Abstract
Objectives
To determine the diagnostic yield of screening patients for SARS-CoV-2 who were admitted with a diagnosis unrelated to COVID-19 and to identify risk factors for positive tests.
Design
Cohort from the Canadian COVID-19 Emergency Department Rapid Response Network registry.
Setting
30 acute care hospitals across Canada.
Participants
Patients hospitalised for non-COVID-19-related diagnoses who were tested for SARS-CoV-2 between 1 March and 29 December 2020.
Main outcome
Positive nucleic acid amplification test for SARS-CoV-2.
Outcome measure
Diagnostic yield.
Results
We enrolled 15 690 consecutive eligible adults who were admitted to hospital without clinically suspected COVID-19. Among these patients, 122 tested positive for COVID-19, resulting in a diagnostic yield of 0.8% (95% CI 0.64% to 0.92%). Factors associated with a positive test included presence of fever, being a healthcare worker, having a positive household contact or institutional exposure, and living in an area with higher 7-day average incident COVID-19 cases.
Conclusions
Universal screening of hospitalised patients for COVID-19 across two pandemic waves had a low diagnostic yield and should be informed by individual-level risk assessment in addition to regional COVID-19 prevalence.
Trial registration number
Keywords: COVID-19, EPIDEMIOLOGY, Organisation of health services, Diagnostic microbiology
Strengths and limitations of this study.
This is a pan-Canadian study including over 30 academic, non-academic, rural and urban hospitals.
The study included patient partners and the public, who assisted in the development and presentation of the manuscript.
The study included pertinent clinical variables, as well as relevant demographic and community-level variables.
The study excluded non-nucleic acid amplification tests due to their infrequent use in the Canadian context.
Introduction
Healthcare institutions initiated widespread testing of admitted patients for COVID-19 in the spring of 2020.1 Patients without any reported symptoms of COVID-19 were routinely tested even in jurisdictions where COVID-19 rates were low in order to identify asymptomatic carriers and prevent hospital outbreaks.2 Some jurisdictions have continued this practice without robust evidence to support it. An Alberta study of 3375 patients admitted to hospital for alternate diagnoses during the first wave of the pandemic when COVID-19 prevalence was very low found that none of the patients tested positive.3 In contrast, other studies from times and regions with higher COVID-19 prevalence reported positive tests in between 2.6% and 15.5% of otherwise asymptomatic patients.4–8
Universal testing has several potential downsides if diagnostic yield is low. First, it may worsen emergency department (ED) crowding, as admitted patients with pending COVID-19 tests are boarded in EDs until their test results are reported. While ED volumes were lower than usual in the early pandemic such that EDs could absorb this delay, high patient volumes have since returned, exacerbating the impact of this practice on hospital crowding.9 This in turn increases patient morbidity and mortality.10 In addition, diagnostic work-ups and therapeutic interventions may be delayed until COVID-19 test results are back, as it takes longer to move patients on isolation precautions through the system. This can further exacerbate patient outcomes and hospital crowding. Third, diagnostic testing capacity may be limited, potentially delaying processing of tests for symptomatic patients. In addition, the use of personal protective equipment (PPE) may be increased as institutions have placed patients with pending COVID-19 tests under isolation precautions. The use of PPE during resuscitation has been associated with worse patient outcomes.11 Lastly, there is also an opportunity cost for hospitals as money spent on universal testing could be allocated to other areas. These unintended consequences of liberal testing policies need to be weighed carefully against the anticipated diagnostic yield and potential benefits.
As for any diagnostic test, rational COVID-19 testing guidelines should be informed by the level of risk of the patient, such that testing is reduced in situations when risk is low and more widespread when risk is high. Based on expert opinion, the Infectious Diseases Society of America (IDSA) recommended a testing strategy based on the prevalence of the disease in the community.12 They recommended universal testing of asymptomatic hospitalised patients in times and places of high disease prevalence, defined as ≥10% or >10 000 active cases per 100 000 population, and did not recommend universal testing in times and places of low prevalence, defined as under 2% prevalence of disease or less than 2000 active cases per 100 000. Most jurisdictions never met the proposed screening threshold as public health measures were enacted to reduce disease prevalence to avoid overwhelming hospital capacity. The IDSA was unable to provide further guidance due to lack of available evidence. Our aim was to determine the diagnostic yield of screening patients for SARS-CoV-2 who had been admitted with a diagnosis unrelated to COVID-19 and identify risk factors for positive tests.
Methods
Study design and setting
The Canadian COVID-19 Emergency Department Rapid Response Network (CCEDRRN, pronounced ‘sedrin’) is a pan-Canadian population-based registry that has enrolled consecutive eligible patients presenting with suspected or confirmed COVID-19 from EDs across Canada starting on 1 March 2020. The study population, data collection, data quality assurance, management and governance structure are described in the network’s methods paper.13 Thirty CCEDRRN sites in seven provinces contributed data to this study (online supplemental appendix A). Data are available on reasonable request and can be shared after approval by the Executive Committee through a process outlined on our website (https://www.ccedrrn.com/).
bmjopen-2021-057852supp001.pdf (110.6KB, pdf)
Patient and public involvement
CCEDRRN has an active patient engagement committee with patient partners who have lived experience with COVID-19 from geographically representative areas of Canada. Patient partners provided input into the development of this research question and study protocol and the final manuscript.
Study patients
Participating sites needed to demonstrate ≥99% compliance in enrolling consecutive eligible patients for their data to be included in this study. Data from sites and periods that did not meet this quality threshold were excluded. We included consecutive eligible patients who were admitted to hospital and swabbed for SARS-CoV-2 using a nucleic acid amplification test (NAAT) within 24 hours of ED arrival. We enrolled patients between 1 March 2020 and 29 December 2020. To identify a population of admitted patients in whom COVID-19 was not suspected, we excluded patients with ED diagnoses that would have been clinically suspicious for COVID-19. These included all patients with ED diagnoses of suspected or confirmed COVID-19, influenza-like illness, upper respiratory infections, and pneumonia or viral pneumonia for which testing would have been indicated based on clinical suspicion. We excluded patients who were discharged directly from the ED, diagnosed with COVID-19 before ED arrival (based on a NAAT done in the community), those whose first swab occurred more than 24 hours after their arrival and repeat admissions. We also excluded patients in whom initial SARS-CoV-2 testing was negative and repeat testing became positive more than 5 days after arrival as these patients could have contracted nosocomial COVID-19.
Data collection
Trained research assistants collected data retrospectively from electronic and/or paper-based medical records into a central, web-based REDCap database (Vanderbilt University; Nashville, Tennessee, USA). Research assistants captured the demographics, infection risk, ED vital signs, presenting symptoms, comorbid conditions and results of COVID-19 tests. The coordinating centre implemented regular data quality checks, including logic checks in REDCap, as well as site-level record verifications for non-sensical or outlying values.
In addition to these clinical variables, we calculated the 7-day moving average incident COVID-19 case count for the health region of each participating site using publicly available epidemiological data.14 For each calendar day within each health region represented in the study, we calculated the average daily incident rate of new infections per 100 000 population over the preceding 7 days. This 7-day moving average incidence was assigned to each patient based on the date of their index ED encounter and the health region of their postal code of residence. We allocated patients with no fixed address to the health region of the hospital in which they were tested. We imputed values for the first 5 weeks of the pandemic by modelling the reported COVID-19 cases that had accumulated in every health region over time using linear interpolation (0.1% missing); COVID-19 case data early in the pandemic were not publicly available. The 7-day moving average incident COVID-19 case count was categorised as 0–1.99 per 100 000 population, 2–7.99 per 100 000 population and ≥8 per 100 000 population based on the relationship between incidence and COVID-19-positive results in a previous analysis.15
Outcome
The primary outcome was a positive NAAT for SARS-CoV-2 in patients admitted with non-COVID-19-related diagnoses.
Data analysis
We divided the cohort into two groups: those without symptoms of COVID-19 and those with symptoms compatible with COVID-19 that were attributed to an alternate diagnosis (ie, Congestive Heart Failure (CHF) and Chronic Obstructive Pulmonary Disease (COPD) etc). We considered cough, dyspnoea, fever, general weakness, chest pain, diarrhoea, nausea and vomiting, headache, chills, myalgia, sore throat, altered level of consciousness, and dysgeusia/anosmia to be COVID-19-compatible symptoms. We used descriptive statistics to describe the population. We calculated the diagnostic yield by dividing the number of positive NAATs over the total number of NAATs performed. We calculated the 7-day average of NAAT positivity over the study period by dividing the number of positive NAATs over all tests performed and averaging over a 7-day period. We calculated the exact binomial proportion 95% CI for all proportions and used the modified Clopper-Pearson interval for small samples. We completed a planned subgroup analysis for patients presenting with and without COVID-19-compatible symptoms to determine the associated factors for a positive test. The initial multivariable logistic regression model to identify factors associated with a positive NAAT considered candidate variables with a p value cut-off point of 0.20 based on the Wald test from univariable analyses. From the full model, a step-down procedure reduced the model to key predictors based on Akaike’s information criterion (AIC) scores (eg, chose the model with the smallest AIC score). Candidate variables included 7-day moving average incident COVID-19 case count category, patient age, gender, infection risk and presenting symptoms. We limited the number of predictor variables in the model to one variable for every 10 outcomes in our data to avoid overfitting. Statistical analysis was preformed using Stata V.16.1.
Results
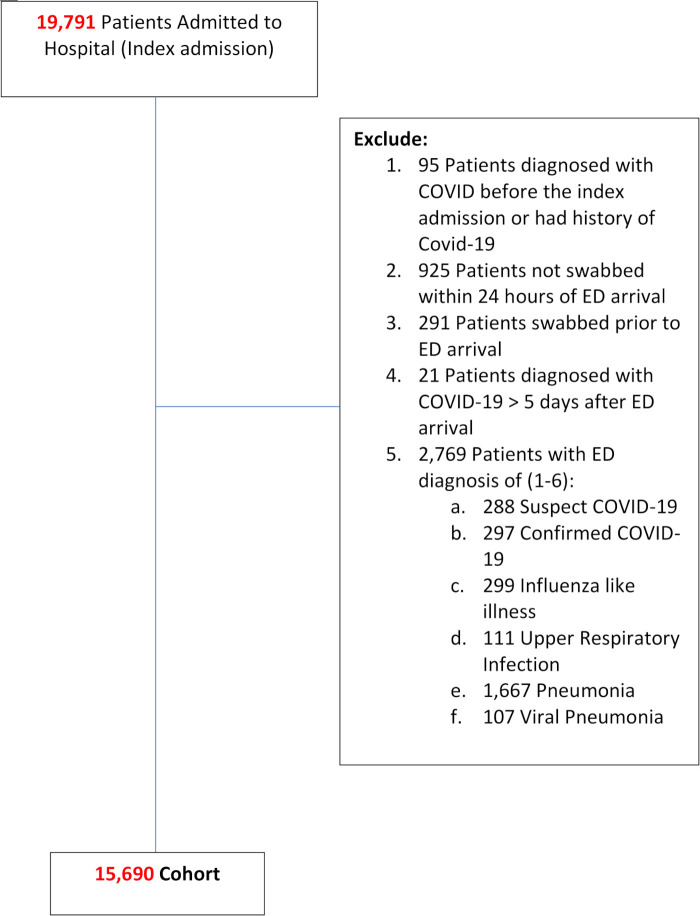
We identified 19 791 patients admitted to hospital who presented to a participating ED between 1 March 2020 and 29 December 2020 (figure 1). We excluded 4101 patients, of whom 2769 had ED diagnoses that were clinically suspicious for COVID-19 and warranted SARS-CoV-2 testing on clinical grounds. The final cohort contained 15 690 patients. During the study period Canada experienced two pandemic waves, with the local 7-day average incident case count ranging from between 0 and 42.6 cases per 100 000 population across sites. The 7-day average diagnostic test positivity varied between 0% and 2.9% across sites during the study period (figure 2).
Figure 1.
Patient flow diagram. ED, emergency department.
Figure 2.
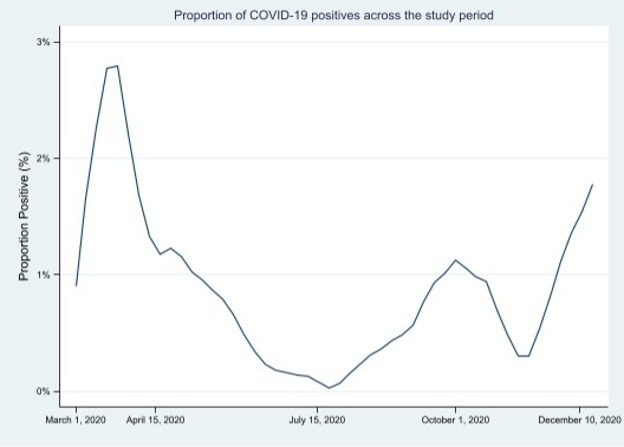
The 7-day working average of COVID-19 NAAT positivity over the study period across sites. NAAT, nucleic acid amplification test.
We divided the cohort into two groups: those without any COVID-19-compatible symptoms and those with COVID-19-compatible symptoms that were attributed to an alternate diagnosis in the ED (table 1). Most patients arrived from home and were in full code. The most common comorbidities were hypertension, diabetes and mental health illness.
Table 1.
Baseline characteristics of admitted patients without clinical suspicion of COVID-19 (N=15 690)
| Patients without COVID-19 symptoms (n=3113) |
Patients with COVID-19-compatible symptoms attributed to an alternate diagnosis (n=12 570) |
|
| Demographics | ||
| Age, mean (SD) | 57.6 (22.6) | 64.6 (20.4) |
| Female (%) | 1418 (45.6) | 5924 (47.1) |
| Pregnant (%) | 18 (0.6) | 45 (0.4) |
| Tobacco use (%) | 491 (15.8) | 1656 (13.2) |
| Illicit substance use (%) | 421 (13.5) | 967 (7.7) |
| Arrival by ambulance (%) | 1724 (55.4) | 7189 (57.2) |
| Arrival from (%) | ||
| Home | 2552 (82.0) | 10 943 (87.0) |
| Long-term care or rehab facility | 217 (7.0) | 832 (6.6) |
| Unstable housing* | 190 (6.1) | 414 (3.3) |
| Corrections | 7 (0.2) | 14 (0.1) |
| Interfacility transfer | 121 (3.9) | 262 (2.1) |
| Risk for infection (%) | ||
| Travel | 32 (1.0) | 134 (1.1) |
| Institutional (LTC/prison) | 231 (7.4) | 721 (5.7) |
| Household contact | 28 (0.9) | 144 (1.1) |
| Occupational | 10 (0.3) | 38 (0.3) |
| Unknown | 1502 (48.2) | 5377 (42.8) |
| Pre-ED goals of care (%) | ||
| Full code | 2946 (94.6) | 11 259 (89.5) |
| Intermediate GOC | 18 (0.6) | 173 (1.4) |
| Do not resuscitate | 149 (4.8) | 1142 (9.1) |
| Acuity | ||
| CTAS 1 (resuscitation) | 241 (7.7) | 1053 (8.4) |
| CTAS 2 (emergent) | 1000 (32.1) | 5786 (46.0) |
| CTAS 3 (urgent) | 1527 (49.1) | 5086 (40.4) |
| CTAS 4 (less urgent) | 295 (9.5) | 572 (4.6) |
| CTAS 5 (non-urgent) | 40 (1.3) | 59 (0.5) |
| Arrival vital signs, mean (SD) | ||
| Heart rate, beats per minute | 91.2 (21.2) | 95.5 (23.9) |
| Systolic BP, mm Hg | 134.7 (25.1) | 133.6 (27.9) |
| Oxygen saturation (%) | 96.6 (3.4) | 95.7 (4.1) |
| Respiratory rate, breaths per minute | 18.6 (4.4) | 21.2 (6.3) |
| Temperature, °C | 36.6 (0.6) | 36.8 (0.9) |
| Comorbidities (%) | ||
| Hypertension | 951 (30.6) | 5321 (42.3) |
| Psychiatric condition | 728 (23.4) | 2134 (17.0) |
| Dyslipidaemia | 425 (13.6) | 2434 (19.4) |
| Diabetes | 427 (13.7) | 2577 (20.5) |
| Chronic neuro disorder | 322 (10.3) | 1406 (11.2) |
| Coronary artery disease | 284 (9.1) | 1796 (14.3) |
| Rheumatological disorder | 229 (7.4) | 1249 (9.9) |
| Dementia | 199 (6.4) | 696 (5.5) |
| Active cancer | 231 (7.4) | 1647 (12.9) |
| Chronic kidney disease | 195 (6.3) | 1319 (10.5) |
| Chronic lung disease (not asthma) | 199 (6.4) | 1691 (13.5) |
| Congestive heart failure | 159 (5.1) | 1392 (11.1) |
| Asthma | 125 (4.0) | 712 (5.7) |
| Obesity | 57 (1.8) | 344 (2.7) |
| Symptoms (%) | ||
| Cough | – | 2763 (22.0) |
| Dyspnoea | – | 4757 (37.8) |
| Fever | – | 2531 (20.1) |
| General weakness | – | 3183 (25.3) |
| Chest pain | – | 2714 (21.6) |
| Diarrhoea | – | 1339 (10.7) |
| Nausea/vomiting | – | 3345 (26.6) |
| Headache | – | 784 (6.2) |
| Chills | – | 957 (7.6) |
| Myalgia | – | 466 (3.7) |
| Sore throat | – | 374 (3.0) |
| Altered consciousness | – | 2502 (19.9) |
| Dysgeusia/anosmia | – | 41 (0.3) |
| ED diagnosis (%) | ||
| Respiratory disease, not specified | 8 (0.3) | 118 (0.9) |
| COPD exacerbation | 11 (0.4) | 648 (5.2) |
| Asthma exacerbation | <5 | 97 (0.8) |
| Congestive heart failure | 44 (1.4) | 1003 (8.0) |
| Shortness of breath, NYD | – | 466 (3.6) |
| Cough, NYD | – | 63 (0.5) |
| Fever, NYD | – | 482 (3.8) |
| Outcome (%) | ||
| Positive SARS-CoV-2 NAAT | 13 (0.4) | 109 (0.9) |
*Unstable housing includes no fixed address, shelter, or single room occupancy.
BP, blood pressure; COPD, chronic obstructive pulmonary disease; CTAS, Canadian Triage and Acuity Scale; ED, emergency department; GOC, goals of care; LTC, long term care; NAAT, nucleic acid amplification test; NYD, not yet determined.
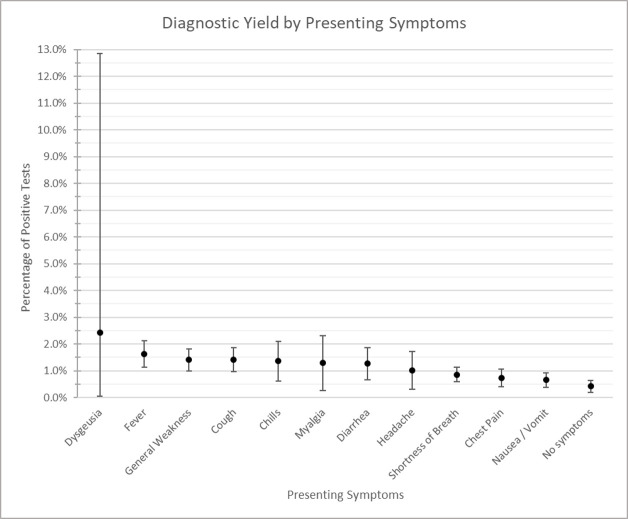
Of 3113 patients admitted without COVID-19-compatible symptoms, 13 (0.4%, 95% CI 0.19% to 0.64%) tested positive for COVID-19. Of the 12 570 with COVID-19-compatible symptoms, 109 patients (0.9%, 95% CI 0.70% to 1.03%) tested positive for COVID-19. Among the 122 individuals who tested positive for COVID-19, 33 (27.0%, 95% CI 19.0% to 35.0%) were from a geographical region that had a moving average daily incident rate of ≥8 infections per 100 000 population. The diagnostic yield of testing among patients with COVID-19-compatible symptoms admitted for alternative diagnoses did not vary substantially by presenting symptom (figure 3) or ED diagnosis (figure 4).
Figure 3.
Diagnostic yield by presenting symptoms.
Figure 4.
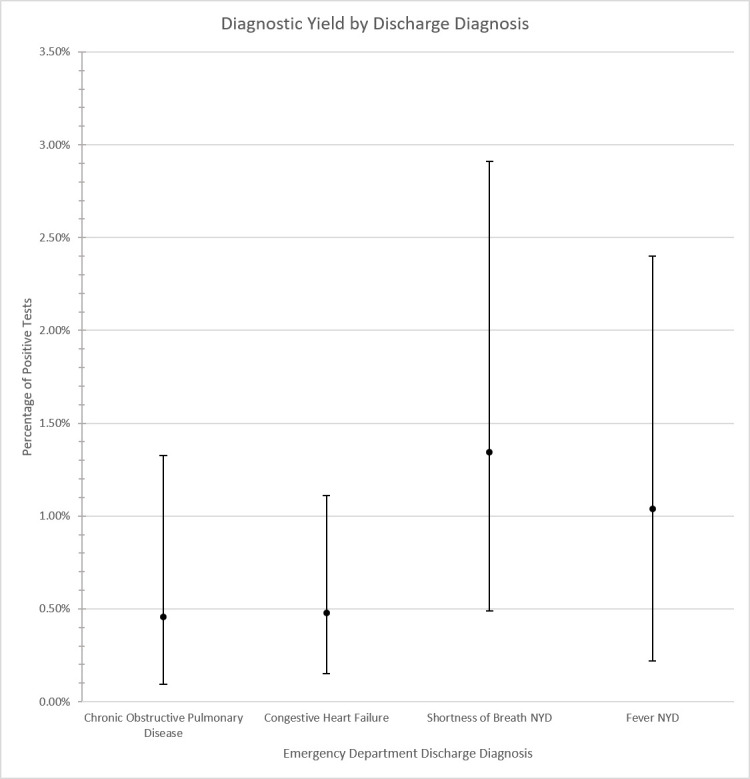
Diagnostic yield by ED diagnosis. ED, emergency department; NYD, not yet determined.
When examining the association between patient factors and screening positive, self-reported fever, being a healthcare worker, having a positive household contact or institutional exposure, and being from an area where the 7-day moving average incident COVID-19 case count was ≥8 per 100 000 population were associated with a greater risk of testing positive (table 2). The most important risk factor was reporting a household contact or being the caregiver of a known COVID-19 case.
Table 2.
Multivariate analysis of factors associated with positive SARS-CoV-2 nucleic tests (N=15 690)
| Univariate analysis, OR (95% CI) | Final model with fully adjusted OR (95% CI)* | P value | |
| Sex | |||
| Male | Reference | Reference | 0.18 |
| Female | 0.84 (0.59 to 1.21) | 0.78 (0.54 to 1.12) | |
| Age | |||
| 1.00 (1.00 to 1.02) | 1.00 (0.99 to 1.01) | 0.27 | |
| 7-day average incident COVID-19 cases | |||
| 0–1.99 daily cases per 100 000 population | Reference | Reference | <0.001 |
| 2–7.99 daily cases per 100 000 population | 1.42 (0.91 to 2.22) | 1.47 (0.94 to 2.31) | |
| ≥8 daily cases per 100 000 population | 2.99 (1.95 to 4.59) | 3.17 (2.05 to 4.89) | |
| COVID-19-compatible symptoms present | |||
| No | Reference | Reference | 0.08 |
| Yes | 2.08 (1.71 to 3.71) | 1.65 (0.90 to 3.00) | |
| Self-reported fever or temperature ≥37.5°C | |||
| No | Reference | Reference | <0.001 |
| Yes | 2.72 (1.89 to 3.90) | 2.53 (1.74 to 3.67) | |
| Diarrhoea present | |||
| No | Reference | Reference | 0.11 |
| Yes | 1.74 (1.04 to 2.92) | 1.57 (0.93 to 2.67) | |
| Healthcare worker | |||
| No | Reference | Reference | 0.06 |
| Yes | 5.62 (1.35 to 23.43) | 4.67 (1.05 to 20.54) | |
| Household contact or caregiver | |||
| No | Reference | Reference | <0.001 |
| Yes | 9.48 (5.01 to 17.96) | 7.74 (3.98 to 15.04) | |
| Institutional exposure | |||
| No | Reference | Reference | <0.001 |
| Yes | 3.46 (2.17 to 5.52) | 3.39 (2.10 to 5.47) | |
| Dysgeusia or anosmia present | |||
| No | Reference | – | |
| Yes | 3.21 (0.43 to 23.52) | – | |
| Dyspnoea present | |||
| No | Reference | – | |
| Yes | 1.16 (0.80 to 1.70) | – | |
| Nausea or vomiting present | |||
| No | Reference | – | |
| Yes | 0.81 (0.51 to 1.29) | – | |
*Final model determined by including variables with p<0.20 from univariable analyses and using the Akaike’s information criterion to determine additional variables to exclude from the final model. Variables adjusted for all other variables present in the final model.
Discussion
Our aim was to evaluate the diagnostic yield of screening non-COVID-19 admissions for SARS-CoV-2 across Canada in 2020 and identify patient-level risk factors for positive tests. The diagnostic yield of screening patients with non-COVID-19-related ED diagnoses who were admitted to hospital was low overall, and extremely low in patients without COVID-19-compatible symptoms. The most important patient factors associated with a positive test were having a positive household contact, being a healthcare worker or having had an institutional exposure to COVID-19. These factors were more important than a high (≥8 daily cases per 100 000 population) 7-day moving average incident COVID-19 case count.
Our study has several strengths. We used data from a large pan-Canadian registry that enrols from large geographically and culturally diverse areas and is one of the largest registries in the world. CCEDRRN’s patient enrolment and data verification protocols are rigorous, ensuring consecutive eligible patients and high-quality clinical data.13 We have previously demonstrated the inter-rater reliability for our data collection methods, including for symptoms.13
Prior studies have examined the diagnostic yield of universal screening in single centres with varied diagnostic yield estimates between 0% and 15.5%.3–8 Many of these were case series with limited methods from the early pandemic. There is one known multicentre study which examined the benefit of universal screening for elective and emergent surgical admissions at 14 centres in the Netherlands.1 Like our study, the authors found that the overall COVID-19 NAAT positivity varied with community prevalence. Our finding that positive SARS-CoV-2 tests were associated with self-reported or measured fever is in keeping with a prior Cochrane systematic review that noted considerable variability in COVID-19-associated symptoms.16
Our study is interesting in the context of current IDSA recommendations, which were based on expert opinion and of ‘very low certainty’.12 The IDSA panel recommended avoiding universal screening for COVID-19 in times and areas of low COVID-19 prevalence, defined as a disease prevalence of under 2% or fewer than 2000 active cases per 100 000 population, a threshold so high that it was never met at any of our study sites, even though multiple sites were in COVID-19 hotspots in 2020.12 The IDSA threshold would have equated to over 6 million cases of active COVID-19 infection in the USA at any given time, which would have vastly overwhelmed hospital capacity, and thus represents an untenable threshold for hospitals. It is therefore not surprising that the prevalence of COVID-19 during the study period was far below the IDSA recommended threshold for initiating screening. While the number needed to screen to identify one positive case among admitted patients in our study was between 110 and 250 among unvaccinated patients, we propose that new screening thresholds need to be adopted which would ideally be based on readily available measures of local incident cases or test positivity.
A limitation of our study is that we only considered NAATs and did not consider the diagnostic yield of antigen-based COVID-19 tests as they were not widespread in Canada in 2020.16 We were unable to examine the sensitivity and specificity of the SARS-CoV-2 NAATs as we were unable to define false positive tests, so it is possible that some of the positive test results we encountered were false positives, leading to an overestimation of diagnostic yield. Additionally, our study was performed before the newer COVID-19 variants, such as Omicron, circulated widely. However, our methods are easily replicated and we intend to repeat our study in a recent data set reflective of new COVID-19 variants. While our study is based on a Canadian population without international sites, we believe our findings are generalisable given their wide geographical spread and the cultural and racial diversity of our patient population. Finally, as data become available on the fourth wave of the pandemic, a future study should examine the impact of widespread vaccination on the yield of screening. As a larger proportion of the population is protected from severe disease and death through vaccination, decision makers should carefully consider the low diagnostic yield of a universal testing strategy going forward.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Mr Rajan Bola in the preparation of this manuscript and thank the UBC clinical coordinating centre staff, the UBC legal, ethics, privacy and contract staff, and the research staff at each of the participating institutions in the network outlined in online supplemental tables 1–4. The network would not exist today without the dedication of these professionals. Thank you to all of our patient partners who shared their lived experiences and perspectives to ensure that the knowledge we co-create addresses the concerns of patients and the public. Creating the largest network of collaboration across Canadian emergency departments would not have been feasible without the tireless efforts of emergency department chiefs and the research coordinators and research assistants at the participating sites. Finally, our most humble and sincere gratitude to our colleagues in medicine, nursing and the allied health professions who have been on the front lines of this pandemic from day 1, staffing our ambulances, emergency departments, hospitals and ICUs and bravely facing the risks of COVID-19 to look after our fellow citizens and after one another. We dedicate this network to you.
Footnotes
Contributors: All authors conceived and planned the study together and iteratively refined the study objectives and analytic plan. PD, RR, ADM, EL and CMH obtained funding for the study. PD, IC, ADM, RD, JT, JK, PTF, MS and BB supervised the data collection. JPH analysed the data under the supervision of RR. All authors interpreted the analysis. PD drafted the manuscript. All authors were actively involved in reporting out the work by revising the manuscript for content. PD is responsible for the overall content as the guarantor. All authors take responsibility for the manuscript as a whole.
Funding: The Canadian Institutes of Health Research (447679), Ontario Ministry of Colleges and Universities (C-655-2129), Saskatchewan Health Research Foundation (5357), Genome BC (COV024), Fondation du CHU de Québec (octroi no 4007) and the Public Health Agency of Canada provided peer-reviewed funding. The BC Academic Health Science Network and BioTalent Canada provided non-peer-reviewed funding. These organisations are not-for-profit and had no role in study conduct, analysis or manuscript preparation.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data is available upon request after Ethics approval and review of the proposed use of the data by PRPC and DAMC of CCEDRRN. Anyone who would like access to data can contact the coordinating centre (https://www.ccedrrn.com/).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the research ethics boards of all participating institutions with a waiver of informed consent for data collection and linkage (UBC REB: H20-01015). We were granted a waiver for informed consent by the UBC Ethics Board as participants were enrolled retrospectively.
References
- 1. Puylaert CAJ, Scheijmans JCG, Borgstein ABJ, et al. Yield of screening for COVID-19 in asymptomatic patients before elective or emergency surgery using chest CT and RT-PCR (SCOUT): multicenter study. Ann Surg 2020;272:919–24. 10.1097/SLA.0000000000004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sutton D, Fuchs K, D'Alton M, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020;382:2163–4. 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravani P, Saxinger L, Chandran U, et al. COVID-19 screening of asymptomatic patients admitted through emergency departments in Alberta: a prospective quality-improvement study. CMAJ Open 2020;8:E887–94. 10.9778/cmajo.20200191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldfarb IT, Diouf K, Barth WH, et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol 2020;41:1095–6. 10.1017/ice.2020.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ossami Saidy RR, Globke B, Pratschke J, et al. Successful implementation of preventive measures leads to low relevance of SARS‐CoV‐2 in liver transplant patients: observations from a German outpatient department. Transpl Infect Dis [Internet] 2020;22 https://onlinelibrary.wiley.com/doi/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gruskay JA, Dvorzhinskiy A, Konnaris MA, et al. Universal testing for COVID-19 in essential orthopaedic surgery reveals a high percentage of asymptomatic infections. J Bone Joint Surg Am 2020;102:1379–88. 10.2106/JBJS.20.01053 [DOI] [PubMed] [Google Scholar]
- 7. London V, McLaren R, Atallah F, et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol 2020;37:991–4. 10.1055/s-0040-1712164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bianco A, Buckley AB, Overbey J, et al. Testing of patients and support persons for coronavirus disease 2019 (COVID-19) infection before scheduled deliveries. Obstet Gynecol 2020;136:283–7. 10.1097/AOG.0000000000003985 [DOI] [PubMed] [Google Scholar]
- 9. Lee DD, Jung H, Lou W, et al. The impact of COVID-19 on a large, Canadian community emergency department. West J Emerg Med 2021;22:572-579 https://escholarship.org/uc/item/0xk8j1fj 10.5811/westjem.2021.1.50123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med 2013;61:605–11. 10.1016/j.annemergmed.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao F, Xu S, Ma X, et al. In-Hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation 2020;151:18–23. 10.1016/j.resuscitation.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanson KE, Caliendo AM, Arias CA, et al. The infectious diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Clin Infect Dis 2021:ciab048. 10.1093/cid/ciab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hohl CM, Rosychuk RJ, McRae AD, et al. Development of the Canadian COVID-19 emergency department rapid response network population-based registry: a methodology study. CMAJ Open 2021;9:E261–70. 10.9778/cmajo.20200290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Health Regional Archive (Public View) . [Internet]. [cited 2021 Jun 4]. Available: https://resourcescovid19canada.hub.arcgis.com/datasets/3aa9f7b1428642998fa399c57dad8045/data?layer=1
- 15. McRae AD, Hohl CM, Rosychuk RJ, et al. Development and validation of a clinical risk score to predict SARS-CoV-2 infection in emergency department patients: The CCEDRRN COVID-19 Infection Score (CCIS) [Internet]. Emergency Medicine 2021. http://medrxiv.org/lookup/doi/ (cited 2021 Aug 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease.. In: Cochrane Database Syst Rev [Internet, 2020. https://onlinelibrary.wiley.com/doi/. (cited 2021 Jul 26). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057852supp001.pdf (110.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data is available upon request after Ethics approval and review of the proposed use of the data by PRPC and DAMC of CCEDRRN. Anyone who would like access to data can contact the coordinating centre (https://www.ccedrrn.com/).