Abstract
Objective: To evaluate the caries-predictive validity of a screening Cariogram model without saliva tests in Chinese young adults with past caries experience. Methods: Two-hundred and fifteen Chinese young adults seeking dental care were included in this observational study, with informed consent obtained. All participants were 18–29 years of age and with past caries experience. A caries risk assessment was made at baseline using a full-blown Cariogram model and a screening Cariogram model without saliva tests; this assessment included bacterial count, saliva secretion and buffer capacity. Participants were divided into five risk groups according to ‘the chance of avoiding caries’ expressed in Cariogram software with two models. Each participant was required to receive re-examination after 2 years and then the actual caries increment (ΔDMFS) was calculated. The correlation between ΔDMFS and the ratings of caries risk was analysed using Spearman rank correlation. Differences between the full-blown and screening models were expressed as area under the receiver–operating characteristics (ROC) curve (AUC). Results: One-hundred and ninety-two participants finished the 2-year follow-up study, after 23 dropped out. The mean 2-year caries increment was 0.67 ± 1.03. Both Cariogram models displayed a statistically significant relationship with caries development (P < 0.001): more new caries was found among those assessed with high risk compared with those assessed with low risk. No statistically significant difference of the AUC was found between the two Cariogram models (P > 0.05). Conclusions: The Cariogram model without saliva tests does not significantly decrease the caries-predictive ability in young adults with a history of caries. This screening model is a potential tool for rapid caries risk assessment for such populations.
Key words: Caries risk, Cariogram, screening model, saliva test, validity
Introduction
Dental caries is highly prevalent around the world and the vast majority of people currently have, or previously have experienced, this disease. In addition to treating teeth with dental decay, it is important to manage such patients to avoid new caries. By identifying individuals who are most likely to develop dental caries through caries risk assessment, practitioners can provide those individuals with suitable preventive measures to interrupt the disease process. Thus, effective caries risk assessment and graded management are necessary to control new caries development.
Many factors should be assessed in new caries prediction because dental caries is a disease with a multifactorial aetiology1. Cariogram is a computerised program used for caries risk assessment: it considers the interaction of nine different caries risk factors; demonstrates the caries risk of an individual graphically; and shows the risk for developing new caries, expressed as ‘chances to avoid new caries in the near future’. Patients are scored on diet (contents and frequency), plaque, caries experience, bacterial counts, related diseases, fluoride programme, saliva secretion and buffer capacity, and the results are shown as a pie-chart risk profile2. Cariogram has been used to investigate the caries risk profiles in different populations. Moreover, the predictive validity of Cariogram has been confirmed among schoolchildren3., 4., teenagers5, young adults6., 7. and elderly adults8.
However, the application of Cariogram may be limited by the inclusion of saliva tests that involve microbial culture, bacterial counts, measurement of the saliva secretion rate and saliva buffer capacity. These saliva tests are costly and time-consuming, and are considered a burden for clinicians. Thus, a simplified model without supplemental laboratory tests may be more suitable for busy clinicians. If Cariogram could be used without time-consuming saliva tests, risk prediction and graded management of patients with dental caries in clinics would become easier.
Although Petersson9 showed that the accuracy of caries prediction in schoolchildren was significantly impaired when the Cariogram model was applied without saliva tests, the subjects were schoolchildren and most of these children had not previously experienced caries. However, such evidence among adults is lacking. As most adult patients seeking care in clinics have already experienced dental caries, caries risk assessment of such a population is still a challenge for clinicians. However, it is unknown whether a screening Cariogram model without saliva tests could be applied to replace the full-blown Cariogram model, particularly in adult patients who have a history of caries.
Thus, the aim of this study was to assess (using Cariogram) the caries risk profiles among 18- to 29-year-old Chinese young adults who seek dental services and to compare the predictive ability of the full-blown Cariogram model with that of the screening Cariogram model.
Materials and Methods
Study sample
Two-hundred and fifteen young adults who visited the Department of Conservative Dentistry and Endodontics (Stomatological Hospital of Chongqing Medical University), from 1 January 2013 to 31 January 2014, for dental caries or pulpitis/pulp necrosis caused by caries, were consecutively recruited in this observational study (Figure 1). This research was conducted in full accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from each participant. Approval for the study was obtained from the Institutional Ethics Committee of Stomatological Hospital of Chongqing Medical University. The sample was determined using sample size software (Pass 11; NCSS, Kaysville, UT, USA) with an alpha risk of 0.05 and a power of 0.80. Inclusion criteria were: (i) age ranging from 18 to 29 years; and (ii) the presence of dental caries or restoration. Those who did not provide informed consent, had no caries, had a diagnosis of psychiatric disease, were younger than 18 years or older than 29 years of age, had taken antibiotics within 3 days of the start of the study or were completely illiterate, were excluded from this study.
Figure 1.
Flow diagram of the recruitment of participants.
Questionnaire and interview
Information concerning related general diseases, fluoride exposure, diet and oral-hygiene habits of each participant were collected using a questionnaire and an interview, according to the Cariogram manual.
Clinical examination
Intra-oral and radiographic examinations were carried out by two skilled dentists in the clinic. Dental caries was identified and classified based on the World Health Organization (WHO) criteria10. A tooth was recorded as decayed when there was a visible cavitation (detectable softened floor, undermined enamel, softened wall) or an approximal translucency in the dentin as viewed on bitewing radiographs. Decayed, missing or filled tooth/surface (DMFT/DMFS) indices for each subject were recorded. The amount of plaque was evaluated according to the Silness-Löe Plaque Index11. The two examiners were trained and calibrated before the study, and the intra- and inter-examiner reliability was assessed using the kappa test. Kappa values of 0.880/0.850 for intra-examiner reliability and 0.849 for inter-examiner reliability were obtained.
Salivary and microbiological tests
Saliva secretion rates were estimated according to the paraffin-stimulated saliva secretion method. Saliva samples were collected from each participant, and the secretion rate was expressed as ml/minute. Buffer capacity was estimated using the Dentobuff test. Then, the levels of mutans streptococci and lactobacilli were evaluated using the Strip mutans test and the Dentocult LB test (Orion Diagnostica, Espoo, Finland) according to the instructions of the manufacturer, respectively.
Risk assessment using Cariogram software
The data collected through the questionnaire, clinical evaluation and saliva tests were input into the boxes according to the Cariogram manual. Caries risk assessment was carried out using the full-blown Cariogram model and the screening Cariogram model. Compared with the full-blown Cariogram, the screening Cariogram was processed without the risk factors obtained from saliva sampling (i.e. bacterial count, secretion rate and buffer capacity). The three boxes in the Cariogram software were not filled with any risk values. Moreover, the variable ‘Diet, contents’ was scored without taking the results of the lactobacillus test count into consideration. The participants were classified into five risk groups according to the chance of avoiding caries: 81–100% (very low); 61–80% (low); 41–60% (medium); 21–40% (high); and 0–20% (very high).
Re-examination after 2 years
All participants were recalled and re-examined by the same two examiners after 2 years. Data on caries experience (DMFT, DMFS) were extracted from the dental records and the actual caries increment (ΔDMFS) for each participant during the 2-year period was calculated.
Statistical analysis
All data were processed using SPSS (version 17.0) and Medcalcu (version 16.4.2) software. Spearman rank correlation was carried out to assess the strength of association between actual new caries increment and caries risk ratings, according to Cariogram models. For comparison between the full-blown and screening Cariogram models, the area under the receiver–operating characteristics (ROC) curve (AUC) was calculated and the differences were tested according to Delong et al.12. Values of P<0.05 were considered to be statistically significant.
Results
One-hundred and ninety-two participants (mean age ± standard deviation: 23.30 ± 3.06 years) received re-examination after 2 years, with a dropout of 23 participants. The reasons for dropout were: out of contact (10 subjects); refusal to participate in the follow-up study (seven subjects); or migration to other cities (six subjects).
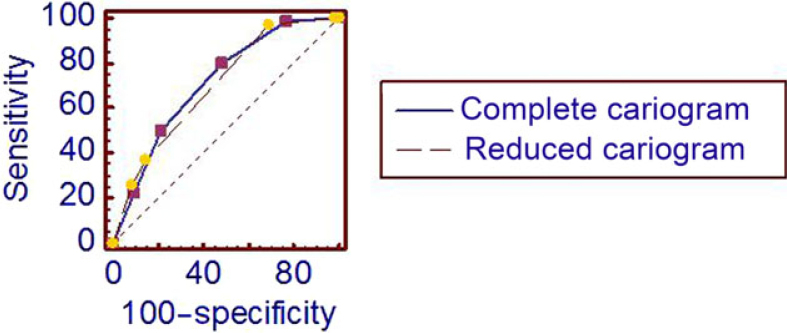
The mean DMFT and DMFS scores in the study population at baseline were 3.41 ± 2.41 and 5.67 ± 4.81, respectively, and after 2 years 39.6% of the subjects had developed new lesions. The mean 2-year caries increment (ΔDMFS) was 0.67 ± 1.03. According to the full-blown Cariogram model, 14.6%, 24.5%, 28.1%, 18.2% and 14.6% of all participants were assessed with very low, low, moderate, high and very high caries risk, respectively, while, based on the screening Cariogram model, 1.0%, 18.8%, 56.8%, 7.8% and 15.6% were assessed with very low, low, moderate, high and very high caries risk, respectively. Both Cariogram models displayed a statistically significant relationship with the caries increment (P < 0.001). More new caries was found among those assessed with high caries risk compared with those with low caries risk, according to either the full-blown or the screening model. The actual caries incidence (ΔDMFS > 0) over 2 years in the five risk groups, assessed using the full-blown and screening Cariogram models, are shown in Table 1. This is further displayed in the ROC curve shown in Figure 2 and in the calculated AUC, as presented in Table 2. No statistically significant difference in AUC was found in predictive ability between full-blown and screening Cariogram models (P > 0.05).
Table 1.
Mean caries increments over 2 years in each caries-risk category of the full-blown and screening Cariogram models
| Risk group at baseline | n | ΔDMFS | ΔDMFS > 0 (%) | |||
|---|---|---|---|---|---|---|
| Full-blown | Screening | Full-blown | Screening | Full-blown | Screening | |
| 81–100 (very low risk) | 28 | 2 | 0.04 ± 0.19 | 0.00 ± 0.00 | 3.57 | 0 |
| 61–80 | 47 | 36 | 0.38 ± 0.64 | 0.11 ± 0.52 | 29.79 | 5.56 |
| 41–60 | 54 | 109 | 0.72 ± 1.02 | 0.68 ± 0.99 | 42.59 | 42.20 |
| 21–40 | 35 | 15 | 1.06 ± 1.21 | 0.80 ± 0.94 | 60 | 53.33 |
| 0–20 (very high risk) | 28 | 30 | 1.21 ± 1.37 | 1.30 ± 1.34 | 60.71 | 66.67 |
ΔDMFS, mean increment of decayed, missing or filled surfaces over 2 years.
Figure 2.
Receiver–operating characteristics (ROC) curve for full-blown and screening Cariogram models. No statistically significant difference in the area under the ROC curve was found between full-blown (0.714) and screening (0.701) Cariogram models (P > 0.05).
Table 2.
Mean area under the receiver–operating characteristics curve (AUC) for full-blown and screening Cariogram models
| Risk model | AUC | SE | 95% CI | Sens. + Spec. at cut-off point | ||||
|---|---|---|---|---|---|---|---|---|
| 81-100% | 61-80% | 41–60% | 21–40% | 0–20% | ||||
| Full-blown | 0.714 | 0.0357 | 0.645–0.777 | 11.6 + 100 | 26.2 + 90.7 | 56.0 + 80.1 | 83.6 + 56.0 | 99.3 + 23.5 |
| Screening | 0.701 | 0.0322 | 0.631–0.765 | 9.8 + 100 | 28.4 + 94.1 | 37.3 + 87.1 | 97.9 + 37.3 | 100 + 8.7 |
95% CI, 95% confidence interval; SE, standard error; Sens., sensitivity; Spec., specificity.
No significant difference was found between the two Cariogram models (P < 0.05)12.
Discussion
The results of this study show that the accuracy of caries prediction was not significantly impaired when the Cariogram model was applied in the absence of salivary tests. A previous study by Petsi et al.13 showed a similar result and supports application of the modified Cariogram, without saliva tests, for caries risk prediction in orthodontic practice. Based on these findings, a screening Cariogram model without saliva tests can be a useful tool for clinical assessment of caries risk under certain conditions. Many clinicians, especially those in developing countries, see a large number of patients each day and when they detect newly developed caries in teeth, they treat such teeth with fillings. It is unwise not to screen high-risk populations with the aim to prevent new caries. The failure to screen could be a result of the time-consuming and costly nature of the adjunct laboratory tests involved in the risk-assessment model. When patients visit dental clinics, most already have dental caries and seem to be more likely to develop new caries. Based on the present study, a screening Cariogram model may be a good choice in graded management of individuals in this population, all of whom have a history of caries.
The findings were based on the conditions of this study. All participants were relatively young adults (18–29 years of age). Besides, all people included were patients seeking dental services in the Department of Conservative Dentistry and Endodontics, and the caries prevalence in the study population at baseline was 100%. The situation was largely different from some previous studies in which participants were volunteers recruited from school or community3., 4., 6.. The screening Cariogram without saliva tests may be valid only for this age group of young adults with past caries history, as the validity of this model for caries risk assessment of populations of different ages or races, or from different areas, was unclear. For instance, a previous study9 demonstrated that the accuracy of caries prediction was significantly impaired when the Cariogram model was applied without enumeration of saliva tests, which drew the opposite conclusion from the present study. In that study, the study population consisted of Swedish schoolchildren, 10–11 years of age, and the caries prevalence at baseline was only 40%. Another study14 that also used a modified Cariogram without laboratory tests was not particularly useful in identifying high-caries-risk individuals. It is noteworthy that the subjects in the study were schoolchildren recruited from a low-caries community. The difference in study population may contribute to the different results obtained among these studies. Therefore, the findings of the present study have certain limitations and may be valid only for this age group of young adults with a mainly uncompromised saliva secretion rate and buffer capacity. Thus, the validity of screening models needs to be investigated in different populations (e.g. elderly or schoolchildren populations) in future studies.
The simplified caries risk prediction model is economical and time-saving when used in dental clinics. The risk-assessment strategies involving too many factors, especially laboratory tests, may limit its application in clinics worldwide. In some developing countries (e.g. China), laboratory tests are only applied in some small-scale epidemiological studies; however, it is not suitable for predicting the caries risk of each patient on a daily basis. Sometimes, the full-blown Cariogram seems to be too time-consuming for incorporating into daily routine in dental clinics when many patients are waiting for treatment. Two other caries-risk prediction systems, namely the American Dental Association (ADA)15 and The International Caries Classification and Management System (ICCMS™)16, also tend to assess the caries risk without laboratory tests; however, both are visual computerised systems, as well as clinical guidelines.
The levels of caries risk were more centralised if saliva tests were not considered, which is similar to the finding of a previous study13. A larger proportion of subjects (56.8%) were assigned to the medium-risk category ‘41–60% chance of avoiding caries’ in the screening model compared with the full-blown model (28.1%). The screening model had a higher predictive accuracy for caries increment in the higher risk group compared with the full-blown model. Two-thirds (66.7%) of subjects in the very-high-risk group in the screening model developed new caries over 2 years compared with 60.7% in the full-blown model. The predictive ability of the screening Cariogram at the cut-off point had lower specificity, while higher specificity was found for the ‘0–20% chance of avoiding caries’. With lower specificity, some individuals with actual low caries risk may be categorised into the high-risk group, and unnecessary preventive measures may be taken. Overall, the different risk groups of the screening Cariogram exhibited a significant relationship to caries increment (P < 0.001). The advantage of the screening Cariogram is rapid assessment of caries risk in the dental clinic.
Cariogram shows the risk for developing new caries using an estimation of ‘chances to avoid new caries in 12 months’, according to the manual. Notably, the actual follow-up period was 24 months in the present study. Compared with 12 months of follow up, more new caries developed within 24 months of follow up. The influence of this modification was considered to be slight because some previous studies4., 6., 9. had validated the long-term (>12 months) predictive ability of the Cariogram model, which broadens the application of Cariogram. The Cariogram model can be used not only for a 12-month caries risk-assessment period but also for a longer-term risk assessment.
Conclusions
With the limits of this study, the Cariogram model without saliva tests did not significantly decrease caries-predictive ability in young adults with a history of caries. This screening model may be used for rapid caries assessment of certain populations in the dental clinic. However, the validity of this screening model needs to be evaluated in other population groups in future studies.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31571508), Chongqing Research Program of Basic Research and Frontier Technology (No. cstc2016jcyjA0162) and Project Supported by Scientific and Technological Research Program of Chongqing Municipal Education Commission (No. KJ1600214).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Maheswari SU, Raja J, Kumar A, et al. Caries management by risk assessment: a review on current strategies for caries prevention and management. J Pharm Bioallied Sci. 2015;7:S320–S324. doi: 10.4103/0975-7406.163436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratthall D, Hänsel Petersson G. Cariogram – a multifactorial risk assessment model for a multifactorial disease. Community Dent Oral Epidemiol. 2005;33:256–264. doi: 10.1111/j.1600-0528.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 3.Utreja D, Simratvir M, Kaur A, et al. An evaluation of the Cariogram as a predictor model. Int Dent J. 2010;60:282–284. [PubMed] [Google Scholar]
- 4.Campus G, Cagetti MG, Sale S, et al. Cariogram validity in schoolchildren: a two-year follow-up study. Caries Res. 2012;46:16–22. doi: 10.1159/000334932. [DOI] [PubMed] [Google Scholar]
- 5.Hänsel Petersson G, Twetman S, Bratthall D. Evaluation of a computer program for caries risk assessment in schoolchildren. Caries Res. 2002;36:327–340. doi: 10.1159/000065963. [DOI] [PubMed] [Google Scholar]
- 6.Petersson GH, Twetman S. Caries risk assessment in young adults: a 3 year validation of the Cariogram model. BMC Oral Health. 2015;15:17. doi: 10.1186/1472-6831-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celik EU, Gokay N, Ates M. Efficiency of caries risk assessment in young adults using Cariogram. Eur J Dent. 2012;6:270–279. [PMC free article] [PubMed] [Google Scholar]
- 8.Hänsel Petersson G, Fure S, Bratthall D. Evaluation of a computer-based caries risk assessment program in an elderly group of individuals. Acta Odontol Scand. 2003;61:165–170. doi: 10.1080/00016350310002261. [DOI] [PubMed] [Google Scholar]
- 9.Petersson GH, Isberg PE, Twetman S. Caries risk assessment in school children using a reduced Cariogram model without saliva tests. BMC Oral Health. 2010;10:5. doi: 10.1186/1472-6831-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation . 3rd ed. World Health Organisation; Geneva: 1987. Oral Health Surveys: Basic Methods. [Google Scholar]
- 11.Macgregor ID. Comparison of the Silness-Loe (1964) Index with gravimetric measurement of dental plaque. Clin Prev Dent. 1987;9:9–12. [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 13.Petsi G, Gizani S, Twetman S, et al. Cariogram caries risk profiles in adolescent orthodontic patients with and without some salivary variables. Angle Orthod. 2014;84:891–895. doi: 10.2319/080113-573.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holgerson PL, Twetman S, Stecksèn-Blicks C. Validation of an age-modified caries risk assessment program (Cariogram) in preschool children. Acta Odontol Scand. 2009;67:106–112. doi: 10.1080/00016350802714734. [DOI] [PubMed] [Google Scholar]
- 15.Tellez M, Gomez J, Pretty I, et al. Evidence on existing caries risk assessment systems: are they predictive of future caries? Community Dent Oral Epidemiol. 2013;41:67–78. doi: 10.1111/cdoe.12003. [DOI] [PubMed] [Google Scholar]