Abstract
Introduction: Increasing prevalence of diabetes and periodontal disease is prompting identification of additional clinical settings to identify patients at risk for dysglycaemia. A systematic review of studies that have examined feasibility of screening for at-risk patients in general dentistry settings at point-of-care (POC) was undertaken. Materials and methods: Systematic review of pragmatic clinical field trials piloting POC screening for dysglycaemia risk in dental settings was undertaken in studies whose primary objective was to explore rates of dysglycaemia among undiagnosed patient populations. Results: Among 17 dental clinical field trials identified, 10 were systematically reviewed. High rates of undiagnosed dysglycaemia were detected among dental patients by biological screening in all trials. Notably, substantive differences in study design and population characteristics were identified, precluding meta-analysis. Conclusion: Screening for dysglycaemia in dental offices effectively identified high-risk patients requiring triage for glycaemic management. Considerations for future clinical trial design were advanced to establish an evidence base amenable to meta-analysis of the relative translational value of glycaemic screening in dental settings.
Key words: Diabetes, dental office, screening, pragmatic clinical trials, oral health
Introduction
Overview
The primary objective of this study was to conduct a systematic review of clinical and field trials in the past 10 years that examined dysglycaemia screening at point-of-care (POC) in dental practices. The primary outcome was rate of dysglycaemia reported in the dental setting. Triage for medical evaluation and compliance were examined as secondary outcomes. Further, study design, glycaemic measure evaluated and instrumentation employed for glycaemic assessment were compared across studies.
Epidemiology of dysglycaemia and periodontitis
Epidemiological surveys have indicated that the rate of both type 2 diabetes (T2DM) and periodontal disease (PD) have achieved epidemic proportions in many countries worldwide. In the USA, 30.2 (9.4%) and 84.1 (33.9%) million people are impacted by diabetes (diagnosed and undiagnosed) and prediabetes, respectively1. Diabetes ranks seventh among the top 10 causes of mortality in the USA, as the principal driver of renal failure, amputations, and a clinically significant contributor to cardio/cerebrovascular diseases2. Prevalence rates in the USA for diabetes/pre-diabetes in adults are projected to achieve 21–33% by 2050, contingent on mortality rate3. The USA ranked third in the world for the largest number of affected adults in 2014, with China (18.9%) and India (11%) ranking first and second, respectively4. Between 1980 and 2014, substantive increases in diabetes have been seen in countries with predominantly Black (Egypt), Asian (Indonesia, Pakistan, Japan) and Hispanic (Brazil, Mexico) populations, causing these countries to displace European populations previously ranked among the top 10 countries contributing to the global burden associated with diabetes. Shifts in prevalence are especially striking in Africa and South East Asia4. The projected direct annual cost globally for diabetes is estimated at international (Intl)$825 billion, with 60% of the cost of care borne by low-to-middle-income countries and the substantial cost burden directly impacting affected individuals as out-of-pocket expense5.
Recent surveillance data, refined assessment approaches and updated definitions of PD collectively point to an underestimation of historic disease prevalence of ≥ 30%6., 7.. Nearly 65 million Americans (46% of the population) are impacted by PD, with higher rates observed in Hispanic and Black populations7. In European countries, periodontitis prevalence of ≥ 50% is projected, with 70–85% prevalence in populations > 60 years old8. While PD prevalence varies by country, substantive increases from 10–15% global prevalence in 1997 to rates of 58% and 77% in Southeast Asia and Western Pacific, respectively, in 2010 were noted, mainly in developing and low-to-middle-income countries. Increased prevalence was attributed to socio-environmental shifts, aging populations, increased burden of diabetes, detrimental dietary changes promoting obesity, sedentary lifestyle, increased tobacco use, and limited access to oral healthcare9.
Oral consequences of two interactive chronic conditions
Diabetes progression is characterised by adverse micro- and macro-vascular processes driven by inflammatory processes. Diabetic progression contributes to co-morbid complications, including PD, which further contribute to a decline in quality of life, increase morbidity and mortality, and associated healthcare cost. PD simultaneously contributes to systemic inflammation.
A growing evidence base supports that underlying pathological processes common to PD and T2DM potentiate disease progression in a bidirectional manner. Bidirectional interaction is supported by a meta-analysis10 (2014) and review11 (2014) that reported reductions in haemoglobin A1C (HbA1C) measures following periodontal treatment. Moreover, sustained dysglycaemia in association with unresolved PD has been reported10., 12., 13.. Clinical consequences associated with dysglycaemia may include persistence of PD. Underlying infectious and inflammatory processes have been posited to contribute to the pathophysiology of both chronic conditions14., 15., 16..
An estimated 25% and 90% of individuals with diabetes and prediabetes, respectively, remain unaware of their dysglycaemic status17. Such a high prevalence of undiagnosed dysglycaemia in dental settings imposes on dentists the often-unachievable task of controlling PD in affected patients, trapping them in a cycle of PD and unresolved dysglycaemia, and putting them at risk for oral and systemic disease progression. Patients with undiagnosed or poorly managed diabetes and diabetes-associated complications seen in the dental setting may exhibit other oral pathology, including root caries, xerostomia (dry mouth), oral mucosal disease including increased candidiasis susceptibility, oral neurosensory disorders18, and implant complications19. A modest association between diabetes history and head and neck cancers has been reported20., 21., but remains controversial due to confounding factors such as environmental exposures (smoking).
Status of screening for dysglycaemia in the dental setting
Historical perspective
Importantly, both conditions represent potentially modifiable diseases responsive to available, evidence-based, relatively low-cost interventions if the at-risk population is identified, ideally in early stages of disease to stem progression and onset of co-morbid complications. In 2008, the US Preventive Services Task Force (USPSTF) recommendations were advanced for population-based screening for diabetes in asymptomatic patients diagnosed with, or under pharmacological management for, hypertension (defined as 135/80 mmHg)22, in internal medicine settings23. However, screening in dental settings was precluded pending achievement of evidence to support recommendation for screening as set forth by the 2013 National Screening Committee24. A systematic review of clinical trials conducted to inform further updates to USPSTF recommendations concluded that screening contributed to delayed disease progression25. Challenges in defining optimal screening criteria were captured by Bullard et al.17, who compared 2008 USPSTF criteria with the expanded criteria set by the American Diabetes Association (ADA) screening guidelines that include: body mass index (BMI) > 25 kg/m2; physical inactivity; race/ethnicity; hypertension (> 140/90 mmHg); gestational diabetes or birth weight ≥ 4,000 g; self-reported prediabetes; or cardiovascular disease. Applying both sets of guidelines to National Health and Nutrition Survey Examination Survey (NHANES) data of ~5,800 participants (2007–2012) to project screening rates, considerable variability in sensitivity across ADA and USPSTF criteria for detection of dysglycaemia in individuals with no diabetes diagnosis (88.8% and 31%, respectively) was noted. The authors noted that incorporating additional risk assessment to mitigate over-screening introduced new challenges in the absence of optimised risk assessment tools to identify high-risk candidates, and emphasised the need to validate tool performance in a population-centric manner. Authors identified urgency in defining best practices surrounding screening in light of high rates of co-morbid complications already present in patients newly diagnosed with diabetes and prediabetes, and escalating disease prevalence17. USPSTF recommendations amended in 2015 included hypertension, age range 40–70 years, and obese status23. ADA screening criteria updated in 2017 currently include: age ≥ 45 years; testing in patients who meet overweight or obesity criteria with one or more of the following risk factors: (i) first-degree relative with diabetes; (ii) high-risk ethnicity; (iii) women with history of gestational DM; (iv) cardiovascular disease history; (v) hypertension or pharmaceutically managed hypertension; (vi) high-density lipoprotein (HDL) cholesterol < 35 mg/dL and/or triglyceride > 250 mg/dL; (vii) polycystic ovary syndrome in women; and (viii) physical inactivity; other clinical conditions associated with insulin resistance26.
Gestational birth weight of ≥ 9 pounds has been removed as a risk factor for T2DM26. Guidelines still lack uniform standard definitions and include some overlap. USPSTF guidelines are generally applied for population-based screening, whereas ADA criteria recommendations are applied for clinical care and assessing risk factor profiles.
Screening for dysglycaemia in the dental setting: clinical and field trials
This review focused on systematic examination of the cumulative evidence emerging from clinical/field trials conducted to date that examined the feasibility of glycaemic screening at POC in the dental setting to define the prevalence of dysglycaemia in order to test alternative approaches to interdisciplinary care delivery for patients impacted by these conditions. The goal is to improve patient outcomes. Implications of the current evidence were reviewed.
Materials and methods
Systematic review approach
Systematic review was conducted on studies investigating the feasibility of conducting POC biological glycaemic screening in clinical dental settings to detect undiagnosed dysglycaemia. Key word searches were conducted by study investigators and the institutional reference librarian, who has extensive experience in performing literature searches to support systematic reviews. No language exclusion was applied if English abstracts were available to assess eligibility for inclusion. Table 1 summarises search strategies, terms, and databases or other resources queried. Additional relevant literature was identified by reviewing citations of relevant articles.
Table 1.
Search strategy overview
| Search terms | Search targets |
|---|---|
| Prediabetic state* OR prediabet* OR dysglycem* AND | PubMed [National Center for Biotechnology Information (NCBI) at US National Library of Congress (NLM); www.opengrey.eu; (European multidisciplinary database of grey literature sources)] |
| Dental* or dentist*, AND | |
| Risk* OR assess* OR model* OR screen* OR algorithm* AND | |
| Risk assessment OR risk factors; | |
| Glucose* OR hemoglobin A1C* AND dental; | |
| Glucose screening test* AND dental office*: OR | |
| HbA1C screening test* AND dental office* |
High-level screening inclusion criteria were:
-
•
Articles published within the last 10 years whose aims included conduct of chair-side POC glycaemic evaluation in a clinical dental setting representing clinical trials, pilot studies or field reports
-
•
Articles published within the last 10 years reporting rates of dysglycaemia detected in the dental setting among patients with no historical diabetes/prediabetes diagnoses or recent glycaemic evaluations.
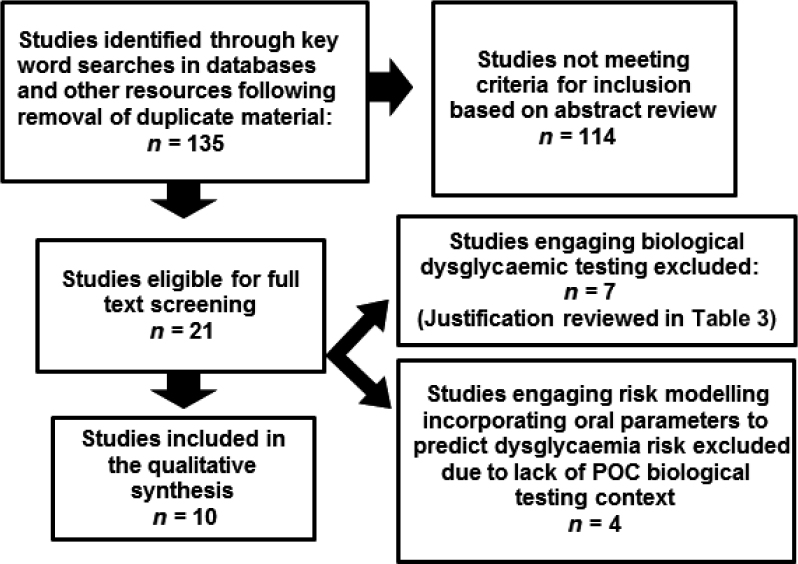
A flow diagram adapted from Moher et al.27 (Figure 1) illustrates the vetting process applied for identifying articles eligible for inclusion in systematic analyses.
Figure 1.
Literature search outcome summary.
Results
Systematic review
Of 135 articles identified by the search strategy, 21 qualified for further screening for potential inclusion. Two studies were reviews, and were excluded based on publication type. While two relevant press releases were excluded, publications cited therein were considered for inclusion. Four of 21 articles28., 29., 30., 31. focused on dysglycaemia risk prediction modelling in the dental setting utilising prospectively-acquired, self-reported data or retrospective analyses of historical data in the absence of biological testing, and were excluded from review. However, the relative merits of risk modelling were discussed with comparisons to the gold standard of biological screening as undertaken in the systematically reviewed studies (Table S1). Seven studies32., 33., 34., 35., 36., 37., 38. were excluded based on justifications presented in Table 2.
Table 2.
Studies conducting biological glycaemic testing in dental setting excluded from systematic review and justification
| Excluded study | Study objective and justification for exclusion |
|---|---|
| 1. Beikler et al. (2002)32 | Study objective: compare HbA1C POC testing performed on blood obtained from gingival crevicular fluid and capillary fingerstick using glucose level self-monitoring device |
| Why excluded: study enrolled patients with known diabetic status including patients with DM dx | |
| 2. Ojehanon and Akhiobare (2006)33 | Study objective: screen oral health in patients with blood glucose > 126 mg/dL to determine PD status; periapical periodontitis was most frequent diagnosis |
| Why excluded: due to lack of POC blood test; study screened urine samples with dip stick and triaged to medical setting for further testing | |
| 3. Nibali et al. (2007)34 | Study objective: monitoring dysmetabolic status in dental patients with severe PD |
| Why excluded: glycaemic measures were not made at POC | |
| Screening was performed on urine and blood | |
| 4. Barasch et al. (2013)35 | Study objective: screening for dysglycaemia at POC in the dental setting |
| Why excluded: due to inclusion of patients with DM dx and pre-DM dx | |
| 5. Miller et al. (2014)36 | Study objective: comparison of glycaemic level determination by a commercial laboratory to patient self-report |
| Why excluded: glycaemic analyses were not conducted at POC in dental settings | |
| 6. Srinivasa et al. (2015)37 | Study objective: compare POC HbA1C levels in patients with or without PD |
| Why excluded: study did not report on DM status of study patients | |
| 7. Harase et al. (2015)38 | Study objective: observe POC HbA1C levels among dental patients with PD stratified by PD severity |
| Why excluded: only subjects with a DM dx were included |
DM, diabetes mellitus; dx, diagnosis; HbA1C, haemoglobin A1C; PD, periodontal disease; POC, point-of-care.
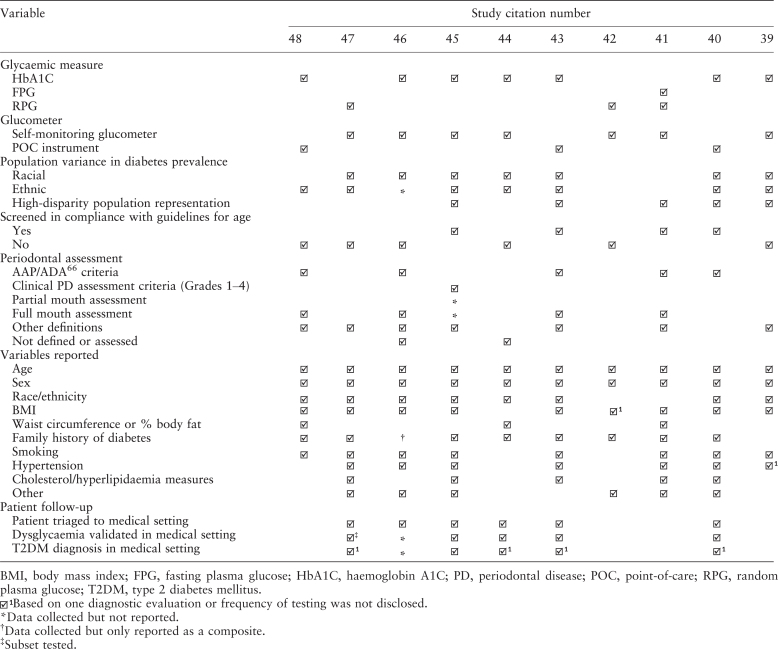
Ten studies39., 40., 41., 42., 43., 44., 45., 46., 47., 48., presented in ascending chronological order, were ultimately reviewed systematically (see summary: Table S1). These trials were largely classifiable as Level II.1 evidence49. Rates of dysglycaemia among patients achieving diabetic range reported across studies ranged between 1.3% and 14%, while rates of patients achieving prediabetic range varied from 19% to 90%. Only 50% of studies provided results of diagnostic assessment following triage to further assess the validity of screening results. The relative frequencies with which co-morbid or demographic variables were tracked across these studies is summarised in Table 3, and reveals variability in glycaemic parameters selected for screening trials, instrument used to conduct screening, dental variables tracked (e.g. some studies reported on dental measures used to define periodontal health status), population differences including ages of patients screened, sample size, ethnic and racial differences, and relative representation of disparity populations in the study cohort. Screening eligibility criteria applied in 60% of studies did not comply with current guidelines for screening eligibility (e.g. age). Further, variables tracked across populations ranged widely across studies (Table S1).
Table 3.
Reporting variability across studies included in systematic analysis in Table S1
Discussion
The evidence base continues to support escalating prevalence rates of DM and PD. These chronic health conditions are associated with high oral and systemic morbidity risk, poor quality of life and high cost of care, and constitute a growing global health concern. The need for early identification and intervention in stemming the tide of the diabetes epidemic has promoted population-based screening for dysglycaemia in the medical domain, especially in the primary care settings.
For patients meeting screening eligibility as defined by current guidelines, dental practices represent an additional primary care setting for dysglycaemia screening and appropriate triage to medical settings for diagnostic follow-up and management. Dental settings are especially opportune for identifying patients with no recent medical encounters and no primary care provider or medical home, who are less likely to be aware of their diabetic status.
This study undertook systematic review of outcomes of clinical trials examining glycaemic screening at POC in dental settings in order to characterise the strength and quality of the emergent evidence base targeting the evidence gap.
Qualitative systematic review findings
The main outcome variable in this study was rate of dysglycaemia defined in the dental setting applying POC glycaemic screening. The prevalence of dysglycaemia in dental settings estimated by applying biological POC screening in undiagnosed patients across the 10 studies systematically reviewed ranged from 1.3% to 14%, and from 19% to 90% for prediabetes (Table S1). Rates reported likely reflected variability across studies surrounding factors including age, ethnicity, proportion of disparity populations across dental settings, screening devices used, and variability in definitions of dysglycaemia. Only a small subset of studies pursued clinical diagnostic laboratory testing as a follow-up to validate screening results, thus diagnosis of diabetes could not be examined as a study endpoint. Moreover, some studies equated positive screening results with ‘diabetes diagnosis’, without reporting whether diagnostic validation in the medical setting was performed.
Meta-analysis was precluded by substantial differences across studies, including population under study, glycaemic parameter evaluated for screening and POC methodology applied for glycaemic evaluation, process and documentation of longitudinal follow-up to screening test for purposes of validating diagnosis and extent of dysglycaemia, and definition of laboratory approaches to diagnostic determination. Collectively these studies raised important considerations in designing future studies investigating the relative clinical merits and cost-effectiveness of POC screening for dysglycaemia in a dental setting as presented below.
Considerations in screening measure selection
Notably, HbA1C and fasting plasma glucose (FPG) measurement for glycaemic screening are associated with variability in performance across populations and capacity to identify true positive cases. For example, the definition of prevalence of prediabetes and undiagnosed diabetes in a nationally representative Canadian population sample (n = 3,494), applying both measures, reported higher estimates with HbA1C, especially in younger patients with lower BMI compared with FPG, which identified prediabetes and diabetes mainly in the older population subset50. The accuracy of HbA1C for POC screening may be constrained due to clinical, demographic or racial/ethnic characteristics51, as demonstrated in the disparate outcomes obtained in studies done in a Chinese52 and African population53. Thus, population characteristics should be weighed in selecting the glycaemic parameter and measurement approach. However, future meta-analyses may only be supportable across analogously-screened, similar populations.
Applying FPG for dysglycaemia screening holds practicable challenges in the dental setting. Importantly, statistical modelling of continuous glucose measures and HbA1C demonstrated comparable capacity of both measures for estimating glucose levels across time, validating the potential use of either measure54., 55. when appropriate in the population being screened. A meta-analysis concluded that while increased stringency in use of high-normal levels did not improve the capacity of HbA1C and FPG for identifying diabetic risk, both demonstrated good capacity for detecting undiagnosed diabetes56.
Considerations in POC screening instrument selection
Previous studies demonstrated considerable variability surrounding the accuracy of testing outcomes depending on whether POC instruments57 or blood glucose self-monitoring (BGSM) devices were used58., 59.. Device selection for screening trials is critical and should consider criteria issued by the US Food and Drug Administration (FDA)60 and performance specifications61. Concerns with off-label use of BGSM devices in a clinical setting have also been raised, citing potential for patient risk in the absence of performance specifications62. Although planning trials with FDA-approved POC devices appears costly, cost-free placement option of POC devices is offered by some vendors57.
Considerations for designing future clinical screening trials
Based on targets for study design improvements identified and summarised in Table 3, the following considerations are proposed when designing and reporting on future screening studies:
-
•
Planning for effective patient triage and follow-up to support analysis of true false-positive and -negative rates of screening, informed by clinical determination of diagnostic status (i.e. diagnosis of diabetes based on two glycaemic measures performed by a CLIA-certified laboratory; a single positive test is sufficient for determining a pre-diabetes diagnosis)
-
•
Applying screening in compliance with current medical guidelines
-
•
Standardising risk factor assessment and reporting to include data on race, ethnicity and disparity status using appropriate surrogate indicators (e.g. insurance status)
-
•
Standardising study designs surrounding inclusion/exclusion criteria, POC instrumentation, and glycaemic measure informed by population characteristics
-
•
Standardising reporting of oral/periodontal screening measures informed by outcomes reported by studies reviewed herein.
Notably, several studies systematically reviewed herein additionally employed risk modelling of clinical and oral health-related, environmental and demographic factors to identify variables with capacity to contribute to relative risk or dysglycaemia39., 40., 43., 46., and/or predict relative risk for dysglycaemia compared with biological testing as the gold standard40., 43.. While some of these models appeared to show good sensitivity and specificity, relevance and portability to populations outside of the population in which they were developed remains to be tested. Such alternative approaches for identifying individuals at risk for dysglycaemia may merit further exploration.
Cost-effectiveness analyses overview
Cost-effectiveness of screening in the dental setting, even in the defined subset of patients currently recommended for glycaemic tracking, has been a concern. Recommendations for screening are presently preempted pending demonstration of a stronger evidence base. Recently, Neidell et al.63 undertook simulation modelling to evaluate the cost-effectiveness of POC screening in a dental setting in dysglycaemic patients being managed by a weight reduction intervention. Investigators estimated the cost of one additional quality-adjusted life year (QALY) for both patients with pre-diabetes and diabetes engaging weight loss interventions over time and projected costs within or below $50,000–60,000/QUALY, a range currently deemed cost-effective. Authors also projected that cost savings stemming from averted future healthcare cost associated with diabetes would further offset costs of screening and weight loss intervention63. This model provides preliminary support that incorporation of POC glycaemic screening into diabetes mellitus integrated care delivery models represents a viable interdisciplinary approach to early identification of high-risk patients requiring intervention. To further validate cost-effectiveness prospectively, de Graaf et al.64 proposed an alternative approach that entails modelling cost per case detected by applying a stepwise screening approach that incorporates evaluation of three parameters: accuracy of the screening tool; cost of distribution and response rate; and incremental cost of each case detected associated with clinical follow-up to confirm diagnosis.
Summary
The removal of barriers inherent in currently-established diabetes care delivery paradigms defined across dental and medical domains65 will require a new evidence base for demonstrating value, patient centricity and cost-effectiveness of alternative paradigms in care delivery, including integrated medical and dental care delivery models that effectively bridge these domains. Apropos to such models is the capacity to identify subpopulations at risk for dysglycaemia in the dental setting and triage them to medical care. Based on the high prevalence of dysglycaemia, especially prediabetes, among dental patients reported in studies included in this systematic review, the collective evidence supports the expansion of glycaemic screening to dental offices as an additional primary care setting for patients meeting screening criteria pending refinement of the approach. Studies demonstrated capacity to identify at-risk individuals with especially high rates of undiagnosed prediabetes identified. Future well-designed screening protocols integrated into DM-ICMs would support earlier detection and opportunity for inter-disciplinary intervention. However, the overall efficacy of screening and triage across medical-dental domains remains contingent on demonstrating referral efficacy, follow-up across medical-dental settings, and patient compliance.
Acknowledgements
The authors thank Marie Fleisner in the Office of Scientific Writing and Publications for assistance with formatting the final manuscript. Funding to support manuscript development was provided by Delta Dental of Wisconsin, Inc. Delta Dental of Wisconsin did not actively participate in the conduct of this systematic review.
Conflict of interest
The authors have no conflict of interest to report.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Overview of study parameters, approaches, outcomes and limitations of studies included in the systematic review (n = 10)
References
- 1.Center for Disease Control National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation. National Diabetes Statistics Report, 2017: Estimates of Diabetes and its Burden in the United States. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 13 November 2017.
- 2.Centers for Disease Control and Prevention. National Center for Health Statistics: Diabetes. Available from: https://www.cdc.gov/nchs/fastats/diabetes.htm last updated: 5/3/2017. Accessed 13 November 2017.
- 3.Boyle JP, Thompson TJ, Gregg LB, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1,513–1,530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015;33:811–831. doi: 10.1007/s40273-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eke PI, Page RC, Wei L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1,449–1,454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009–2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.König J, Holtfreter B, Kocher T. Periodontal health in Europe: future trends based on treatment needs and the provision of periodontal services-position paper 1. Eur J Dent Educ. 2010;14:4–24. doi: 10.1111/j.1600-0579.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- 9.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Jen I, Chou C, et al. Effect of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta analysis. Medicine (Baltimore) 2014;93:e292. doi: 10.1097/MD.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genco RJ, Genco FD. Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. J Evid Base Dent Pract. 2014;14S:4–16. doi: 10.1016/j.jebdp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Borgnakke WS, Ylostalo PV, Taylor GW, et al. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40:S135–S152. doi: 10.1111/jcpe.12080. [DOI] [PubMed] [Google Scholar]
- 13.Corbella S, Francetti L, Taschierri S, et al. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Invest. 2013;4:502–509. doi: 10.1111/jdi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 15.Hasturk H, Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000. 2015;69:255–273. doi: 10.1111/prd.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Periodontol. 2013;84:S113–S134. doi: 10.1902/jop.2013.134005. [DOI] [PubMed] [Google Scholar]
- 17.Bullard KM, Ali MK, Imperatore G, et al. Receipt of glucose testing and performance of two US diabetes screening guidelines, 2007–2012. PLoS ONE. 2015;10:e0125249. doi: 10.1371/journal.pone.0125249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamster IB, Lalla E, Borgnacke WS, et al. The relationship between oral health and diabetes mellitus. JAMA. 2008;139:19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 19.Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol. 2017;44:636–648. doi: 10.1111/jcpe.12724. [DOI] [PubMed] [Google Scholar]
- 20.Vegh D, Banyai D, Hermann P, et al. Type-2 diabetes mellitus and oral tumors in Hungary: a long-term comparative epidemiological study. Anticancer Res. 2017;37:1853–1857. doi: 10.21873/anticanres.11521. [DOI] [PubMed] [Google Scholar]
- 21.Stott-Miller M, Chuang SC, Lee YC, et al. History of diabetes and risk of head and neck cancer: a pooled analysis from the international head and neck epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2012;21:294–304. doi: 10.1158/1055-9965.EPI-11-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris SL, Kansagara D, Bougatsos C, et al. Agency for Healthcare Research and Quality (US); Rockville, MD: 2008. Screening for Type 2 Diabetes Mellitus: Update of 2003 Systematic Evidence Review for the US Preventive Services Task Force. Report No.: 08-05116-EF-1. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. [PubMed] [Google Scholar]
- 23.Siu AL, US Preventive Services Task Force Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:861–868. doi: 10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 24.Waugh NR, Shyangdan D, Taylor-Phillips S, et al. Screening for type 2 diabetes: a short report for the National Screening Committee. Health Technol Assess. 2013;17:1–90. doi: 10.3310/hta17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selph S, Dana T, Blazina I, et al. Screening for type 2 diabetes mellitus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162:765–776. doi: 10.7326/M14-2221. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association 2. Classification and diagnosis of diabetes Table 2.3. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetziaff J, et al. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright D, Muirhead V, Weston-Price S, et al. Type 2 diabetes risk screening in dental practice settings: a pilot study. Br Dent J. 2014;216:E15. doi: 10.1038/sj.bdj.2014.250. [DOI] [PubMed] [Google Scholar]
- 29.Borrell LN, Kunzel C, Lamster I, et al. Diabetes in the dental office: using NHANES III to estimate the probability of undiagnosed disease. J Periodont Res. 2007;42:559–565. doi: 10.1111/j.1600-0765.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 30.Strauss SM, Russell S, Wheeler A, et al. The dental office visit as a potential opportunity for diabetes screening: an analysis using NHANES 2003–2004 data. J Public Health Dent. 2010;70:156–162. doi: 10.1111/j.1752-7325.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Williams PL, Douglass CW. Development of a clinical guideline to predict undiagnosed diabetes in dental patients. J Am Dent Assoc. 2011;142:28–37. doi: 10.14219/jada.archive.2011.0025. [DOI] [PubMed] [Google Scholar]
- 32.Beikler T, Kuczek A, Petersilka G, et al. In-dental-office screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol. 2002;29:216–218. doi: 10.1034/j.1600-051x.2002.290306.x. [DOI] [PubMed] [Google Scholar]
- 33.Ojehanon PI, Akhiobare O. Prevalence of undiagnosed diabetes mellitus among dental patients in Edo State, Nigeria. J Med Biomed Res. 2006;5:24–28. [Google Scholar]
- 34.Nibali L, D’Aiuto F, Griffiths G, et al. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007;34:931–937. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 35.Barasch A, Gilbert GH, Spurlock N, et al. Random plasma glucose values measured in community dental practices: findings from the Dental Practice-based Research Network. Clin Oral Invest. 2013;17:1383–1388. doi: 10.1007/s00784-012-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CS. Implications of medical screenings of patients arriving for dental treatment: the results of a comprehensive laboratory screening. J Am Dent Assoc. 2014;145:1,027–1,035. doi: 10.14219/jada.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasa TS, Agrawal P, Goyal P, et al. Comparative clinical evaluation of glycosylated haemoglobin level in healthy and chronic periodontitis patients: a chairside diagnostic method. Indian J Dent Res. 2015;26:504–507. doi: 10.4103/0970-9290.172049. [DOI] [PubMed] [Google Scholar]
- 38.Harase TS, Nishida W, Hamakawa T, et al. Clinical implication of blood glucose monitoring in general dental offices: the Ehime Dental Diabetes Study. BMJ Open Diabetes Res Care. 2015;3:e000151. doi: 10.1136/bmjdrc-2015-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff RE, Wolff LF, Michalowicz BS. A pilot study of glycosylated hemoglobin levels in periodontitis cases and healthy controls. J Periodontol. 2009;80:1,057–1,061. doi: 10.1902/jop.2009.080664. [DOI] [PubMed] [Google Scholar]
- 40.Lalla E, Kunzel C, Burkett S, et al. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J Dent Res. 2011;90:855–860. doi: 10.1177/0022034511407069. [DOI] [PubMed] [Google Scholar]
- 41.Carmagnola D, Demarosi F, Lodi G, et al. Screening for the undiagnosed diabetes at dental chair-side of an Italian university clinic. A pilot prospective study. Minvera Stomatol. 2012;61:467–476. [PubMed] [Google Scholar]
- 42.Opeodu OI, Adeyemi BF. Undiagnosed diabetes mellitus: a survey of dental outpatients in a tertiary hospital. Afr J Med Med Sci. 2013;42:39–45. [PubMed] [Google Scholar]
- 43.Lalla E, Cheng B, Kunzel C, et al. Dental findings and identification of undiagnosed hyperglycemia. J Dent Res. 2013;92:888–892. doi: 10.1177/0022034513502791. [DOI] [PubMed] [Google Scholar]
- 44.Franck SD, Stolberg RL, Bilich LA, et al. Point-of-care HbA1c screening predicts diabetic status of dental patients. J Dent Hyg. 2014;88:42–52. [PubMed] [Google Scholar]
- 45.Genco RJ, Schifferled RE, Dunford RG, et al. Screening for diabetes mellitus in dental practices. J Am Dent Assoc. 2014;145:57–64. doi: 10.14219/jada.2013.7. [DOI] [PubMed] [Google Scholar]
- 46.Hermann WH, Taylor GW, Jacobson JJ, et al. Screening for prediabetes and type 2 diabetes in dental offices. J Pub Health Dent. 2015;75:175–182. doi: 10.1111/jphd.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossart M, Calley KH, Gurrenlian JR, et al. A pilot study of an HbA1c chairside screening protocol for diabetes in patients with chronic periodontitis: a dental hygienist’s role. Int J Dent Hyg. 2016;14:1–7. doi: 10.1111/idh.12140. [DOI] [PubMed] [Google Scholar]
- 48.Holm NC, Belstrom D, Ostergaard JA, et al. Identification of individuals with undiagnosed diabetes and pre-diabetes in a Danish cohort attending dental treatment. J Periodontol. 2016;87:395–402. doi: 10.1902/jop.2016.150266. [DOI] [PubMed] [Google Scholar]
- 49.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:3,015–3,310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosella LC, Lebenbaum M, Fitzpatrick T, et al. Prevalence of prediabetes and undiagnosed diabetes in Canada (2007–2011) according to fasting plasma glucose and HbA1C screening criteria. Diabetes Care. 2015;38:1,299–1,305. doi: 10.2337/dc14-2474. [DOI] [PubMed] [Google Scholar]
- 51.NCD Risk Factor Collaboration (NCD-RisC) Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331,288 participants. Lancet Diabetes Endocrinol. 2015;3:624–637. doi: 10.1016/S2213-8587(15)00129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Liu Y, Li X, et al. Utility of A1c for the identification of individuals with diabetes and prediabetes in a Chinese high risk population. Scand J Clin Lab Invest. 2012;72:403–409. doi: 10.3109/00365513.2012.689324. [DOI] [PubMed] [Google Scholar]
- 53.Mayega RW, Guwatudde D, Makumbi FE, et al. Comparison of fasting plasma glucose and haemoglobin A1c point of care tests in screening for diabetes and abnormal glucose regulation in a rural low income setting. Diabetes Res Clin Pract. 2014;104:112–120. doi: 10.1016/j.diabres.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 54.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2,239–2,244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spanalis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kodama S, Horikawa C, Fujihara K, et al. Use of high-normal levels of haemoglobin A1C and fasting plasma glucose for diabetes screening and for prediction: a meta analysis. Diabetes Metab Res Rev. 2013;29:680–692. doi: 10.1002/dmrr.2445. [DOI] [PubMed] [Google Scholar]
- 57.Whitley HP, Yong EV, Rasinen C. Selecting an A1C point-of-care instrument. Diabetes Spectr. 2015;28:201–208. doi: 10.2337/diaspect.28.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brazg RL, Klaff LJ, Parkin CG. Performance variability of seven commonly used self-monitoring glucose systems: clinical considerations for patients and providers. J Diabetes Sci Technol. 2013;7:144–152. doi: 10.1177/193229681300700117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freckmann G, Baumstark A, Schmid C. Evaluation of 12 blood glucose monitoring systems for self-testing: system accuracy and measurement reproducibility. Diabetes Technol Ther. 2014;16:113–122. doi: 10.1089/dia.2013.0208. [DOI] [PubMed] [Google Scholar]
- 60.Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197:2013 and current FDA recommendations. J Diabetes Sci Technol. 2015;9:885–894. doi: 10.1177/1932296815580160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohlfing CL, Parvin CA, Sacks DB, et al. Comparing analytical performance criteria: evaluation of HbA1C certification criteria as an example. Clin Chim Acta. 2014;433:259–263. doi: 10.1016/j.cca.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department of Health and Human Services: Center for Medicare and Medicaid Services; Center for Clinical Standards and Quality/Survey and Certification Group, Hamilton TE: Temporary withdrawal-S&C: 15-11-CLIA and reissuance as draft with draft clarifications: Off-label/modified use of waived blood glucose monitoring systems.. Available from: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-15-11.pdf. Accessed 15 March 2017.
- 63.Neidell M, Lamster IB, Shearer B. Cost-effectiveness of diabetes screening initiated through a dental visit. Community Dent Oral Epidemiol. 2017;45:275–280. doi: 10.1111/cdoe.12286. [DOI] [PubMed] [Google Scholar]
- 64.de Graaf G, Postmus D, Bakker SJL, et al. Design of stepwise screening for prediabetes and type 2 diabetes based on costs and cases detected. J Clin Epidemiol. 2015;68:1,010–1,018. doi: 10.1016/j.jclinepi.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Glurich I, Nycz G, Acharya A. Status update on translation of integrated primary dental-medical care delivery for management of diabetic patients. Clin Med Res. 2017;15:21–32. doi: 10.3121/cmr.2017.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Dental Association and American Academy of Periodontics . American Dental Association and American Academy of Periodontics; Chicago, IL: 1992. Periodontal Screening and Recording Training Program Kit. [Google Scholar]