Abstract
Aim: To evaluate whether socioeconomic position exerts a mediating and/or moderating effect on the association between oral clinical measures and oral health-related quality of life (OHRQoL) in adolescents. Materials and methods: The study analysed data on 5,445 adolescents aged 15–19 years from the Brazilian Oral Health Survey (SBBrasil Project). The numbers of decayed and missing teeth, number of sextants with gingivitis and malocclusion were assessed through oral clinical examinations. Participant’s age, sex, OHRQoL and socioeconomic position were also collected. Monthly family income was used to indicate the participant’s socioeconomic position, and OHRQoL was assessed using the Oral Impacts on Daily Performance. Moderation was tested using Poisson regression models. Structural equation modelling and Sobel’s test assessed the mediation effects. Results: Oral clinical measures, OHRQoL and socioeconomic position were significantly correlated (P < 0.001). The moderator effect of socioeconomic position on the association between all oral clinical measures and OHRQoL was observed. The impact of all oral clinical conditions on adolescents’ OHRQoL was lower in the low-family-income groups compared with those with a better income. Socioeconomic position partially mediated the relationship between the four oral clinical measures and OHRQoL. Sobel’s test confirmed these findings (P < 0.001). Conclusions: The findings suggest the importance of socioeconomic position as a moderator and mediator factor between oral clinical measures and OHRQoL. Disadvantaged adolescents are likely to experience poor OHRQoL due to oral conditions. The reduction of the impact of oral conditions on quality of life in adolescents may be enhanced by addressing social inequalities related to oral health.
Key words: Oral health, quality of life, socioeconomic status, adolescent
INTRODUCTION
Oral health-related quality of life (OHRQoL) is a subjective multi-dimensional construct measuring the extent to which oral conditions impact on physical functioning, psychological, emotional and social well-being1. The importance of OHRQoL for dental practice and oral health policies has been acknowledged, reflecting the increase of OHRQoL measures in oral health research1., 2.. Aspects related to such expansion include the recognition that OHRQoL measures are ‘reliable’, the development of validated instruments, and the responsiveness of OHRQoL measures to meaningful clinical changes3. During recent decades, OHRQoL indicators have been collected in different types of dental studies, including surveys, studies involving specific population groups, clinical trials, health services research and cost-utility analysis4., 5..
The identification of predictors of OHRQoL has been considered a relevant topic in dental research. Poor clinical oral health status, demographic characteristics, psychosocial factors and socioeconomic conditions have been identified as predictors of OHRQoL in different age groups6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23.. Previous studies involving children and adolescents have reported the potentially harmful influences of dental caries, dental trauma, missing teeth, gingivitis and malocclusion on OHRQoL6., 7., 9., 10., 11., 12., 14., 15., 20., 21., 22., 23.. Oral diseases may affect people’s well-being and OHRQoL through symptoms status (e.g. dental infection, dental pain), functional status (e.g. eating and speech impairments), and emotional and social negative influences (e.g. social interaction problems due to dental aesthetic concerns)3., 7., 8.. Recent evidence has shown different mediating effects of the link between poor oral conditions and OHRQoL. For example, personal health practices and use of services mediated the relationship of decayed teeth and perceived treatment need with OHRQoL7. The influence of decayed and missing teeth on quality of life was mediated by functional status8. In addition, the association between caries experience and OHRQoL was mediated through psychological characteristics11.
There is robust evidence to support that oral health disparities are influenced by social inequalities. Socioeconomic characteristics are important environmental factors that can negatively impact children and adolescent’s OHRQoL. A recent systematic review on the impact of parental socioeconomic status and home environment characteristics on children’s OHRQoL suggests that children from more affluent families and with higher parental education had better OHRQoL16. In addition, low education and low household income were associated with worse OHRQoL in adolescents8., 13., 20.. Studies on social determinants of oral diseases suggest that health inequalities related to clinical oral conditions are more common in socially deprived population groups24. Children and adolescents from lower socioeconomic families have poorer oral health compared with those who are better off when different clinical outcomes (e.g. dental caries, gingivitis) and measures of social status (e.g. family income, parental education and occupational background) were considered25., 26., 27..
The relationships between socioeconomic conditions, clinical oral status and OHRQoL have been investigated using different analytical strategies, including multivariable regression analysis13., 17., 18., 19., 20., 22. and structural equation modeling (SEM)7., 8., 11., 15., 19.. In the latter statistical method, theoretical models have been tested to identify direct and indirect effects between biological, individual and environmental variables and OHRQoL outcomes3. However, the main aim of previous studies has been to investigate the determinants of OHRQoL and well-being.
Socioeconomic status has been considered an important predictor of oral clinical status and OHRQoL7., 8., 10., 12., 13., 15., 17., 18., 20.. In addition, socioeconomic status has been acknowledged as a potential confounder for the relationship between dental status and OHRQoL10., 12., 13., 17., 18., 20.. However, far too little attention has been paid to other possible roles of socioeconomic position on this relationship. It can be argued that the influence of oral clinical status on OHRQoL may vary according to different socioeconomic groups, suggesting a moderator effect of socioeconomic status on the association between oral clinical status and OHRQoL. Adolescents from better socioeconomic background may experience greater impact on their OHRQoL despite having better oral health. This might be explained by the greater awareness of factors related to oral health and the pervasiveness effect of oral diseases on OHRQoL in these individuals. Previous research shows the relationship between clinical oral status and OHRQoL is alleviated in socially privileged populations6., 7., 8., 18.. A different perspective is to speculate that socioeconomic status mediates the impact of oral clinical status on OHRQoL. Based on this mediational model, adolescent’s oral health status is a consequence of their social position, which in turn deteriorates their OHRQoL, suggesting the mediator effect of socioeconomic status. People living in social disadvantage are more exposed to economic deprivation and poor social ties, such as low social support and social isolation, which in turn are associated with poor oral health in adolescents28., 29.. The available socioeconomic resources may buffer the impact of oral clinical status on OHRQoL. For instance, adolescents with high socioeconomic status tend to live in higher health-promoting areas (e.g. access to preventive dental care and water fluoridation), increasing the opportunities to engage in oral health-promoting behaviours.
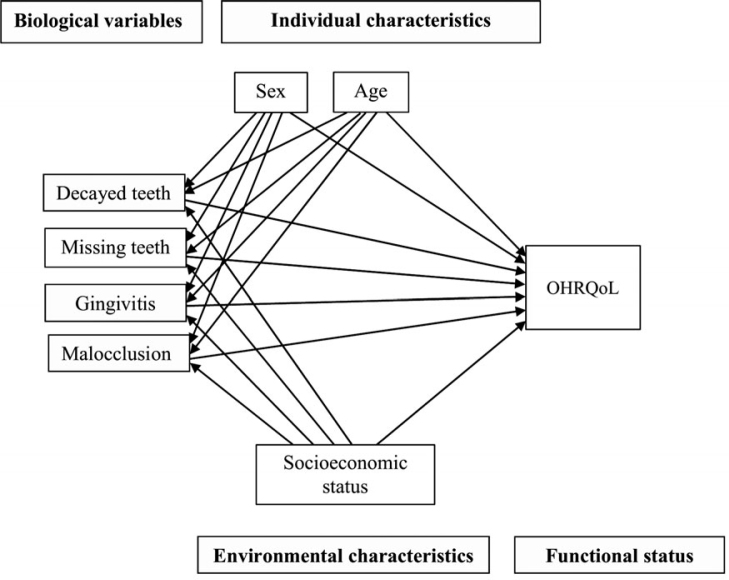
Exploring the moderation and mediating effects of socioeconomic position on the link between dental status and OHRQoL is relevant for healthcare professionals and policy makers for different reasons. One reason is to raise the awareness and to highlight the importance of the development of strategies to reduce social inequalities to minimise the negative direct and indirect effects of oral diseases on OHRQoL. From the point of view of health policies, actions aiming to tackle socioeconomic disparities would result in an overall improvement of population oral health and also would reduce the oral health gap between different socioeconomic groups. With these points in mind, the present study explored the mediation and modification effects of socioeconomic status on the relationship between oral clinical measures on OHRQoL in adolescents using the Wilson and Cleary conceptual model (Figure 1)3. Therefore, we can hypothesise that the association between clinical oral health measures and OHRQoL varies according to socioeconomic status (moderator hypothesis). Another hypothesis is that socioeconomic status mediates the impact of poor oral health on OHRQoL. The above-mentioned gaps in knowledge prompted us to conduct a study using a representative sample of Brazilian adolescents to test: (i) whether dental caries, missing teeth, gingivitis and malocclusion are associated with poor OHRQoL; (ii) whether socioeconomic position modifies the association between oral clinical measures on OHRQoL (effect of modification); and (iii) whether socioeconomic status mediates the relationship between oral clinical measures and OHRQoL (mediation effect).
Figure 1.
Full theoretical model on relationships between oral clinical measures, individual and environmental characteristics, and oral health-related quality of life (OHRQoL) in adolescents according to Wilson and Cleary conceptual model3.
MATERIALS AND METHODS
Participants and sampling procedures
The present study was carried out using data from the Brazilian Oral Health Survey (SBBrasil Project) in 201030. A representative sample of Brazilian adolescents from urban areas of the country was clinically examined in their homes to estimate their oral health status. In addition, demographic data, socioeconomic characteristics and OHRQoL were registered through interviews30., 31.. This study included all individuals aged between 15 and 19 years from the state capitals, the Federal District and Interior Municipalities who participated in the SBBrasil Project.
The sample size of the Brazilian Oral Health Survey was calculated to estimate the mean of the number of decayed, missing and filled teeth (DMFT) using data from the 2003 survey considering 95% of precision [confidence interval (CI) of 95%] in each domain, a response rate of 80% and design effect equal to 2 to account for complex sampling31. A probabilistic cluster-sampling method was used in the SBBrasil Project to obtain a representative sample of the country, according to 32 domains, including the 26 state capitals, the Federal District and five domains of the interior municipalities of each macro-region. A two-stage sampling strategy was adopted in the state capitals and the Federal District. Thirty interior municipalities of the five macro-regions were randomly selected through a three-stage process using the probability proportion-to-size method. Sampling weights were calculated using the inverse probability equation and incorporated into the dataset afterwards. The weights (w) were calculated for each primary sampling unit of the sample. Further details of the sampling process are given elsewhere31.
Data collection and pilot study
Five-hundred and seventy fieldwork teams collected the data through structured interviews and clinical oral examinations. Each fieldwork team was composed of one dentist and one healthcare worker (interviewer) from the Brazilian Public Health Care System. The interviewers underwent 40 hours of training before the fieldwork data collection in order to conduct standardised interviews. Examiners also underwent 40 hours of clinical training before the clinical calibration study. There were 10 teams in each state capital and the Federal District, whereas the number of fieldwork teams in the interior municipalities varied from two to six, depending on the size of the city30. Pre-tested questionnaires were used to register demographic data, OHRQoL and socioeconomic position using Personal Digital Assistant equipments (PDA model P550) in participant’s households. The head of the family provided the information about socioeconomic status.
A pilot study was conducted in two state capitals before the main survey to evaluate questionnaires’ feasibility and understanding, and to check the logistic procedures. In addition, the examiner’s consistency concerning clinical oral measurements was assessed using the consensus technique for standardisation28. Dental examinations were performed by 570 calibrated dentists who achieved Kappa coefficient equal to or over 0.65 during the calibration study30., 32..
Measures
Oral health-related quality of life
Oral health-related quality of life was measured using the Oral Impacts on Daily Performance (OIDP) questionnaire validated for the Brazilian population33., 34.. The OIDP questionnaire assesses the impact of oral health status on daily life activities in the preceding 6 months according to nine items related to the performances on ‘eating and enjoying food’; ‘speaking and pronouncing clearly’; ‘cleaning teeth’; ‘sleeping and relaxing’; ‘smiling, laughing and showing teeth without embarrassment’; ‘maintaining usual emotional state without being irritable’; ‘carrying out major work or social role’; ‘enjoying contact with people’; and ‘doing sports’33. The participants were asked to inform whether each activity was affected by their teeth (code 1) or not (code 0). The OIDP severity score was analysed as a discrete variable (OIDP extent) by counting the activities affected by oral health, ranging from 0 (no impact) to 9 (impact in all activities). The Cronbach’s α for the OIDP in the 15–19-years-old age group was 0.78, 95% CI 0.77–0.79.
Socioeconomic position and demographic data
Demographic data were age and sex. Socioeconomic position was assessed using monthly family income, which is considered a relevant measure for social stratification to reflect material conditions in epidemiological studies35. Monthly family income was registered based on the number of Brazilian minimum wages per family, and recorded as ≤ 500 Brazilian reals (R$), R$ 501–1,500, R$ 1,501–2,500 and > R$ 2,500. The Brazilian minimum wage was R$ 500 when the study was conducted and one Brazilian real corresponded to 0.586 US dollars.
Oral clinical measures
All oral clinical measures collected from adolescents who participated in the Brazilian Oral Health Survey (SBBrasil Project) were analysed30. They included dental caries, missing teeth, gingivitis and malocclusion, and were collected according to the WHO guidelines for oral health surveys32. Components ‘decayed’ and ‘missing’ teeth of the DMFT index were employed to assess the number of decayed and missing teeth. Component ‘filled’ was not considered in the present study as filled teeth does not represent a diagnosis of a dental clinical condition or a measure of a current dental disease. Furthermore, there is no sound evidence to suggest that treated teeth may affect OHRQoL, and therefore this measure should not be considered a biological factor according to Wilson and Cleary’s theoretical model. Gingivitis was recorded using the component bleeding on probing of the Community Periodontal Index (CPI). The number of sextants with bleeding on probing for each sextant, ranging from 0 (no sextant with bleeding on probing) to 6 (all 6 sextants with bleeding on probing), was registered using a ball-end CPI probe32. The Dental Aesthetic Index (DAI) was used to assess malocclusion. The DAI score is computed by multiplying each of the 10 components (occlusal traits) by their coefficients and then summing up the final score to a constant. Participants were classified as no abnormality or minor malocclusion (score ≤ 25), definite malocclusion (score 26–30), severe malocclusion (score 31–35), and very severe or handicapping malocclusion (score ≥ 36)32.
Statistical analysis
Descriptive results are presented for the total sample and according to monthly family income groups: ‘≤ R$ 1,500 = low family income’ and ‘> R$ 1,500 = high family income’. Participants from low- and high-family-income groups were compared for continuous and categorical variables using Mann–Whitney test and Pearson Chi-square test, respectively. Non-parametric Spearman linear correlation was used to identify possible correlations between age, monthly family income, dental caries, missing teeth, gingivitis, malocclusion and OIDP extent. Monthly family income was analysed using the four categories in the correlation analysis. All other analyses considered monthly family income as a dichotomous measure, namely low (≤ R$ 1,500) and high (> R$ 1,500) family income. The modification effect of socioeconomic position on the association between each oral clinical measure and OHRQoL was assessed using Poisson regression according to three statistical models as follows. Model 1, unadjusted association between each oral clinical measure and OIDP extent. Model 2, Model 1 adjusted for age, sex and monthly family income; and Model 3, Model 2 plus the interaction term ‘oral clinical measure × monthly family income’. The statistical significance of interaction was tested using Likelihood Ratio tests between Model 2 (without interaction term) and Model 3 (with interaction term). Dental attendance was not included in the analysis as this variable was not statistically associated with OIDP extent. The median of OIDP extent did not differ between the categories of dental attendance variable (< 1 year, 1–2 years, > 2 years; P > 0.05). Stratified analyses through multivariable Poisson regression were used to estimate rate ratios (RRs) and 95% CI between each clinical oral measure and OHRQoL adjusted by age and sex according to different socioeconomic position groups (low- and high-family-income). Sample weights were used in the analyses due to the complex nature of the survey.
The socioeconomic position mediator effect of oral clinical measures and OHRQoL was assessed according to Baron and Kenny criteria36: (i) oral clinical measure should predict OHRQoL; (ii) socioeconomic position should predict OHRQoL; (iii) oral clinical measure should predict socioeconomic position; and (iv) controlling for socioeconomic position, the association between oral clinical measure and OHRQoL should be reduced or no longer significant. Perfect mediation is established if the association between oral clinical measure and OHRQoL is reduced to zero. A series of SEMs was carried out considering the sample weights (STATA command ‘[pweight =], nocapslatent’) to individually examine the mediation effect of socioeconomic position for each clinical oral measure adjusted for sex and age37. The Sobel’s test was used to assess indirect effects of the mediation models38. Mediation was assessed by testing the significance of the indirect effects using the bias-corrected bootstrap confidence intervals where multiple samples (n = 900) are randomly drawn from the original sample39. This method produces less biased estimates under conditions of non-normality. The bootstrap estimates and standard errors were estimated. SEM models were considered of good fit according to the coefficient of determinantion and standardised root mean square (SRMR) < 0.08 criterion40. The significance level for all analyses was 5% (P = 0.05). All statistical analyses were conducted using STATA version 14.0 (Stata, College Station, TX USA).
Ethical aspects
The project was approved by the Brazilian National Council of Ethics in Research under protocol no. 15,498, in compliance with Brazilian National Health Council Resolution 196/96. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants aged 18 years and over or participants’ parents or guardians of those under 18 years old included in the study.
RESULTS
The final sample comprised 5,445 adolescents (mean age = 16.84 years), 67.1% of whom were from low-income families. Missing data on monthly family income, DMFT and DAI were identified in 320, 78 and 1,046 participants, respectively. Thus, the analysed sample comprised 5,052 participants for decayed teeth and missing teeth, 5,445 participants for gingivitis, and 4,168 for malocclusion with complete data for the investigated variables.
Descriptive characteristics of the sample according to family monthly income are presented in Table 1. The majority of participants were females (51.7%), and the means of decayed teeth, missing teeth and the number of sextants with gingivitis were 1.71, 0.38 and 0.97, respectively. The mean OIDP extent was 0.99 and the prevalence of one impact on OHRQoL was 39.4%. The most prevalent performances affected by oral health status were ‘eating’ (20.8%), followed by ‘cleaning teeth’ (16.2%) and ‘emotional state’ (14.8%). The least affected performances were ‘sports’ (4.4%) and ‘social role’ (4.6%). Demographic and dental clinical characteristics of adolescents significantly differed between family income groups (Table 1). Female adolescents were more common in the lower income group. The mean OIDP extent, the overall OIDP prevalence, and the prevalence of the oral impacts on cleaning, smiling, emotional state, social contact and sports were higher for adolescents with low family income (Table 1).
Table 1.
Demographic and socioeconomic characteristics, oral clinical measures and OHRQoL (OIDP); comparisons between family income groups
| Total (N = 5,445) | Family monthly income |
P | ||
|---|---|---|---|---|
| Low (N = 3,735) | High (N = 1,710) | |||
| Age (years), mean (SE)† | 16.86 (0.05) | 16.83 (0.05) | 16.91 (0.10) | 0.002* |
| Sex, %‡ | ||||
| Female | 51.7 | 56.1 | 42.8 | < 0.001* |
| Male | 48.3 | 43.9 | 57.2 | |
| Number of decayed teeth, mean (SE)† | 1.71 (0.12) | 2.12 (0.15) | 0.87 (0.10) | < 0.001* |
| Number of missing teeth, mean (SE)† | 0.38 (0.04) | 0.40 (0.04) | 0.33 (0.11) | < 0.001* |
| Gingivitis§, mean (SE)† | 0.97 (0.08) | 1.10 (0.10) | 0.73 (0.10) | < 0.001* |
| Malocclusion¶, mean (SE)† | 23.95 (0.37) | 24.89 (0.45) | 21.98 (0.51) | < 0.001* |
| OIDP | ||||
| OIDP extent††, mean (SE)† | 0.99 (0.06) | 1.15 (0.08) | 0.66 (0.09) | < 0.001* |
| Prevalence (OIDP ≥ 1), %‡ | 39.6 | 44.3 | 30.1 | 0.001* |
| Eating, %‡ | 20.8 | 22.7 | 16.9 | 0.102 |
| Speaking, %‡ | 6.2 | 6.6 | 5.3 | 0.395 |
| Cleaning, %‡ | 16.2 | 19.5 | 9.4 | < 0.001* |
| Sleeping, %‡ | 11.4 | 12.8 | 8.7 | 0.091 |
| Smiling, %‡ | 13.0 | 15.0 | 9.1 | 0.009* |
| Emotional state, %‡ | 14.8 | 18.5 | 7.1 | < 0.001* |
| Social role, %‡ | 4.6 | 5.4 | 3.0 | 0.054 |
| Social contact, %‡ | 7.6 | 9.5 | 3.7 | < 0.001* |
| Doing sports, %‡ | 4.4 | 5.4 | 2.4 | 0.012* |
Compared between family income groups; P < 0.05 is considered significant.
Mann–Whitney test.
Pearson Chi-square test.
Number of sextants with bleeding on probing.
DAI score.
Oral Impacts on Daily Performance (OIDP) extent: number of oral impacts, ranging from 0 (no impact) to 9 (impact in all activities).
Spearman correlation was used to identify possible correlations between age, monthly family income, number of decayed teeth, number of missing teeth, number of sextants with gingivitis, DAI score (malocclusion) and OIDP extent (Table 2). Higher age (ρ = 0.036), low monthly family income (ρ = −0.126), number of decayed teeth (ρ = 0.254), number of missing teeth (ρ = 0.152), number of sextants with gingivitis (ρ = 0.221) and DAI score (maloclussion; ρ = 0.168) were found to be significantly correlated with greater number of oral impacts (P < 0.05). Greater age (ρ = 0.047) and high monthly family income were also correlated (P < 0.05).
Table 2.
Correlation matrix (Spearman coefficient) between age, family monthly income, dental caries, missing teeth, gingivitis, malocclusion and OIDP extent†
| OIDP† (P) | Family monthly income (P) | |
|---|---|---|
| Age | 0.036 (0.009)* | 0.047 (0.001)* |
| Family monthly income‡ | −0.126 (< 0.001)* | 1 |
| Number of decayed teeth | 0.254 (< 0.001)* | −0.250 (< 0.001)* |
| Number of missing teeth | 0.152 (< 0.001)* | −0.130 (< 0.001)* |
| Gingivitis§ | 0.221 (< 0.001)* | −0.093 (< 0.001)* |
| Malocclusion¶ | 0.168 (< 0.001)* | −0.084 (< 0.001)* |
P < 0.05 is considered significant.
Oral Impacts on Daily Performance (OIDP) extent: number of oral impacts, ranging from 0 (no impact) to 9 (impact in all activities).
Family monthly income categories: ≤ R$ 500, R$ 501–1,500, R$ 1,501–2,500 and > R$ 2,500.
Number of sextants with bleeding on probing.
DAI score.
The non-adjusted and adjusted analysis on the association between each oral clinical measure and the number of oral impacts is shown in Table 3. In the first models, decayed teeth, missing teeth, gingivitis and malocclusion were statistically associated with OIDP extent (P ≤ 0.001). In the second models, the association between each oral clinical measure and OIDP extent was adjusted for age, sex and monthly family income. All oral clinical measures remained statistically associated with OIDP extent (P ≤ 0.001). The final models (third models) tested the moderator effect of monthly family income on the association between each oral clinical measure and OHRQoL through incorporating the interaction term between the oral clinical measure and monthly family income. The interaction term and the independent variables were significantly related to OIDP extent for each oral clinical measure.
Table 3.
Multivariate Poisson regression on the associations of decayed teeth, number of missing teeth, gingivitis and malocclusion with OIDP†
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| β (SE) | β (SE) | β (SE) | |
| Number of decayed teeth | 0.096 (0.006)** | 0.089 (0.006)** | 0.127 (0.016)** |
| Age | 0.061 (0.017)** | 0.064 (0.017)** | |
| Sex (ref: male) | 0.274 (0.051)** | 0.277 (0.050)** | |
| Family monthly income (ref: R$ > 1,500)‡ | 0.366 (0.059)** | 0.483 (0.066)** | |
| Decayed teeth × family monthly income | −0.046 (0.017)* | ||
| Likelihood ratio§ | 905.46 | 942.64 | |
| ΔDif = 37.18, df = 5 (χ2 test, P < 0.001)** | |||
| Number of missing teeth | 0.115 (0.019)** | 0.107 (0.019)** | 0.163 (0.021)** |
| Age | 0.045 (0.017)* | 0.047 (0.017)* | |
| Sex (ref: male) | 0.257 (0.051)** | 0.255 (0.051)** | |
| Family monthly income (ref: R$ ≥ 1,500)‡ | 0.456 (0.060)** | 0.489 (0.061)** | |
| Missing teeth × family monthly income | −0.008 (0.002)* | ||
| Likelihood ratio§ | 552.02 | 562.75 | |
| ΔDif = 10.73, df = 5 (χ2 test, P = 0.030)* | |||
| Gingivitis¶ | 0.171 (0.011)** | 0.162 (0.011)** | 0.221 (0.024)** |
| Age | 0.064 (0.017)** | 0.064 (0.017)** | |
| Sex (ref: male) | 0.261 (0.050)** | 0.260 (0.050)** | |
| Family monthly income (ref: R$ ≥ 1,500)‡ | 0.427 (0.059)** | 0.532 (0.071)** | |
| Gingivitis × family monthly income | −0.074 (0.027)* | ||
| Likelihood ratio§ | 790.15 | 807.53 | |
| ΔDif = 17.38, df = 5 (χ2 test, P = 0.002)* | |||
| Malocclusion†† | 0.444 (0.052)** | 0.417 (0.057)** | 0.666 (0.117)** |
| Age | 0.069 (0.019)** | 0.069 (0.019)** | |
| Sex (ref: male) | 0.259 (0.058)** | 0.263 (0.058)** | |
| Family monthly income (ref: R$ > 1,500)‡ | 0.413 (0.067)** | 0.551 (0.090)** | |
| Malocclusion × family monthly income | −0.318 (0.133)* | ||
| Likelihood ratio§ | 383.87 | 399.11 | |
| ΔDif = 15.24, df = 5 (χ2 test, P = 0.004)* |
Model 1: unadjusted.
Model 2: adjusted for age, sex and family monthly income.
Model 3: model 2 + interaction term (clinical oral measure × family monthly income).
P < 0.05 and **P ≤ 0.001 are considered significant.
Oral Impacts on Daily Performance (OIDP) extent: number of oral impacts, ranging from 0 (no impact) to 9 (impact in all activities).
Family monthly income categories: ≤ R$ 1,500 and > R$ 1,500.
Interaction was assessed through comparison likelihood ratio between models 2 and 3; P < 0.05 suggests interaction.
Number of sextants with bleeding on probing.
DAI score.
Table 4 reports the adjusted Poisson regression on the association of decayed teeth, missing teeth, gingivitis and malocclusion with OIDP extent, stratified by monthly family income. Adolescents with worst clinical measures were more likely to have a higher mean of OIDP extent in both family income strata. However, the impact of the investigated oral clinical conditions on adolescents’ quality of life was lower in the low-family-income groups compared with those who were better off. Each additional tooth with caries was associated with an estimated 8% increase in the mean number of impacts in the low-income group and 14% increase in the high-income group. The mean number of oral impacts increased by 11% and 18% for each additional missing tooth in the low- and high-income groups, respectively. The number of sextants with gingivitis increased the likelihood of a higher mean of number of impacts in adolescents by 16% and 25% in the low- and high-income groups. Adolescents with a greater DAI score were 42% and 94% more likely to have a higher mean of OIDP extent compared with those with normal occlusion in the high- and low-income groups.
Table 4.
Adjusted Poisson regression models on the associations of decayed teeth, number of missing teeth, gingivitis and malocclusion, and OIDP† stratified by socioeconomic position
| β | SE | RR‡ | 95% CI | |
|---|---|---|---|---|
| Number of decayed teeth | ||||
| Low family monthly income | 0.080** | 0.006 | 1.08 | 1.07–1.10 |
| High family monthly income | 0.127** | 0.015 | 1.14 | 1.10–1.17 |
| Number of missing teeth | ||||
| Low family monthly income | 0.104** | 0.018 | 1.11 | 1.07–1.23 |
| High family monthly income | 0.163** | 0.021 | 1.18 | 1.13–1.23 |
| Gingivitis§ | ||||
| Low family monthly income | 0.147** | 0.013 | 1.16 | 1.13–1.19 |
| High family monthly income | 0.221** | 0.024 | 1.25 | 1.19–1.31 |
| Malocclusion¶ | ||||
| Low family monthly income | 0.348* | 0.064 | 1.42 | 1.25–1.61 |
| High family monthly income | 0.662* | 0.118 | 1.94 | 1.54–2.44 |
P < 0.05 and **P ≤ 0.001 are considered significant.
All analyses were controlled for age and sex.
Oral Impacts on Daily Performance (OIDP) extent: number of oral impacts, ranging from 0 (no impact) to 9 (impact in all activities).
RR: rate ratio.
Number of sextants with bleeding on probing.
DAI score.
The SEM assessed the mediating effect of family income (mediator) between each oral clinical measure and OHRQoL (Table 5). Fit indices were SRMR = 0.030 for decayed teeth model; SRMR = 0.031 for missing teeth model; SRMR = 0.030 for gingivitis model; and SRMR = 0.038 for malocclusion model. Poor oral clinical measures were significantly associated with an increase in OIDP extent: decayed teeth, β = 0.241 (P < 0.001); missing teeth, β = 0.261 (P < 0.001); gingivitis, β = 0.197 (P < 0.001); and malocclusion, β = 0.165 (P < 0.001). When mediation was controlled, oral clinical measures were associated with OIDP extent: decayed teeth, β = 0.223 (P < 0.001); missing teeth, β = 0.257 (P < 0.001); gingivitis, β = 0.189 (P < 0.001); and malocclusion, β = 0.145 (P < 0.001; Table 5). The Sobel test indicated that the role of socioeconomic position as a mediator of the effect of oral clinical measure on OHRQoL was significant (decayed teeth, z = 4.94, P < 0.001; missing teeth, z = 4.69, P < 0.001; gingivitis, z = 4.64, P < 0.001; malocclusion, z = 4.18, P < 0.001). Because the four criteria were met and the standardised beta coefficient was reduced from 0.241 (without mediation) to 0.223 (with mediation controlled) for decayed teeth; from 0.261 to 0.257 for missing teeth; from 0.197 to 0.189 for gingivitis; and from 0.165 to 0.145 for malocclusion, this is evidence for a partial mediation effect of socioeconomic position between all oral clinical measures and OHRQoL. Thus, the effect of poor oral clinical conditions on OHRQoL was partially mediated by family income. The indirect effect of family income on the clinical measures–quality of life pathway was 0.018 for decayed teeth (‘without mediation’ minus ‘with mediation’), 0.004 for missing teeth, 0.008 for gingivitis, and 0.020 for malocclusion. This suggests that about 7% of the effect of decayed teeth on quality of life went through the mediator (socioeconomic position) and 93% (0.223/241) of the effect was direct. Similarly, 98% (0.257/0.261) of the effect of missing teeth, 96% of the effect of gingivitis (0.189/0.197) and 88% of the effect of malocclusion (0.145/0.165) on OHRQoL was direct, and 2%, 4% and 12% went through family income, respectively.
Table 5.
SEM for the effects of socioeconomic position on the oral clinical measures–OHRQoL relationship
| β† | Bootstrap SE‡ | Bias-corrected 95% CI§ | P-value | |
|---|---|---|---|---|
| Decayed teeth | ||||
| Decayed teeth – OHRQoL | 0.241 | 0.028 | 0.186, 0.296 | < 0.001* |
| Socioeconomic position – OHRQoL | −0.094 | 0.028 | −0.148, −0.040 | 0.001* |
| Decayed teeth – socioeconomic position | −0.197 | 0.022 | −0.240, −0.154 | < 0.001* |
| Decayed teeth – OHRQoL/socioeconomic position | 0.223 | 0.029 | 0.167, 0.279 | < 0.001* |
| Missing teeth | ||||
| Missing teeth – OHRQoL | 0.261 | 0.067 | 0.130, 0.391 | < 0.001* |
| Socioeconomic position – OHRQoL | −0.130 | 0.026 | −0.181, −0.080 | < 0.001* |
| Missing teeth – socioeconomic position | −0.031 | 0.042 | −0.114, 0.053 | 0.472 |
| Missing teeth – OHRQoL/socioeconomic position | 0.257 | 0.071 | 0.119, 0.396 | < 0.001* |
| Gingivitis | ||||
| Gingivitis – OHRQoL | 0.197 | 0.031 | 0.137, 0.258 | < 0.001* |
| Socioeconomic position – OHRQoL | −0.114 | 0.028 | −0.168, −0.060 | < 0.001* |
| Gingivitis – socioeconomic position | −0.113 | 0.028 | −0.167, −0.059 | < 0.001* |
| Gingivitis – OHRQoL/socioeconomic position | 0.189 | 0.031 | 0.127, 0.250 | < 0.001* |
| Malocclusion | ||||
| Malocclusion – OHRQoL | 0.165 | 0.031 | 0.105, 0.225 | < 0.001* |
| Socioeconomic position – OHRQoL | −0.123 | 0.024 | −0.170, −0.075 | < 0.001* |
| Malocclusion – socioeconomic position | −0.159 | 0.030 | −0.119, −0.059 | < 0.001* |
| Malocclusion – OHRQoL/socioeconomic position | 0.145 | 0.031 | 0.085, 0.206 | < 0.001* |
P < 0.05 is considered significant.
β = bootstrapped standardised estimate.
SE = standard error.
CI = confidence interval.
DISCUSSION
Our findings support the hypotheses that decayed teeth, missing teeth, gingivitis and malocclusion are associated with poor OHRQoL in individuals age 15–19 years. The results further uphold the hypotheses that the negative effects of worst oral clinical conditions on OHRQoL among adolescents are influenced by socioeconomic context. The influence of oral clinical conditions on OHRQoL is more intense in the high socioeconomic group. In addition, the effect of worst oral conditions on quality of life was partially mediated by socioeconomic position. These findings are consistent with the clinical oral measures investigated. To our knowledge, this is the first study to explore the mediation and moderation of socioeconomic position influences on the relationship between oral clinical conditions and OHRQoL in adolescents.
The result of the association between oral clinical conditions and OHRQoL is consistent with previous research in children6., 9., 10., 12., 14., 15., 22., adolescents8., 11., 13., 20., 21., 23. and adults7., 18., 19.. For example, Foster Page reported a direct relationship between malocclusion and poor OHRQoL in adolescents, and that OHRQoL was indirectly predicted by dental caries experience11. In addition, more decayed and missing teeth directly predicted an adolescent’s low OHRQoL functional status8. The other two previous studies involving oral clinical conditions and OHRQoL in adolescents also used the data from the Brazilian Oral Health Survey13., 20.. Although these studies have distinct objectives and adopted different statistical approaches, their findings also reported that untreated dental caries, missing teeth, gingivitis and malocclusion were associated with poor OHRQoL13., 20.. Other studies did not find poor oral health to be associated with OHRQoL in children41., 42. and adolescents8. The lack of association between oral clinical conditions and OHRQoL might be related to the low levels of oral diseases in the studied samples, the statistical power of the studies or the low discriminative ability of the OHRQoL instruments used.
Oral health-related quality of life has been associated with individual and environmental factors6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23.. Of them, socioeconomic position has been investigated as a relevant predictor as previous research consistently reports the link between low socioeconomic status and poor OHRQoL in children10., 15., 16., adolescents8., 13., 20. and adults7., 18., 19.. Socioeconomic position has been assessed as a predictor of OHRQoL according to the Wilson and Cleary model3, and tested through SEM7., 8., 15., 19., as an independent associated factor of OHRQoL using regression analysis10., 12., 13., 18., and as a confounder on the relationship between contextual determinants and OHRQoL assessed by multilevel modelling17., 20.. However, other studies on OHRQoL have not assessed participant’s or their family’s social status6., 9., 11., 14., 21., 22., 23.. The present study indicates that the role of environmental conditions in studies investigating the predictors of OHRQoL is not simple as family income was both moderator and mediator of the relationship between oral clinical measures and OHRQoL. The findings provide new insights and suggest that socioeconomic position may influence the extent and nature of the relationship between oral clinical measures and OHRQoL in adolescents.
The association between poor oral health and OHRQoL was consistently stronger in the high-income group when different oral clinical measures were tested. This finding was somewhat unexpected as there is a vast literature on the inverse relationship of socioeconomic status with oral health and OHRQoL7., 8., 10., 13., 15., 16., 18., 19., 20., 21., 22., 23., 24.. There are possible explanations for these findings. Adolescents from disadvantaged families, despite their poor oral health, may have not been attending dental services regularly as a consequence of low educational level and low health literacy43., 44.. As a consequence, they tend to be less aware of their oral problems and are less likely to perceive their oral condition as an issue for their OHRQoL compared with those from better-off families whose dental attendance is more frequent43., 44.. Socioeconomic position does appear to mediate the pathway from dental clinical measures and OHRQoL, even though dental clinical measures have stronger direct effects on OHRQoL.
The findings of the present study have important implications for dental care services, oral health promotion and public policies. Nonetheless, they should be interpreted taking into account the structure and organisation of the national healthcare system and the high levels of social inequalities in Brazil. Our results point out that the provision of oral healthcare aiming to improve adolescent’s dental clinical status and OHRQoL should consider that both large oral health inequalities, and large variations of income inequality between cities, existing in the country18. For instance, regions and cities with high levels of social inequalities and therefore with poor population oral health may experience a lower or similar demand for dental care services compared with those with low levels of oral diseases. This is because the considerable variation in oral healthcare needs between cities may be due to socioeconomic discrepancies rather than oral diseases disparities. However, this may be a fallacy resulting from the moderating effect of socioeconomic status between dental clinical status and OHRQoL as the latter is a reliable measure of oral health needs33., 34.. Improving access to primary dental care and oral health promotion actions to underprivileged population can reduce the social inequalities related to oral health disparities across socioeconomic groups. Nonetheless, the limitations of primary and secondary dental services availability in explaining dental treatment needs in Brazil have been acknowledged45. Adolescents living in deprived areas and those from low-income families are more likely to engage in damaging oral health-related behaviours (e.g. smoking, poor diet), which reinforces the importance of the social environment on shaping the oral health of the population. Thus, inter-sectoral public policies through collaborative efforts involving public health professionals and researchers, policy-makers, practitioners and the public are required to reduce oral health inequalities, and therefore enhance adolescent’s oral health status and OHRQoL at the population level.
Adolescents from wealthy families might notice a worst emotional state and face more social interaction problems due to dental aesthetic concerns, which in turn result in poorer OHRQoL. This suggests that there may be other individual and environmental aspects that are also important mediators and/or moderators of the relationship between oral clinical conditions and OHRQoL. For example, psychological characteristics, including mental health, self-esteem, propensity to somatisation and perceptions of body image, mediated the effect of decayed, missing or filled surfaces on OHRQoL in adolescents11. In addition, other psychological factors such as sense of coherence, self-esteem, dental coping beliefs and dental anxiety were directly and/or indirectly linked to OHRQoL7., 8., 15.. The mediating and/or moderating effect of psychological characteristics on the influence of physical health on health-related quality of life (HRQoL) was reported. Parent depressive symptoms and family structure moderated the effect of body mass index (BMI) on children’s HRQoL46. Body image and self-esteem mediated the relationship between BMI and HRQoL in adolescents47. Future research should examine psychological variables as moderators and mediators of the effects of clinical oral measures on OHRQoL.
Some limitations must be acknowledged. Firstly, demographic and socioeconomic characteristics, oral clinical measures and OHRQoL were obtained using a cross-sectional design, which limits possible causal inference. Secondly, the OIDP questionnaire is considered a distinct OHRQoL instrument that evaluates ultimate functional impacts on the ability to perform physical, psychological and social activities2. Thus, although OIDP considers relevant aspects of an individual’s performance influenced by oral conditions, the discriminative ability of OIDP is subject to criticism. Thirdly, monthly income was not adjusted for the number of people in the household. Fourthly, oral health-related behaviours and dental insurance were not considered in the analyses as these variables were not obtained in the Brazilian Oral Health Survey. Finally, our results cannot be generalised to adolescents from other age groups.
Our results suggest that socioeconomic position influences the strength and partially explains the impact of different oral conditions on OHRQoL in adolescents. The reduction of social inequalities and narrowing of income differentials seems particularly relevant to improve oral conditions and OHRQoL as well as to reduce the oral health gap between socioeconomic groups. Oral health policies and dental care planning could be more effective in the prevention and reduction of oral diseases and to improve OHRQoL through intersectoral actions aiming to alleviate social inequalities.
Acknowledgement
None.
Competing interest
The authors declare that they have no conflict of interest.
Funding statement
None.
References
- 1.Sischo L, Broder HL. Oral health-related quality of life: what, why, how, and future implications. J Dent Res. 2011;90:1,264–1,270. doi: 10.1177/0022034511399918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locker D, Allen F. What do measures of ‘oral health-related quality of life’ measure? Community Dent Oral Epidemiol. 2007;35:401–411. doi: 10.1111/j.1600-0528.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 4.Allen P. Assessment of oral health related quality of life. Health Qual Life Outcomes. 2003;1:40. doi: 10.1186/1477-7525-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick R, Fletcher A, Gore D, et al. Quality of life measures in health care. I: Application and issues in assessment. BMJ. 1992;305:1,074–1,077. doi: 10.1136/bmj.305.6861.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gherunpong S, Tsakos G, Sheiham A. The prevalence and severity of oral impacts on daily performances in Thai primary school children. Health Qual Life Outcomes. 2004;2:57. doi: 10.1186/1477-7525-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker SR. Applying Andersen’s behavioural model to oral health: what are the contextual factors shaping perceived oral health outcomes? Community Dent Oral Epidemiol. 2009;37:485–494. doi: 10.1111/j.1600-0528.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Baker SR, Matm A, Robinson PG. What psychosocial factors influence adolescents’ oral health? J Dent Res. 2010;89:1,230–1,235. doi: 10.1177/0022034510376650. [DOI] [PubMed] [Google Scholar]
- 9.Castro RA, Portela MC, Leão AT, et al. Oral health-related quality of life of 11- and 12-year-old public school children in Rio de Janeiro. Community Dent Oral Epidemiol. 2011;39:336–344. doi: 10.1111/j.1600-0528.2010.00601.x. [DOI] [PubMed] [Google Scholar]
- 10.Paula J, Leite I, Almeida A, et al. The influence of oral health conditions, socioeconomic status and home environment factors on schoolchildren’s self-perception of quality of life. Health Qual Life Outcomes. 2012;10:6. doi: 10.1186/1477-7525-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster Page LA, Thomson WM, Ukra A, et al. Clinical status in adolescents: is its impact on oral health-related quality of life influenced by psychological characteristics? Eur J Oral Sci. 2013;121:182–187. doi: 10.1111/eos.12034. [DOI] [PubMed] [Google Scholar]
- 12.Kramer PF, Feldens CA, Ferreira SH, et al. Exploring the impact of oral diseases and disorders on quality of life of preschool children. Community Dent Oral Epidemiol. 2013;4:327–335. doi: 10.1111/cdoe.12035. [DOI] [PubMed] [Google Scholar]
- 13.Peres KG, Cascaes AM, Leão AT, et al. Sociodemographic and clinical aspects of quality of life related to oral health in adolescents. Rev Saude Publica. 2013;47:19–28. doi: 10.1590/s0034-8910.2013047004361. [DOI] [PubMed] [Google Scholar]
- 14.Bakhtiar M, Mohammadi TM, Hajizamanim A, et al. Association of oral health indicators with quality-of-life related to oral health among Iranian adolescent. J Int Oral Health. 2014;6:5–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Gururatana O, Baker SR, Robinson PG. Determinants of children’s oral-health-related quality of life over time. Community Dent Oral Epidemiol. 2014;42:206–215. doi: 10.1111/cdoe.12080. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Kroon J, Lalloo R. A systematic review of the impact of parental socio-economic status and home environment characteristics on children’s oral health related quality of life. Health Qual Life Outcomes. 2014;12:41. doi: 10.1186/1477-7525-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarca GA, Leal MC, Leao ATT, et al. The different roles of neighbourhood and individual social capital on oral health-related quality of life during pregnancy and postpartum: a multilevel analysis. Community Dent Oral Epidemiol. 2014;42:139–150. doi: 10.1111/cdoe.12062. [DOI] [PubMed] [Google Scholar]
- 18.Vettore MV, Aqeeli A. The roles of contextual and individual social determinants of oral health-related quality of life in Brazilian adults. Qual Life Res. 2016;25:1,029–1,042. doi: 10.1007/s11136-015-1118-0. [DOI] [PubMed] [Google Scholar]
- 19.Rebelo MA, de Castro PH, Rebelo Vieira JM, et al. Low social position, periodontal disease, and poor oral health-related quality of life in adults with systemic arterial hypertension. J Periodontol. 2016;87:1,379–1,387. doi: 10.1902/jop.2016.160204. [DOI] [PubMed] [Google Scholar]
- 20.Alwadi MA, Vettore MV. Are school and home environmental characteristics associated with oral health-related quality of life in Brazilian adolescents and young adults? Community Dent Oral Epidemiol. 2017;45:356–364. doi: 10.1111/cdoe.12298. [DOI] [PubMed] [Google Scholar]
- 21.Bastos RS, Carvalho ES, Xavier A, et al. Dental caries related to quality of life in two Brazilian adolescent groups: a cross-sectional randomised study. Int Dent J. 2012;62:137–143. doi: 10.1111/j.1875-595X.2011.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisdapong S, Prasertsom P, Rattanarangsima K, et al. Using associations between oral diseases and oral health-related quality of life in a nationally representative sample to propose oral health goals for 12-year-old children in Thailand. Int Dent J. 2012;62:320–330. doi: 10.1111/j.1875-595x.2012.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitas AR, Aznar FD, Tinós AM, et al. Association between dental caries activity, quality of life and obesity in Brazilian adolescents. Int Dent J. 2014;64:318–323. doi: 10.1111/idj.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locker D. Deprivation and oral health: a review. Community Dent Oral Epidemiol. 2000;28:161–169. doi: 10.1034/j.1600-0528.2000.280301.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwendicke F, Dörfer CE, Schlattmann P, et al. Socioeconomic inequality and caries: a systematic review and meta-analysis. J Dent Res. 2015;94:10–18. doi: 10.1177/0022034514557546. [DOI] [PubMed] [Google Scholar]
- 26.Skinner J, Johnson G, Blinkhorn A, et al. Factors associated with dental caries experience and oral health status among New South Wales adolescents. Aust N Z J Public Health. 2014;38:485–489. doi: 10.1111/1753-6405.12245. [DOI] [PubMed] [Google Scholar]
- 27.Tomazoni F, Vettore MV, Zanatta FB, et al. The associations of socioeconomic status and social capital with gingival bleeding among schoolchildren. J Public Health Dent. 2017;77:21–29. doi: 10.1111/jphd.12166. [DOI] [PubMed] [Google Scholar]
- 28.Fontanini H, Marshman Z, Vettore M. Social support and social network as intermediary social determinants of dental caries in adolescents. Community Dent Oral Epidemiol. 2015;43:172–182. doi: 10.1111/cdoe.12139. [DOI] [PubMed] [Google Scholar]
- 29.de Araújo Teixeira Silva C, Rebelo Vieira JM, Rebelo MA, et al. The association between participation of adolescents in community groups and dental caries in a deprived area in Brazil. Caries Res. 2015;49:540–547. doi: 10.1159/000438726. [DOI] [PubMed] [Google Scholar]
- 30.Roncalli AG, Silva NN, Nascimento AC, et al. Relevant methodological issues from the SBBrasil 2010 Project for national health surveys. Cad Saude Publica. 2012;28:S40–S57. doi: 10.1590/s0102-311x2012001300006. (in Portuguese) [DOI] [PubMed] [Google Scholar]
- 31.Silva NN, Roncalli AG. Sampling plan, weighting process and design effects of the Brazilian Oral Health Survey. Rev Saude Publica. 2013;47:3–11. doi: 10.1590/s0034-8910.2013047004362. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 1997. Oral Health Surveys. Basic Methods. [Google Scholar]
- 33.Adulyanon S, Vourapukjaru J, Sheiham A. Oral impacts affecting daily performance in a low dental disease Thai population. Community Dent Oral Epidemiol. 1996;24:385–389. doi: 10.1111/j.1600-0528.1996.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 34.Castro RAL, Cortes MI, Leão AT, et al. Child-OIDP index in Brazil: cross-cultural adaptation and validation. Health Qual Life Outcomes. 2008;6:68. doi: 10.1186/1477-7525-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch J, Kaplan G. In: Social Epidemiology. Berkman LF, Kawachi I, editors. Oxford University Press; New York: 2003. Socioeconomic position; pp. 13–35. [Google Scholar]
- 36.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;6:1,173–1,182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 37.Kline RB. The Guildford Press; New York: 2005. Principles and Practice of Structural Equation Modeling. [Google Scholar]
- 38.Sobel M. Some new results on indirect effects and their standard errors in covariance structure models. Sociol Methodol. 1986;16:159–186. [Google Scholar]
- 39.MacKinnon DP, Lockwood CM, Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 41.Marshman Z, Rodd H, Stern M, et al. An evaluation of the Child Perceptions Questionnaire in the UK. Community Dent Health. 2005;22:151–155. [PubMed] [Google Scholar]
- 42.Gururatana O, Baker S, Robinson PG. Psychometric properties of long and short forms of the Child Perceptions Questionnaire (CPQ11-14) in a Thai population. Community Dent Health. 2011;28:232–237. [PubMed] [Google Scholar]
- 43.Araújo CS, Lima Rda C, Peres MA, et al. Use of dental services and associated factors: a population-based study in southern Brazil. Cad Saude Publica. 2009;25:1,063–1,072. doi: 10.1590/s0102-311x2009000500013. [DOI] [PubMed] [Google Scholar]
- 44.Broadbent JM, Zeng J, Foster Page LA, et al. Oral health-related beliefs, behaviors, and outcomes through the life course. J Dent Res. 2016;95:808–813. doi: 10.1177/0022034516634663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roncalli AG, Tsakos G, Sheiham A, et al. Social determinants of dental treatment needs in Brazilian adults. BMC Public Health. 2014;14:1,097. doi: 10.1186/1471-2458-14-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YP, Chang MY, Chi YL, et al. Health-related quality of life in father of children with or without development disability: the mediating effect of parental stress. Qual Life Res. 2014;23:175–183. doi: 10.1007/s11136-013-0469-7. [DOI] [PubMed] [Google Scholar]
- 47.Kolodziejczyk JK, Gutzmer K, Wright SM, et al. Influence of specific individual and environmental variables on the relationship between body mass index and health-related quality of life in overweight and obese adolescents. Qual Life Res. 2015;24:251–261. doi: 10.1007/s11136-014-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]