association between these two infectious diseases may lie in the possible immune response triggered by dental caries. Innate and adaptive immunity are two fundamental aspects of the immune system response against infectious disease, including dental caries and URI28., 29.. In line with this, some possible mechanisms might be involved in the inverse association between dental caries and URI. The innate immune system in the oropharynx of children with dental caries may be in an active state. Such activation of the first line of non-specific defence against infection may contribute to protection against URI. On the other hand, the adaptive mucosal immune responses of caries-affected children may also be in a heightened state. Previous studies have reported higher antibody titres, such as secretory IgA immunoglobulins (s-IgA), in children with carious lesions compared with children without caries30., 31., 32., 33., 34.. The adaptive immune system activated by dental caries is likely to be another protective factor against URI. It has been reported in many mucosal vaccination experiments in animals that mucosal s-IgA in nasal washes and/or bronchoalveolar fluid is the primary defence compound against nasal influenza35., 36.. The presence of low levels of nasal antiviral s-IgA and neutralising antibody were noted at the onset of influenza infection37. In addition, it was found that the secretion rate of s-IgA was significantly lower in subjects who experienced URI than among others38. The findings of a review also indicated that s-IgA plays a role in protection against URI39. Considering the role of s-IgA in both dental caries and URI, the s-IgA induced by dental caries might insert a protective effect against URI. Furthermore, the mechanism by which dental caries might inhibit URI may involve the possibility that the cariogenic microflora influences the oral cavity environment to reduce susceptibility to URI. For instance, cariogenic organisms might acidify saliva and thus alter the mucosal surface in a way to inhibit the adhesion, proliferation or production of virus40., 41..
Dental plaque is a biofilm harbouring various microbiomes including both pathogenic and non-pathogenic species and its amount cannot be equated with the amount of cariogenic pathogens42. Although the fewer URI episodes in caries-affected children may be attributable to the immunological response triggered by cariogenic bacteria, these children may not necessarily have worse oral hygiene. This possibly may explain the non-significant relationship between plaque index and URI observed in the present study.
As the first study revealing an inverse association between oral health and URI among children, this study only provides some preliminary evidence and generates several speculations on the possible mechanism. The innate and adaptive immune responses induced by dental caries should be further investigated in animal and epidemiological studies. A greater systemic immune responses may occur when dental caries progresses to induce pulpal inflammation of the tooth43. Further observations of the entire spectrum of immune responses related to dental caries and its complications would be useful to clarify the inverse association between oral health and URI.
Conclusion
Our findings showed that children’s caries experience was associated with reduced episodes of URI. Whether this inverse association is attributable to the immune response induced by dental caries should be investigated.
Acknowledgements
The participating kindergartens, parents and children supported this project. This study was financially supported by the General Research Fund (#HKU 766012M), Research Grant Council, Hong Kong.
Conflict of interest
The authors declared no conflict of interest associated with this study.
References
- 1.Fourrier F, Duvivier B, Boutigny H, et al. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Crit Care Med. 1998;26:301–308. doi: 10.1097/00003246-199802000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Didilescu AC, Skaug N, Marica C, et al. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9:141–147. doi: 10.1007/s00784-005-0315-6. [DOI] [PubMed] [Google Scholar]
- 3.Mojon P, Budtz-Jorgensen E, Michel JP, et al. Oral health and history of respiratory tract infection in frail institutionalised elders. Gerodontology. 1997;14:9–16. doi: 10.1111/j.1741-2358.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- 4.Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 5.Sjogren P, Nilsson E, Forsell M, et al. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc. 2008;56:2124–2130. doi: 10.1111/j.1532-5415.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 6.Adachi M, Ishihara K, Abe S, et al. Effect of professional oral health care on the elderly living in nursing homes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:191–195. doi: 10.1067/moe.2002.123493. [DOI] [PubMed] [Google Scholar]
- 7.Houston S, Hougland P, Anderson JJ, et al. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11:567–570. [PubMed] [Google Scholar]
- 8.Mossad SB. Upper respiratory tract infections. Cleveland Clinic: Centers for Continuing Education 2013 Available from http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/upper-respiratory-tract-infection/. Accessed 8 July 2016.
- 9.Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol. 1998;3:251–256. doi: 10.1902/annals.1998.3.1.251. [DOI] [PubMed] [Google Scholar]
- 10.Estimates NIoH Chronic illness self-management in children. 2010. http://grants.nih.gov/grants/guide/pa-files/PA-03-159.html. Accessed 7 May 2016.
- 11.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 12.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 13.Sands KM, Twigg JA, Lewis MAO, et al. Microbial profiling of dental plaque from mechanically ventilated patients. J Med Microbiol. 2016;65:147–159. doi: 10.1099/jmm.0.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands KM, Wilson MJ, Lewis MA, et al. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J Crit Care. 2017;37:30–37. doi: 10.1016/j.jcrc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Bridger RC. Haemophilus influenzae: the relationship to upper respiratory tract infection. N Z Med J. 1974;80:19–22. [PubMed] [Google Scholar]
- 16.Guzek A, Rybicki Z, Korzeniewski K, et al. Etiological factors causing lower respiratory tract infections isolated from hospitalized patients. Adv Exp Med Biol. 2015;835:37–44. doi: 10.1007/5584_2014_23. [DOI] [PubMed] [Google Scholar]
- 17.Gao XL, Hsu CY, Loh T, et al. Dental caries prevalence and distribution among preschoolers in Singapore. Community Dent Health. 2009;26:12–17. [PubMed] [Google Scholar]
- 18.Gao XL, Di Wu I, Lo EC, et al. Validity of caries risk assessment programmes in preschool children. J Dent. 2013;41:787–795. doi: 10.1016/j.jdent.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 20.WHO . 4th ed. WHO; Geneva: 1997. Oral Health Surveys: Basic Methods. [Google Scholar]
- 21.Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Ng DMW, Seto WH, et al. Seroprevalence of antibody to pandemic influenza A (H1N1) 2009 among healthcare workers after the first wave in Hong Kong. J Hosp Infect. 2011;78:308–311. doi: 10.1016/j.jhin.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206:1862–1871. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longmier E, Barrett B, Brown R. Can patients or clinicians predict the severity or duration of an acute upper respiratory infection? Fam Pract. 2013;30:379–385. doi: 10.1093/fampra/cmt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soltis BW, Sanders JW, Putnam SD, et al. Self reported incidence and morbidity of acute respiratory illness among deployed US military in Iraq and Afghanistan. PLoS One. 2009;4:e6177. doi: 10.1371/journal.pone.0006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skouteris H, McCabe M, Swinburn B, et al. Healthy eating and obesity prevention for preschoolers: a randomised controlled trial. BMC Public Health. 2010;10:220. doi: 10.1186/1471-2458-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Cássia Negrini T, Duque C, Höfling JF, et al. Fundamental mechanisms of immune response to oral bacteria and the main perspectives of a vaccine against dental caries: a brief review. Revista Odonto Ciência. 2009;24:198–204. [Google Scholar]
- 29.Skinner MA, Bentley-Hewitt K, Rosendale D, et al. Effects of kiwifruit on innate and adaptive immunity and symptoms of upper respiratory tract infections. Adv Food Nutr Res. 2013;68:301–320. doi: 10.1016/B978-0-12-394294-4.00017-1. [DOI] [PubMed] [Google Scholar]
- 30.Al Amoudi N, Al Shukairy H, Hanno A. A comparative study of the secretory IgA immunoglobulins (s. IgA) in mothers and children with SECC versus a caries free group children and their mothers. J Clin Pediatr Dent. 2007;32:53–56. doi: 10.17796/jcpd.32.1.l338366jw54634q5. [DOI] [PubMed] [Google Scholar]
- 31.Bagherian A, Asadikaram G. Comparison of some salivary characteristics between children with and without early childhood caries. Indian J Dent Res. 2012;23:628. doi: 10.4103/0970-9290.107380. [DOI] [PubMed] [Google Scholar]
- 32.Twetman S, Lindner A, Modeer T. Lysozyme and salivary immunoglobulin A in caries-free and caries-susceptible pre-school children. Swed Dent J. 1981;5:9–14. [PubMed] [Google Scholar]
- 33.Ranadheer E, Nayak UA, Reddy NV, et al. The relationship between salivary IgA levels and dental caries in children. J Indian Soc Pedod Prev Dent. 2011;29:106–112. doi: 10.4103/0970-4388.84681. [DOI] [PubMed] [Google Scholar]
- 34.Priya PG, Asokan S, Karthick K, et al. Effect of dental treatments on salivary immunoglobulin A of children with and without dental caries: a comparative study. Indian J Dent Res. 2013;24:394. doi: 10.4103/0970-9290.118004. [DOI] [PubMed] [Google Scholar]
- 35.Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004;57:236–247. [PubMed] [Google Scholar]
- 36.Takahashi E, Kataoka K, Fujii K, et al. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect. 2010;12:778–783. doi: 10.1016/j.micinf.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto C, Takeda N, Matsunaga A, et al. Induction and maintenance of anti-influenza antigen-specific nasal secretory IgA levels and serum IgG levels after influenza infection in adults. Influenza Other Respir Viruses. 2012;6:396–403. doi: 10.1111/j.1750-2659.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gleeson M, Bishop N, Oliveira M, et al. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 39.Williams JE. Portal to the interior: viral pathogenesis and natural compounds that restore mucosal immunity and modulate inflammation. Altern Med Rev. 2003;8:395–410. [PubMed] [Google Scholar]
- 40.Düzgüneş N, de Lima MC, Stamatatos L, et al. Fusion activity and inactivation of influenza virus: kinetics of low pH-induced fusion with cultured cells. J Gen Virol. 1992;73:27–37. doi: 10.1099/0022-1317-73-1-27. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig S, Planz O. Influenza viruses and the NF-κB signaling pathway-towards a novel concept of antiviral therapy. Biol Chem. 2008;389:1307–1312. doi: 10.1515/BC.2008.148. [DOI] [PubMed] [Google Scholar]
- 42.Vratsanos SM, Mandel ID. Comparative plaque acidogenesis of caries-resistant vs. caries-susceptible adults. J Dent Res. 1982;61:465–468. doi: 10.1177/00220345820610030401. [DOI] [PubMed] [Google Scholar]
- 43.van Gemert-Schriks MC, van Amerongen EW, Aartman IH, et al. The influence of dental caries on body growth in prepubertal children. Clin Oral Investig. 2011;15:141–149. doi: 10.1007/s00784-010-0380-3. [DOI] [PubMed] [Google Scholar]