Abstract:
Objective: To review the evidence regarding the mechanisms of silver diamine fluoride (SDF) for arresting caries. Methods: A literature search was conducted using the keywords silver diamine fluoride, and its alternative names, in seven databases: PubMed, Embase and Scopus (English); China National Knowledge Infrastructure (Chinese); Bilioteca Virtual em Saude (Portuguese); Biblioteca Virtual en Salud Espana (Spanish); and Ichushi-Web (Japanese). The titles and abstracts were screened. Full texts were retrieved for publications that studied mechanisms of actions of SDF, including its effects on remineralisation of carious lesions and on cariogenic bacteria. Results: A total of 1,123 publications were identified. Twenty-nine articles were included and they investigated the effect of SDF on cariogenic bacteria and dental hard tissues. Eleven studies investigated the antibacterial properties of SDF. They found that SDF was bactericidal to cariogenic bacteria, mainly Streptococcus mutans. It inhibited the growth of cariogenic biofilms on teeth. Twenty studies reported the remineralisation of demineralised enamel or dentine by SDF. They found that mineral loss of demineralised enamel and dentine was reduced after SDF treatment. A highly mineralised surface rich in calcium and phosphate was formed on arrested carious lesions. Four studies examined the effect of SDF on dentine collagen. They found that SDF inhibited collagenases (matrix metalloproteinases and cysteine cathepsins) and protected dentine collagen from destruction. Conclusion: SDF is a bactericidal agent and reduces the growth of cariogenic bacteria. It inhibits demineralisation and promotes the remineralisation of demineralised enamel and dentine. It also hampers degradation of the dentine collagen.
Key words: Silver diamine fluoride, caries, mechanism, review
Introduction
Dental caries is a localised chemical dissolution of dental hard tissues that is caused by acidic by-products of the metabolic processes of the biofilm (dental plaque) covering an affected tooth surface1. Margolis and Moreno2 suggested that the dental plaque fluid is an important factor affecting caries development. Following exposure to fermentable carbohydrate, the amounts of tooth mineral and other calcium phosphates in plaque fluid decrease rapidly; this is primarily because of lactic acid production and reduction in the volume of plaque fluid, which can result in caries formation2. Caries progression in enamel and dentine is different. Enamel caries refers to the dissolution of highly mineralised tissue as a result of attack by bacterial acid3, whereas that in dentine involves both mineral demineralisation and organic matrix degradation of the type I collagen fibre network4.
Silver diamine fluoride (SDF) is used to prevent and arrest caries, and “silver diamine fluoride” is the most common spelling/keyword for this compound in the dental literature. There are a number of different nomenclatures for this dental product: ‘silver diamine fluoride’5., 6., 7., 8. ‘diammine silver fluoride’9 ‘silver diammine fluoride’10 ‘diamine silver fluoride or silver fluoride’11., 12. and ‘silver ammonium fluoride’13. SDF is a colourless alkaline solution containing silver and fluoride, which forms a complex with ammonia14. SDF is not merely a simple salt of silver, ammonium and fluoride ions. Rather, it is a mixed heavy-metal halide coordination complex. Ammonia can keep the solution at a constant concentration for a certain period of time15. Silver compounds have a long history of use in both medicine and dentistry because of their antimicrobial properties16. Fluoride is used in various forms to prevent and arrest caries14. Hence, the combined effects of silver and fluorides have been hypothesised to have the ability to halt caries progression and prevent the development of new caries simultaneously17. A review concluded that SDF is an effective, efficient, equitable and safe caries-preventive agent that appears to meet the standards of the US Institute of Medicine and the Millennium Goals of the World Health Organization17.
SDF was approved for use as a therapeutic agent in Japan in the 1960s18. It has also been used in Argentina, Australia, Brazil and China for many years to treat dental caries14. In 2014, the US Food and Drug Administration (FDA) cleared the first SDF product for use in the USA4. Since 1969, SDF has been used to arrest caries of the primary teeth in children18, prevent pit and fissure caries of the erupting permanent molars9 and prevent root caries in elderly people19. Apart from caries management, SDF is also used to treat tooth hypersensitivity and to sterilise infected root canals15. It can be applied directly onto a carious lesion to arrest the caries or onto a caries-free surface for prevention. Clinical studies have demonstrated that SDF is effective in reducing enamel carious lesions in first permanent molars20 and dentine caries in the primary anterior teeth8.
Although studies have demonstrated that SDF is effective in arresting dental caries, the mechanism of action is unclear. Studies that investigated the mechanism of SDF vary markedly in terms of perspective, hypotheses, objectives, methodology, experimental conditions, model systems and conclusions. Past literature reviews were performed based on publications in the English language. As SDF has been widely used for dentistry in Argentina, Brazil, China and Japan for many years, a number of studies related to SDF were published in Spanish, Portuguese, Chinese and Japanese. A systematic review was performed on clinical studies of SDF, and a meta-analysis was carried out to evaluate the effectiveness of SDF in arresting dental caries21. To date, there has been no comprehensive review in the literature to evaluate studies, published in different languages, performed to investigate the mechanisms of actions of SDF. This paper is a literature review of SDF on publications published in Chinese, English, Japanese, Portuguese and Spanish. The aim of this paper was to review the evidence on the mechanisms of actions of SDF on dental caries in terms of its effect on carious lesions, including its action on cariogenic bacteria.
Materials and Methods
Search strategy
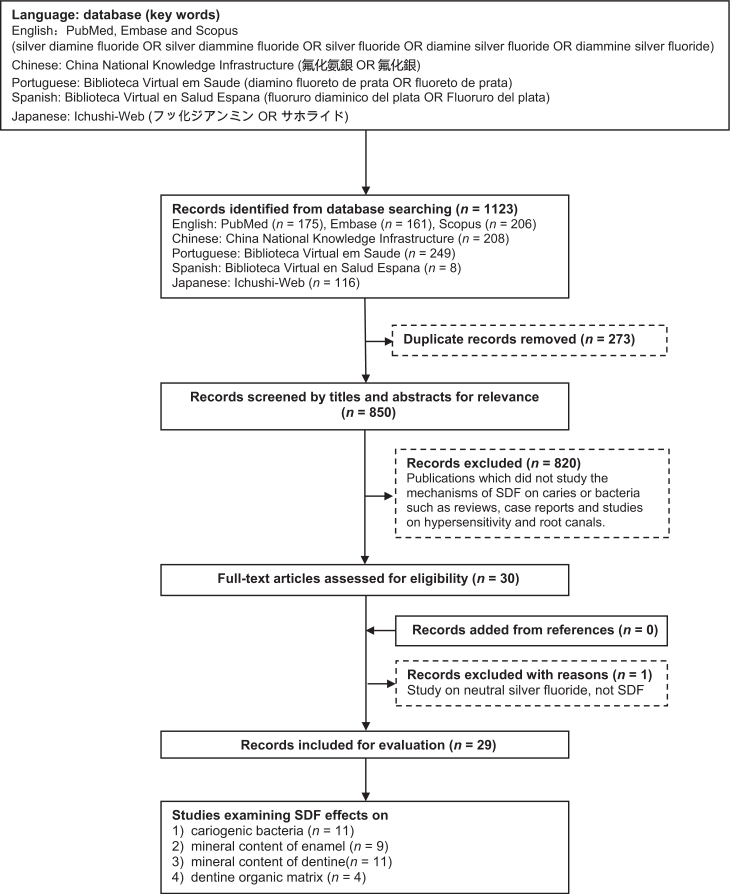
A literature search was conducted to identify papers in seven databases that included references in five different languages. English publications in PubMed, Embase and Scopus were searched using the keywords ‘silver diamine fluoride’ OR ‘silver diammine fluoride’ OR ‘silver fluoride’ OR ‘diamine silver fluoride’ OR ‘diammine silver fluoride’. A search of the Chinese literature in the China National Knowledge Infrastructure (CNKI) was conducted using the keywords ‘氟化氨銀’ OR ‘氟化銀’. Spanish publications in Biblioteca Virtual en Salud Espana (BVSE) were searched using the keywords ‘fluoruro diaminico del plata’ OR ‘fluoruro del plata’. A Portuguese literature search was conducted in Biblioteca Virtual em Saude (BVS) using the keywords ‘diamino fluoreto de prata’ OR ‘fluoreto de prata’. Japanese papers in Ichushi-Web were searched using the keywords ‘フッ化ジアンミン銀’ OR ‘サホライド’. No publication-year limit was set, and the last search was made in March 2016. A potentially eligible list of publications was developed including papers searched using the keywords (Figure 1).
Figure 1.
Flow chart of literature search. SDF, silver diamine fluoride.
Study selection and data extraction
Reference lists identified from searching the seven databases were checked for duplication. After eliminating the duplicate publications, the titles and abstracts of articles initially identified from the potentially eligible list were screened. Publications in which the mechanisms of SDF on caries or bacteria were not studied were excluded via the screening of titles and abstracts. Afterwards, the full texts of the remaining articles were retrieved. Manual screening was performed on the reference lists of all possible eligible papers. Studies were selected for this review in accordance with the following inclusion criterion: studies investigated the properties of SDF on carious lesions, including its action on cariogenic bacteria. If consensus about the inclusion of a study was not reached, the paper was discussed with an independent investigator until agreement was achieved.
Related information of non-English publications included in the final list was translated into English. For the studies included, the following information was recorded: publication details (authors and years); methods; outcome assessments (various criteria for evaluating the remineralisation of caries: lesion depth; mineral loss; calcium and phosphate absorption and release; microhardness of tooth surfaces; surface morphology; collagen degradation; and bacterial counts); and the main findings.
Results
The initial literature search found 1,123 potentially eligible publications up to March 2016 (175 articles in PubMed, 161 articles in Embase, 206 articles in Scopus, 208 articles in CNKI, 249 articles in BVS, eight articles in BVSE and 116 articles in Ichushi-Web). Two-hundred and seventy-three duplicate records were removed (Figure 1). After screening the titles and abstracts, 820 articles that were classified as literature reviews, case reports, studies on hypersensitivity, root canal treatment, cytotoxicity and caries prevention, along with other irrelevant studies, were excluded. In this review, no clinical trial was found to study the mechanism of SDF. Therefore, the publications included were either ex vivo or in vitro studies. Full-text papers were obtained for the remaining 30 publications. Hand searches of the references of the selected papers did not identify any additional publications that met the inclusion criteria. One article was excluded from the final evaluation because it used neutral silver fluoride without ammonia22. The remaining 29 papers were found to meet the eligibility criteria and were included in this review. Among them, 11 studies examined the action of SDF on cariogenic bacteria (Table 1), nine studies investigated the effect of SDF on the mineral content of enamel (Table 2), 11 studies investigated the effect of SDF on the mineral content of dentine (Table 3) and four studies examined the effect of SDF on the dentine organic matrix (Table 4).
Table 1.
Summary of publications studying the action of silver diamine fluoride (SDF) on cariogenic bacteria
| Authors, Year (Language) | Methods | Main findings |
|---|---|---|
| Suzuki et al. 1976 (English)27 | The visual broth microdilution method was used to determine the MICs of SDF, Ag(NH3)2NO3 and NaF against S. mutans | The MIC of SDF was 19.0 μg/ml, which was lower than the MICs of Ag(NH3)2NO3 and NaF |
| Igarashi, 1978 (Japanese)26 | The agar diffusion method was used to study the antibacterial activity of SDF, AgNO3 and NaF against S. mutans | SDF was more effective than AgNO3 and NaF at inhibiting the growth of S. mutans |
| Tsutsumi, 1981 (Japanese)28 | SEM was used to study the adhesion of S. mutans on carious enamel treated with 7.6% SDF incubated in Trypticase Soy Broth | SDF inhibited adherence and growth of S. mutans on the carious enamel surface |
| Li et al., 1984 (Chinese)23 | The agar diffusion method was used to determine the MICs of SDF, Ag(NH3)2NO3 and NaF against S. mutans | The MICs (%) of SDF, Ag(NH3)2NO3 and NaF against S. mutans were <3.3 × 10−11, 3.3 × 10−11 and 5.4 × 10−7, respectively |
| Knight et al., 2005 (English)11 | Spectrophotometry was used to determine the effect of 29% (1.8 mol/l) SDF on bacterial growth (optical density) of S. mutans supplied by the chemostat system | SDF was effective at inhibiting the growth of bacteria (SDF vs. control, P < 0.05) |
| de Almeida et al., 2011 (English)25 | The agar diffusion method was used to study the antibacterial activity (MID) of 12% and 30% SDF against S. mutans | The agar diffusion method showed that 12% and 30% SDF inhibited the growth of S. mutans |
| Targino et al., 2014 (English)29 | The spectrophotometric broth microdilution method and turbidity were used to determine the MICs of SDF and CHX against S. mutans. The MBC was evaluated in plates containing brain–heart infusion agar | The MICs of SDF and CHX were 33.3 μg/ml and 3.3 μg/ml, respectively. The MBCs of SDF and CHX were 50.0 μg/ml and 6.0 μg/ml, respectively |
| Chu et al., 2012 (English)7 | SEM, CFU and CLSM were used to study two biofilms (S. mutans and A. naeslundii) on carious dentine treated with 38% SDF | Silver particles were found on the dentine surface after SDF treatment. Compared with the control, the SDF-treated dentine had fewer CFUs of bacteria (P < 0.001) and more dead bacteria (P < 0.05) |
| Alves et al., 2010 (Portuguese)24 | The agar diffusion method was used to study the antibacterial activity (MID) of 12%, 16% and 30% SDF against S. mutans, S. oralis and L. casei | The agar diffusion method showed that 12%, 16% and 30% SDF inhibited the growth of the three species of bacteria |
| Mei et al., 2013 (English)6 | SEM, CFU and CLSM were used to study a dual-species biofilm (S. mutans and L. acidophilus) on carious dentine treated with 38% SDF | Silver particles were found on the dentine surface after treatment with SDF. Compared with the control, the SDF-treated dentine had fewer CFUs of bacteria (P < 0.01) and more dead bacteria (P < 0.05) |
| Mei et al., 2013 (English)5 | SEM, CFU and CLSM were used to study a multispecies biofilm (S. mutans, S. sobrinus, L. acidophilus, L. rhamnosus and A. naeslundii) on carious dentine treated with 38% SDF | Silver particles were found on the dentine surface after treatment with SDF. Compared with the control, the SDF-treated dentine had lower CFU counts of bacteria (P < 0.01) and more dead bacteria (P < 0.01) |
AgNO3, silver nitrate; Ag(NH3)2NO3, silver ammonium nitrate; CFU, colony-forming unit; CHX, chlorhexidine; CLSM, confocal laser scanning microscopy; NaF, sodium fluoride; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; MID, maximum inhibitory dilution; SDF, silver diamine fluoride; SEM, scanning electron microscopy.
Table 2.
Summary of publications studying the effects of silver diamine fluoride (SDF) on the mineral content of enamel
| Authors, Year (Language) | Methods | Main findings |
|---|---|---|
| Suzuki et al., 1974 (English)37 | (1) Human enamel blocks treated with 38% SDF were immersed in artificial saliva for 1 week before EPMA. (2) Enamel powder treated with 38% SDF was immersed in artificial saliva containing thiocyanate for 20 weeks before XRD |
(1) Fluoride and silver were detected within 20 μm and 10 μm from the enamel surface, respectively. (2) CaF2 was formed but gradually disappeared within 10 weeks. Ag3PO4 was formed but disappeared after 1 week. AgSCN was retained for up to 20 weeks |
| Li et al., 1984 (Chinese)23 | Demineralised enamel blocks with internal control, treated with 38% SDF, were immersed in lactic acid for 2 days before MCR and MHT | SDF-treated blocks had less lesion depth and increased microhardness (P < 0.05) compared with the negative control. SDF stained demineralized, but not sound, enamel black |
| Klein et al., 1999 (English)10 | Demineralised enamel blocks treated with 38% SDF were subjected to challenge with cariogenic biofilm for 2, 4 and 6 weeks before PLM | SDF-treated enamel blocks had less lesion depth compared with control blocks up to 4 weeks of biofilm challenge |
| Li et al., 2001 (Chinese)32 | Demineralised enamel blocks were treated with SDF three times per week for 4 weeks before MHT | SDF-treated enamel blocks had increased microhardness compared with the control |
| Wu et al., 2002 (Chinese)34 | Demineralised enamel blocks, treated and untreated (control) with 38% SDF, were immersed in remineralising solution for 4 days before AAS | SDF-treated blocks took up more calcium than did control blocks from the remineralising solution (P < 0.001) |
| Wu et al., 2002 (Chinese)35 | Demineralised enamel blocks treated with 38% SDF were immersed in demineralising solution for 6 days before AAS | SDF-treated blocks released less calcium into the demineralising solution than did control blocks (P < 0.01) |
| Wang et al., 2005 (Chinese)40 | Demineralised enamel blocks treated with 38% SDF were subjected to pH cycling for 10 days before SEM | Precipitates were formed on SDF-treated surfaces but not on water-treated surfaces |
| Rosas et al., 2014 (Spanish)13 | Demineralised enamel blocks treated with 38% SDF were subjected to pH cycling for 5, 10 and 15 days before PLM | SDF-treated enamel blocks had less mineral loss than the control after 5 and 10 days (P < 0.05) |
| Lou et al., 2011 (English)36 | Hydroxyapatite powder and 10% gelatin were treated with 38% SDF. Treated materials were studied with SEM/TEM, EDX and ED before and after washing with water | CaF2 was formed when SDF reacted with hydroxyapatite powder but disappeared after washing. Metallic silver was produced when SDF reacted with gelatin |
AAS, atomic absorption spectrometry; AgSCN, silver thiocyanate; Ag3PO4, silver phosphate; CaF2, calcium fluoride; ED, electron diffraction; EDX, energy-dispersive X-ray analysis; EPMA, electron probe microanalysis; MCR, micro-contact radiography; MHT, micro-hardness testing; PLM, polarized light microscopy; SEM, scanning electron microsopy; TEM, transmission electron microscopy; XRD, X-ray diffraction.
Table 3.
Summary of publications studying the effects of silver diamine fluoride (SDF) on the mineral content of dentine
| Authors, Year (Language) | Methods | Main findings |
|---|---|---|
| Li et al., 1997 (Chinese)38 | Human dentine powder was immersed in 38% SDF solution. The product after reaction was analysed by XRD | CaF2 and Ag3PO4 were formed |
| Yang et al., 2004 (Chinese)30 | Demineralised human root surfaces treated with 38% SDF were subjected to challenge with cariogenic biofilm for 2 days before MCR | SDF-treated root surfaces had less lesion depth (P < 0.05) and mineral loss than control |
| Yao et al., 2006 (Chinese)31 | Demineralised human root surfaces treated with 38% SDF were immersed in remineralising solution for 7 days before SEM and MCR | Precipitates were formed on SDF-treated surfaces but not on water-treated surfaces.SDF-treated surfaces had less lesion depth (P < 0.05) and mineral loss (P < 0.05) than the control |
| Chu et al., 2008 (English)33 | Primary teeth with arrested dentine caries treated with 38% SDF were extracted and underwent KHN measurements | Within the outer 25–200 μm, the median KHN of arrested carious lesions were greater (no statistics presented) than those of soft carious lesions |
| Knight et al., 2009 (English)12 | Demineralised human dentine disks treated with 29% (1.8 mol/l) SDF were subjected to cariogenic biofilm challenge for 2 weeks before SEM and EPMA | SDF-treated dentine had less calcium (P < 0.05) and phosphorus (P < 0.05) loss and more fluoride uptake than the control |
| Guo et al., 2011 (Chinese)41 | Demineralised human root surfaces treated with 38% SDF were subjected to cariogenic biofilm challenge for 6 days before SEM. The calcium concentration was evaluated at day 2, 4 and 6 by AAS | SDF-treated root surfaces had less calcium release than control (P < 0.05) and precipitates were formed |
| Chu et al., 2012 (English)7 | Demineralised human dentine blocks treated with 38% SDF were subjected to cariogenic biofilm challenge for 7 days before MHT, EDX and FTIR | SDF-treated dentine blocks had increased microhardness and calcium/phosphate weight-percentage than the control (P < 0.05); the ratio of amide I to hydrogen phosphate was reduced (P < 0.05) |
| Mei et al., 2013 (English)6 | Demineralised human dentine blocks treated with 38% SDF were subjected to challenge with cariogenic biofilm for 7 days before XRD and FTIR | SDF-treated dentine blocks had reduced mineral loss and reduced ratio of amide I to hydrogen phosphate (P < 0.05) |
| Mei et al., 2013 (English)5 | Demineralised human dentine blocks treated with 38% SDF were incubated in artificial mouth for 21 days before MHT, EDX and FTIR | SDF-treated dentine blocks had increased microhardness and calcium/phosphate weight percentage (P < 0.05); the ratio of amide I to hydrogen phosphate was reduced (P < 0.01) |
| Mei et al., 2013 (English)3 | Demineralised human dentine blocks treated with 38% SDF were subjected to pH cycling for 8 days before SEM, micro-CT and XRD | SDF-treated dentine blocks had reduced lesion depth (P < 0.01). Silver chloride and metallic silver were formed |
| Mei et al., 2014 (English)39 | Primary teeth with arrested dentine caries, treated with 38% SDF, were extracted and underwent assessments of micro-CT, EDX, SEM and TEM | A highly remineralised surface zone (about 150 μm), rich in calcium and phosphate, was found on the arrested dentinal lesion. Collagens were protected and not exposed as a result of SDF treatment |
AAS, atomic absorption spectrometry; Ag3PO4, silver phosphate; CaF2, calcium fluoride; EDX, energy-dispersive X-ray analysis; EPMA, electron probe microanalysis; FTIR, Fourier transform infrared spectroscopy; KHN, Knoop hardness number; MCR, micro-contact radiography; micro-CT, micro-computed tomography; MHT, micro-hardness testing; SEM, scanning electron microsopy; TEM, transmission electron microscopy; XRD, X-ray diffraction.
Table 4.
Summary of publications studying the effects of silver diamine fluoride (SDF) on the organic content of dentine
| Authors, Year (Language) | Methods | Main findings |
|---|---|---|
| Mei et al., 2012 (English)42 | Fluorescent MMP kits (for MMP-2, MMP-8 and MMP-9) were used to study the inhibition of collagen degradation by AgNO3, NaF and 12%, 30% and 38% SDF | Collagen was degraded less by MMPs in the presence of SDF than in the presence of AgNO3 or NaF (P < 0.001). Collagen was degraded less in the presence of 38% SDF than in the presence of 30% SDF and 12% SDF |
| Mei et al., 2013 (English)6 | Human demineralised dentine blocks treated with 38% SDF were subjected to challenge with cariogenic biofilm. An immunolabelling method was used to detect intact collagen I in dentine | SDF-treated dentine blocks had more intact collagen I than did the control (P < 0.05) |
| Mei et al., 2013 (English)3 | Human demineralised dentine blocks treated with 38% SDF were subjected to pH cycling. The hydroxyproline assay was used to assess the amount of degraded collagen | SDF-treated dentine blocks had less collagen degradation (P < 0.01) |
| Mei et al., 2014 (English)43 | Fluorescent cathepsin kits (cysteine cathepsin B and cysteine cathepsin K) were used to study the inhibition of collagen degradation by AgNO3, NaF and 12%, 30% and 38% SDF | Collagen degradation by cysteine cathepsins was lower in the presence of SDF than in the presence of AgNO3 or NaF (P < 0.001). SDF at different concentrations had no significant difference on inhibiting proteolytic activity of cysteine cathepsins |
AgNO3, silver nitrate; MMP, matrix metalloproteinases; NaF, sodium fluoride; SDF, silver diamine fluoride.
Actions of SDF on cariogenic bacteria
Dentine surfaces treated with SDF had significantly less growth of Streptococcus mutans than did those without SDF treatment11. Colony-forming unit (CFU) counts of monospecies strains of S. mutans and Actinomyces naeslundii were reduced after application of SDF, with very few bacteria found to be alive7. CFU counts of dual-species biofilms containing the cariogenic bacteria S. mutans and Lactobacillus acidophilus were significantly lower on demineralised dentine treated with SDF than when treated with water; the dead-to-live ratios of the bacteria were significantly higher after SDF application than after water application6. A further study used multispecies cariogenic biofilms consisting of S. mutans, Streptococcus sobrinus, L. acidophilus, Lactobacillus rhamnosus and A. naeslundii, with the results showing that CFU counts were reduced with SDF treatment5. The growth of S. mutans, Streptococcus oralis and Lactobacillus casei was reduced after treatment with SDF23., 24., 25., 26.. SDF also inhibited the adherence of S. mutans to tooth surfaces27., 28.. The minimum inhibitory concentration and minimum bactericidal concentration of SDF for S. mutans were 33.3 μg/ml and 50.0 μg/ml, respectively29, showing that SDF was more effective than silver ammonium nitrate and sodium fluoride23., 27..
Effects of SDF on mineral content of enamel and dentine
Demineralised tooth surfaces became black after application of SDF23. The lesion depth of a demineralised tooth surface decreased after the application of SDF3., 23., 30., 31. and it was also effective in slowing down the progression of lesions10. Carious lesions treated with SDF had significantly higher surface microhardness, to a depth of approximately 150 μm, compared with the control lesions treated with deionised water5., 7., 23., 32., 33.. The concentration of calcium in the remineralisation solution was found to be reduced23., 34., which indicates that SDF promotes absorption of calcium. In addition, the concentration of calcium in the demineralisation solution was also decreased35, which shows that SDF inhibited calcium dissolution from enamel. Using a polarised light technique with photo-microscopy, demineralised enamel surfaces treated with SDF had significantly lower mineral loss than did those without SDF treatment13.
A study reported that silver chloride and metallic silver were formed after application of SDF3. In addition, SDF appeared to produce calcium fluoride and metallic silver when reacted with hydroxyapatite36. Other studies discovered calcium fluoride and silver phosphate when enamel powder or dentine powder were mixed with SDF37., 38.. Elemental analysis revealed that the weight percentages of calcium and phosphorus in demineralised dentine treated with SDF were significantly higher than those of calcium and phosphorus in demineralised dentine without SDF treatment (control group)5., 7.. Moreover, demineralised dentine treated with SDF had less mineral loss than did demineralised dentine with no SDF treatment5., 7.. The levels of calcium and phosphorus increased from the surface to a depth of 300 μm12. There was also a significantly higher uptake of fluoride in the SDF-treated dentine samples than in the water-treated dentine samples12. An ex vivo study showed that a highly remineralised zone abundant in calcium and phosphate was detected on arrested dentine carious lesions treated with SDF39. Studies using scanning electron microscopy observed dense precipitates covering tooth surfaces after application of SDF40., 41.. However, the investigators did not mention the content of these precipitates. Cross-section scanning electron micrographs revealed that dense granular structures of spherical grains were found in the intertubular area of dentine after treatment with SDF 3. X-ray diffraction found reduced loss of dentine crystallinity resulting from the dissolution of hydroxyapatite in dentine treated with SDF6.
Effects of SDF on the organic content of dentine
A study using immunolabeling revealed that a larger amount of intact collagen remained on the dentine surface after treatment with SDF than after treatment with water (i.e. the control)6. Dentine treated with SDF showed significantly less liberation of hydroxyproline as a result of collagen degradation than did dentine treated with water3. SDF had an inhibitory effect on matrix metalloproteinases (MMPs), which play an important role in the enzymatic degradation of collagen, by inhibiting the proteolytic activities of MMP-2, MMP-8 and MMP-942. The activities of cysteine cathepsins (or cathepsins), which are proteolytic enzymes that contribute to dentine collagen degradation, were also inhibited by SDF43.
Discussion
Rosenblatt et al.17 performed a literature review of publications on SDF in three languages: English, Portuguese and Spanish. SDF has also been widely used in China and Japan for several decades9., 23.. Thus, a number of research publications on SDF, written in Chinese and Japanese, may have been included in the literature search, making the search comprehensive and providing a wider evidence base. The most common concentration of SDF used for caries management was 38%, but concentrations of SDF of 30% and 12% were also used. In this review, most laboratory studies used 38% SDF for their experiments; however, some older studies did not mention the concentrations of SDF used. A systematic review on clinical studies showed that effectiveness of SDF in caries arrest would be enhanced by increasing the concentration from 12% to 38%, and by increasing the frequency of application from annual to semi-annual21. The findings of the selected laboratory studies generally concur that an SDF concentration of 38% is more effective at inhibiting collagenase activity and preventing collagen degradation than low concentrations42., 43.. As the duration of the selected laboratory studies was relatively short, the long-term caries-arresting effect and the periodicity of the SDF application could not be evaluated. A time limitation was not set for the literature search; there were studies published as early as the 1970s26., 27., 37.. Some of these early laboratory studies did not present their results in a contemporary format. The methodology and outcome assessment varied between studies, making quantitative analysis difficult. Last, but not least, this review summarised the relevant findings of the studies in peer-reviewed publications. It is not the objective of this review to judge the quality of the study or to discuss the limitations of each study. This should be taken into consideration when interpreting the results and in the conclusions of this review.
After analysing the results of the studies, it was found that the possible mode of action of SDF can be related to its antibacterial properties on cariogenic bacteria, its remineralisation effect on the inorganic content of the tooth and its inhibitory effect on the degradation of the organic matrix.
According to this review, it was found that SDF possessed antimicrobial action against cariogenic monospecies strains of S. mutans or A. naeslundii7, dual-species cariogenic biofilms of S. mutans and L. acidophilus6 and multispecies cariogenic biofilms formed on dentine surfaces5. In the caries process, bacterial invasion initially involves Streptococci, Actinomycetes and Lactobacilli. Streptococcus mutans is one of the most important pathogens associated with the initiation and progression of caries. Lactobacillus acidophilus and L. rhamnosus are the most abundant species of bacteria, routinely found in both deep and superficial carious lesions. Actinomyces naeslundii is linked to root caries that has the potential to invade dentinal tubules. It is suggested that the concentrations of antibacterial agents required for inhibiting biofilms are more than 100 times higher than those required for inhibiting planktonic bacteria because biofilms are more resistant to antimicrobial agents than are planktonic bacteria16. Both fluoride and silver ions contained in SDF appear to have the ability to inhibit the formation of cariogenic biofilms5. High-concentration fluorides can inhibit biofilm formation because fluorides can bind to bacterial cellular components and influence enzymes that are related to carbohydrate metabolism as well as to sugar uptake5. Ionised silver can either kill cariogenic microorganisms or interfere with their metabolic processes, depending on its concentration. It has been suggested that silver ions at a concentration of 20 p.p.m. can inhibit the growth of S. mutans12. A review concluded that silver ions have three antimicrobial effects16: first, silver ions can destroy the cell-wall structure of bacteria; second, they can inhibit enzyme activities and influence metabolic processes; and, third, they can inhibit the replication of bacterial DNA.
SDF at a concentration of 38% contains 44,800 p.p.m. fluoride. Its fluoride concentration is the highest among the fluoride agents available for dental use. Fluoride promotes the remineralisation of hydroxyapatite in enamel and dentine. One proposed chemical reaction between SDF and hydroxyapatite of teeth involves the formation of silver phosphate and calcium fluoride3., 38.. The subsequent dissolution of fluoride and calcium facilitates the formation of insoluble fluorapatite, which is a possible reaction product of fluoride ions with hydroxyapatite. It is difficult to confirm the formation of fluorapatite, primarily because its crystal structure is similar to that of hydroxyapatite. Calcium fluoride is less acid resistant than fluorapatite. The calcium fluoride formed after application of SDF is considered to be a pH-regulated slow-release reservoir of fluoride on the tooth surface. Nevertheless, calcium fluoride could be removed easily from a tooth surface by toothbrushing or mastication37. The solubility of silver phosphate (6.4 × 10−3 g/100 ml) is higher than that of silver chloride (8.9 × 10−4 g/100 ml). Therefore, silver phosphate could react with alkali chlorides in remineralisation solutions to form silver chloride. This could explain why silver chloride was detected as the principal precipitate on the tooth surface after SDF treatment. The reaction between SDF and hydroxyapatite also led to the formation of nanoscopic metallic silver particles attached to hydroxyapatite crystals3, while the production of metallic silver was accelerated by exposure to light and high temperature36. Silver nanoparticles have shown a great inhibitory effect on the growth of cariogenic bacteria, which might be an important reason why SDF can arrest caries even without removal of carious tooth structure39. The changes in the microhardness of a tooth are directly linked to its mineral content33. Laboratory studies reported that the microhardness of demineralised enamel and dentine increased after treatment with SDF7., 23.. Laboratory studies suggested that virtually insoluble or less soluble silver chloride and silver phosphate were detected on the dentine surface when treated with SDF3., 38.. An insoluble protective layer was formed, according to these precipitates, to decrease calcium and phosphorous loss from demineralised enamel and dentine. These laboratory studies simulated some factors of clinical conditions by using different in vitro models, while ex vivo studies are more appropriate for representing the complicated oral environment. An ex vivo study found that the microhardness of the outermost dentine surface of an arrested carious lesion was increased after treatment with SDF33. Another ex vivo study reported that a dense and highly remineralised surface layer, rich in calcium and phosphate, was found after application of SDF39, which could directly reflect the clinical situation. This may explain why the surface of the arrested lesion could be hardened with SDF treatment.
The pathogenesis of caries in dentine and enamel are different. By weight, dentine contains approximately 10% fluid, 20% organic matrix and 70% mineral44. Thus, the process of dentine caries cannot be merely explained by mineral loss as a result of attack by bacterial acid. Dentine type I collagen accounts for approximately 90% of the organic component in dentine, and the residual part is composed of noncollagenous proteins. Type I collagen can act as a scaffold for the deposition of mineral crystals, and the organic dentine matrix might inhibit the diffusion of calcium and phosphate for further demineralisation44. In this review, a study showed that SDF can preserve collagen from degradation in demineralised dentine3. In the past, the dentine organic matrix was considered to be destroyed mainly as a result of the action of bacterial collagenases, while recent studies have suggested that collagen can be degraded by MMPs. MMPs are present in saliva and the dentine matrix45. They can be activated by the low pH in carious dentine42. MMP-8 (neutrophil collagenases) cleaves interstitial collagen types I, II and III. It is capable of digesting other extracellular matrix and non-extracellular matrix molecules. MMP-2 (gelatinase A) and MMP-9 (gelatinase B) not only degrade the denatured collagen molecules (gelatin) and type IV collagen, but also other proteins to a lesser degree. Cysteine cathepsins are proteolytic enzymes that contribute to degradation of dentine collagen by breaking down type I collagen and proteoglycans43. Cathepsins can be identified from the degradation of extracellular matrix components and are considered to be associated with MMP activities in teeth. Cathepsin-B cleaves in the non-helical telopeptide extensions of collagens, and Cathepsin-K can catabolise collagen and degrade dentine42., 43.. Therefore, the inhibition of MMP and cysteine cathepsin activities may prevent collagen degradation and contribute to the arrest of the caries process. It is suggested that silver ions may contribute more than fluoride to the inhibitory effect of SDF on cysteine cathepsins43.
Studies have found that removal of caries is not necessary before application of SDF. This suggests that dentists do not need to remove caries from patients’ teeth during treatment with SDF. SDF is a non-invasive, simple and low-cost approach to arresting dental caries. However, the main disadvantage of its use is discoloration of carious teeth23, which can cause patient dissatisfaction. Some researchers have proposed using potassium iodide after topical application of SDF to reduce the staining effect by generating silver iodide11. However, this white product, silver iodide, is considered to be photosensitive and can turn dark with exposure to light. Ammonium hexafluorosilicate has been suggested to exclude silver and its staining effect, but it is less effective than SDF in arresting caries16. A recent study has used nano-silver fluoride, which was found to be effective in arresting dentine caries and did not result in black staining of the carious lesions46. This new agent is shown to have low toxicity to living cells and has antibiotic efficacy similar to that of SDF against S. mutans29. Further research is necessary to find an approach to solve the staining problem of SDF without reducing its effectiveness in arresting dental caries.
Conclusion
This literature review concludes that SDF reduces the growth of cariogenic bacteria. The silver ion is bactericidal. SDF can also remineralise both enamel and dentine caries. The possible mode of action of SDF for arresting caries may be attributed to its inhibition of mineral demineralisation, promotion of mineral remineralisation and protection of the collagen matrix from degradation.
Acknowledgements
The authors thank Dr Leticia Ito for her assistance in the literature search. This study was supported by the Research Grant Council General Research Fund (no. 760413M).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Margolis H, Moreno E. Composition and cariogenic potential of dental plaque fluid. Crit Rev Oral Biol Med. 1994;5:1–25. doi: 10.1177/10454411940050010101. [DOI] [PubMed] [Google Scholar]
- 3.Mei ML, Ito L, Cao Y, et al. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent. 2013;41:809–817. doi: 10.1016/j.jdent.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Calif Dent Assoc. 2016;44:16–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Mei ML, Li QL, Chu CH, et al. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4. doi: 10.1186/1476-0711-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei ML, Chu CH, Low KH, et al. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18:824–831. doi: 10.4317/medoral.18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CH, Mei L, Seneviratne CJ, et al. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22:2–10. doi: 10.1111/j.1365-263X.2011.01149.x. [DOI] [PubMed] [Google Scholar]
- 8.Chu CH, Lo ECM, Lin H. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 9.Nishino M, Ono S, Kita Y, et al. Caries prevention in pits and fissures with diammine silver fluoride solution and fissure sealant. Sealing properties of pits and fissures and adhesive characteristics to enamel. J Osaka Univ Dent Sch. 1974;14:1–7. [PubMed] [Google Scholar]
- 10.Klein U, Kanellis M, Drake D. Effects of four anticaries agents on lesion depth progression in an in vitro caries model. Pediatr Dent. 1999;21:176–180. [PubMed] [Google Scholar]
- 11.Knight G, McIntyre J, Craig G, et al. An in vitro model to measure the effect of a silver fluoride and potassium iodide treatment on the permeability of demineralized dentine to Streptococcus mutans. Aust Dent J. 2005;50:242–245. doi: 10.1111/j.1834-7819.2005.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Knight GM, McIntyre JM, Craig GG, et al. Inability to form a biofilm of Streptococcus mutans on silver fluoride-and potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40:155–161. [PubMed] [Google Scholar]
- 13.Rosas SGP, Téllez MÁA, EspinozaII EV. In vitro efficiency of fluoride-containing compounds on remineralization of carious enamel lesions under cyclic pH conditions. Revista Odontológica Mexicana. 2014;18:96–104. [Google Scholar]
- 14.Chu CH, Lo ECM. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6:315–321. [PubMed] [Google Scholar]
- 15.Mei ML, Lo ECM, Chu CH. Clinical use of silver diamine fluoride in dental treatment. Compend Contin Educ Dent. 2016;37:93–98. [PubMed] [Google Scholar]
- 16.Peng JY, Botelho M, Matinlinna J. Silver compounds used in dentistry for caries management: a review. J Dent. 2012;40:531–541. doi: 10.1016/j.jdent.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblatt A, Stamford T, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res. 2009;88:116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 18.Yamaga R, Yokomizo I. Arrestment of caries of deciduous teeth with diamine silver fluoride. Dent Outlook. 1969;33:1007–1013. [Google Scholar]
- 19.Tan H, Lo E, Dyson J, et al. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89:1086–1090. doi: 10.1177/0022034510375825. [DOI] [PubMed] [Google Scholar]
- 20.Llodra J, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84:721–724. doi: 10.1177/154405910508400807. [DOI] [PubMed] [Google Scholar]
- 21.Gao SS, Zhao IS, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Trans Res. 2016;1:201–210. doi: 10.1177/2380084416661474. [DOI] [PubMed] [Google Scholar]
- 22.Zhi Q, Lo E, Kwok A. An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J. 2013;58:50–56. doi: 10.1111/adj.12033. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Qiu X, Li J, et al. Effect of a silver ammonia fluoride solution on the prevention and inhibition of caries. Zhonghua Kou Qiang Ke Za Zhi. 1984;19:97–100. [PubMed] [Google Scholar]
- 24.Alves S, Maria T, Silva CA, et al. Atividade Antimicrobiana de Produtos Fluoretados sobre Bactérias Formadoras do biofilme Dentário: Estudo in vitro. Pesqui Bras Odontopediatria Clin Integr. 2010;10:209–216. [Google Scholar]
- 25.de Almeida LF, Cavalcanti YW, Valenca A. In vitro antibacterial activity of silver diamine fluoride in different concentrations. Acta Odontol Latinoam. 2011;24:127–131. [PubMed] [Google Scholar]
- 26.Igarashi S. Antibacterial Effect of Ag (NH3)2F solution on Streptococcus mutans in plaque. Jpn J Paediatr Dent. 1978;16:1–18. [Google Scholar]
- 27.Suzuki T, Tsutsum N, Sobue S, et al. Effect of diammine silver fluoride on plaque formation by Streptococcus mutans 2. Mode of action of plaque inhibition. Jpn J Oral Biol. 1976;18:268–278. [Google Scholar]
- 28.Tsutsumi N. Studies on topical application of Ag(NH3)2F for the control of interproximal caries in human primary molars 2. effect of Ag(NH3)2F on Streptococcus mutans on interproximal plaque. Jpn J Paediatr Dent. 1981;19:523–536. [Google Scholar]
- 29.Targino AGR, Flores MAP, dos Santos Junior VE, et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25:2041–2047. doi: 10.1007/s10856-014-5221-5. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Li Y, Ge L. Effect of ammonium oxofluoromolybdate solution on the inhibition of artificial caries on root surface in vitro. J Pract Stomatol. 2004;20:39–42. [Google Scholar]
- 31.Yao N, Tang R. Study of the effect of Chinese nutgall water extract on experimental root caries remineralization. Chin J Conserv Dent. 2006;16:285–287. [Google Scholar]
- 32.Li L, Shi S. Comparison of the remineralizations of three preparations on deciduous enamel. Shanghai Med J. 2001;24:29–31. [Google Scholar]
- 33.Chu CH, Lo ECM. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36:387–391. doi: 10.1016/j.jdent.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Yang F. The effects of three fluoride-containing agents on the remineralization of deciduous teeth in vitro. J Pract Stomatol. 2002;18:347–349. [Google Scholar]
- 35.Wu L, Yang F. Comparison of the effects of three fluoride-containing agents on the demineralization of deciduous teeth in vitro. J Modern Stomatol. 2002;16:216–218. [Google Scholar]
- 36.Lou Y, Botelho M, Darvell B. Reaction of silver diamine fluoride with hydroxyapatite and protein. J Dent. 2011;39:612–618. doi: 10.1016/j.jdent.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki T, Nishida M, Sobue S, et al. Effects of diammine silver fluoride on tooth enamel. J Osaka Univ Dent Sch. 1974;14:61–72. [PubMed] [Google Scholar]
- 38.Li Y, Li J. Study of mechanism of a silver ammonia fluoride solution on inhibition of dentine caries. Beijng J Stomatol. 1997;5:151–152. [Google Scholar]
- 39.Mei ML, Ito L, Cao Y, et al. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent. 2014;42:395–402. doi: 10.1016/j.jdent.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Tang R. Scanning electron microscopy observations of surface morphology of initial artificial lesions of enamel treated with Chinese nutgall. Chin J Conserv Dent. 2005;15:133–135. [Google Scholar]
- 41.Guo J, Zhao M, Qi F, et al. The study of tea polyphenol on the inhibition of artificial caries on root surface in vitro. J Hebei Med Univ. 2011;32:175–178. [Google Scholar]
- 42.Mei ML, Li Q, Chu CH, et al. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater. 2012;28:903–908. doi: 10.1016/j.dental.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Mei ML, Ito L, Cao Y, et al. The inhibitory effects of silver diamine fluorides on cysteine cathepsins. J Dent. 2014;42:329–335. doi: 10.1016/j.jdent.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Hiraishi N, Sono R, Islam M, et al. Effect of hesperidin in vitro on root dentine collagen and demineralization. J Dent. 2011;39:391–396. doi: 10.1016/j.jdent.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Chaussain-Miller C, Fioretti F, Goldberg M, et al. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 46.dos Santos VE, Vasconcelos Filho A, Targino AGR, et al. A new “Silver-Bullet” to treat caries in children–Nano Silver Fluoride: a randomised clinical trial. J Dent. 2014;42:945–951. doi: 10.1016/j.jdent.2014.05.017. [DOI] [PubMed] [Google Scholar]