Abstract
Aims: The objective was to investigate if gender differences exist in the associations between periodontitis and type 2 diabetes. Disproportionate disparities by gender were found to exist in rates of both periodontitis and diabetes with respect to demographics and behavioural predictors that cannot be explained solely by the well-established association between these two diseases. Materials and methods: Multiple datasets were extracted from the National Health and Nutrition Examination Survey (NHANES) 2009–2014, which used a stratified multistage probability sampling to obtain samples from all civilian non-institutionalised people in the USA. Bivariate relationships between each explanatory variable and periodontitis level were assessed with odds ratios (OR) and their 95% confidence intervals (CI). A set of weighted logistic regression models was used to investigate the association differentiations between periodontitis and diabetes by gender. C-statistics measured the goodness-of-fit of weighted logistic regression models. Results: The prevalence of moderate–severe periodontitis was 36.39% and 22.71% among participants with type 2 diabetes and without diabetes, respectively. Type 2 diabetes was significantly associated with moderate–severe periodontitis OR (OR = 1.47, 95% CI: 1.18–1.82) among males even after adjusting for demographics, socioeconomic status and oral health behaviours. The aforementioned relationship was not found in females. Furthermore, different relationships of moderate–severe periodontitis with body mass index and the use of mouthwash were found between the males and females. Conclusions: The current findings suggest that important improvements in the development of gender-specific strategies in prevention, such as oral home-care, to reduce the high prevalence of periodontal disease and maintain good oral health are vital, and are especially important for male diabetic patients and those who are at high risk of developing diabetes, such as those who are obese.
Key words: Oral health behaviours, disproportionate disparities, oral home-care, prevalence, association, periodontal disease
Introduction
Periodontitis and type 2 diabetes are both highly prevalent chronic diseases whose rates are increasing among US adults1., 2.. Periodontitis, mediated by microorganisms, can lead to the irreversible destruction of connective tissue and alveolar bone. As periodontitis progresses, it can result in increased mobility or loss of teeth, compromised orofacial esthetics, and ultimately adversely affect the quality of life. Periodontitis is common, with prevalence rates in the USA of 46% for total periodontitis (mild or moderate or severe periodontitis) and 8.9% for severe periodontitis1. Worldwide, severe periodontitis was the sixth most prevalent disease in 2010, and resulted in large economic burdens for the afflicted and society2. The reduction of moderate and severe periodontitis among American adults is one of the important objectives of Healthy People 2020 and a strategic plan of the US Center for Disease Control and Prevention (CDC) for 2011–20143., 4.. Diabetes is characterised by elevated blood sugar and a set of metabolic disorders. As diabetes progresses it can cause complications, including heart disease, kidney disease and vision loss. Diabetes was the seventh leading cause of mortality in the USA in 20135 and it resulted in an economic burden of more than $322 billion due to medical costs and lost productivity in 20126. The prevalence of diabetes has increased in recent years, and disparities in diabetes rates, including gender differences, still exist among American adults7.
The relationship between periodontitis and diabetes has been a research interest in recent decades. Epidemiological studies have shown that these two conditions are bi-directionally associated. Specifically, diabetes negatively alters the healing and the immune/inflammatory response of the patient and in that way exacerbates/facilitates the destructive disease process, and diabetic individuals are three times more likely to have periodontitis than non-diabetic individuals8. Further, periodontitis has been considered as the sixth complication of diabetes9. On the other hand, treatment of periodontitis can effectively improve metabolic control, such as the control of blood sugar in type 2 diabetes10.
Disparities in rates of periodontal disease have existed for a long time in US adults1., 11.. In particular, there is a disproportionate prevalence of periodontitis in males versus females. Males have continuously tended to carry a considerably larger burden of periodontitis than their female counterparts over several decades in the USA1., 11.. The periodontitis rates for US males and females were 54.9% and 37.4%, respectively, from 2009 to 20121. This phenomenon has also existed in Japan, the Philippines and Brazil12. Recent studies have also found gender differences in other oral health issues, including tooth loss and self-reported oral health status13., 14.. Furthermore, gender differences have also been found in the relationship of periodontal disease to metabolic syndrome and atherosclerosis12., 13..
Previous studies have already identified gender differences in rates of periodontal disease and diabetes, and the relationship between periodontal disease and diabetes has been well established8. To the best of our knowledge, no study has yet examined gender differences in the relationship between periodontitis and diabetes and applied stratified analyses. To fill this gap, the aim of this study is to investigate the existence and nature of gender differences in the association between periodontitis and type 2 diabetes in US adults. The anticipated outcomes of this study are foundational evidence for future customised preventive and therapeutic approaches to improve human health.
Materials and methods
Multiple datasets were extracted from the National Health and Nutrition Examination Survey (NHANES) 2009–2014. NHANES used a stratified multistage probability sampling to obtain samples from all civilian non-institutionalised people in the USA. Oversampling was applied to different subpopulations to improve estimation accuracy. Asian Americans were oversampled in 2011–2014. This current study included dentate participants aged ≥ 30 years who had completed periodontal examinations, body measures, laboratory tests for diabetes, and interviews for demographics and other related health issues. The US National Center for Health Statistics (NCHS) research Ethics Review Board (ERB) approved the study [NCHS IRB/ERB Protocol # 2005–2006 (used for 2009–2010) and #2011–2017 (used for 2011–2012 and 2013–2014)].
Moderate–severe periodontitis
Oral examinations, which took place in mobile examination centres, were conducted by health technologists who had received an intense period of training. Third molars were not evaluated for periodontal condition. Full-mouth periodontal examinations (FMPEs) executed by dental hygienists (2009–2010) and dentists (2011–2014) licensed in at least one US state, where gingival recession and periodontal pocket probing depths (PPDs) were measured at six sites per tooth in dentate individuals, were only started from 2009 to 2010. Inclusion and exclusion criteria, periodontal measurements, and definitions of no, mild, moderate and severe periodontitis were the same as previously described by Eke et al.15 Due to different clinical treatments being needed for mild periodontitis and moderate and severe periodontitis, the ‘moderate–severe’ designation used in the current study combined the moderate and severe conditions. Specifically, individuals with moderate–severe periodontitis included those with ≥ 2 interproximal sites not on the same tooth with PPD ≥ 5 mm or clinical attachment loss (PPD minus gingival recession measurement) ≥ 4 mm.
Type 2 diabetes
Type 1 and type 2 diabetes were not distinguished in NHANES. However, this study excluded those with type 1 diabetes with a high probability by excluding participants who were diagnosed with diabetes at age < 25 years and who were being treated with insulin8., 14., 16.. Three laboratory measures of diabetes, including glycated haemoglobin A1c in blood, fasting plasma glucose (FPG) and a 2-hour plasma glucose (PG), were consecutively collected in three biennial surveys from NHANES 2009 to 2014. A participant having type 2 diabetes was defined by having at least one of four conditions: (i) haemoglobin A1c ≥ 6.5%; (ii) FPG ≥ 126 mg/dl; (iii) PG ≥ 200 mg/dl; (iv) diagnosed with diabetes by a doctor or other health professional7. Non-diabetic individuals had none of the above four conditions.
Oral health behaviours and other related health variables
Two oral heath behaviours were initially documented in NHANES from 2009 to 2010 as dichotomous variables (yes or no). Use of dental floss was represented by two categories: yes (1–7 days) and no (0 days) in response to the question “aside from brushing teeth with a toothbrush, in the last 7 days, how many days did you floss or use any device to clean between your teeth?” Similarly, mouthwash use was represented by two categories: yes (1–7 days) and no (0 days) in response to the question “aside from brushing teeth with a toothbrush, in the last 7 days, how many days did you use mouthwash or other dental rinse product to treat dental disease or dental problems”. Body height and body weight were collected to calculate body mass index (BMI). According to BMI (kg/m2), individuals were categorised into two groups: BMI < 30 and BMI ≥ 30. Participants self-reported whether they were covered by health insurance or not.
Demographics and socioeconomic status (SES)
Demographic variables included three self-reported variables: age (years); gender (male, female); and ethnicity (Hispanic, White, Black and other race). Age was stratified as 30–44, 45–59 and ≥ 60 years. SES included family poverty and education levels. According to federal poverty level (FPL) guidelines, poverty level (PL) was categorised into three groups: PL < 100% FPL; 100% FPL ≤ PL < 300% FPL; and PL ≥ 300% FPL. Education had three categories: < 12; 12; and > 12 years of education.
Statistical analysis
First, the distribution of all explanatory variables stratified by moderate–severe periodontitis was assessed. Bivariate relationships between each explanatory variable and periodontitis level (moderate–severe) were assessed with odds ratios (ORs) and their 95% confidence intervals (CIs). Statistically significant interactions between gender and other explanatory variables were found. To further explore these results, a series of weighted logistic regression models was carried out and analysed separately by gender. Model 1 only contained the categorical variable diabetes and demographics including age and race/ethnicity. Model 2 additionally adjusted for poverty and education levels. The third model additionally adjusted for BMI, health insurance and dental behaviours, in order to assess whether the relationship between moderate–severe periodontitis and type 2 diabetes remained significant or not. ORs and 95% CIs were constructed. A 6-year sample weighted variable was created by combining three biennial national surveys in the analysis. C-statistics, equivalent to areas under receiver operating characteristic curves, were used to measure the goodness-of-fit of weighted logistic regression models. Generally, a value of C > 0.7 indicates a good model. All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.4; Cary, NC, USA). A P-value < 0.05 was interpreted as indicating statistical significance.
Results
The current study included 10,605 adults aged ≥ 30 years who had completed FMPEs and diabetes testing in NHANES 2009–2014. Participants had a mean age (SE) of 53.27 (0.14) years. The overall prevalence of moderate–severe periodontitis was 24.73%. The prevalence of moderate–severe periodontitis for each category of exploratory variable was summarised in Table 1. The prevalence of moderate–severe periodontitis was 36.39% and 22.71% among participants with type 2 diabetes and without diabetes, respectively. Obvious disparities in rates of moderate–severe periodontitis existed in the years 2009–2014 among American adults. Specifically, higher prevalence rates of moderate–severe periodontitis existed in older (≥ 60 years), male and non-white adults, and those who had lower SES status and no health insurance.
Table 1.
Weighted prevalence (%) of moderate–severe periodontitis from 2009 to 2014 among US adults grouped by exploratory variables
| Number | Prevalence (%) | 95% CI of prevalence | |
|---|---|---|---|
| Total | 10,605 | 24.73 | 23.70–25.75 |
| Type 2 diabetes | |||
| Yes | 1983 | 36.85 | 34.00–39.70 |
| No | 8246 | 22.79 | 21.68–23.90 |
| Age (years) | |||
| 30–44 | 3538 | 13.44 | 12.15–14.73 |
| 45–60 | 3165 | 28.43 | 26.43–30.43 |
| ≥ 60 | 3706 | 34.31 | 32.30–36.32 |
| Gender | |||
| Male | 5166 | 30.92 | 29.28–32.56 |
| Female | 5243 | 18.99 | 17.73–20.25 |
| Race/ethnicity | |||
| White | 4702 | 21.56 | 20.22–22.91 |
| Black | 2188 | 34.7 | 32.58–36.81 |
| Hispanic | 2301 | 31.76 | 29.66–33.86 |
| Other | 1218 | 30.56 | 27.05–34.07 |
| PL | |||
| ≥ 300% FPL | 4116 | 18.24 | 16.82–19.67 |
| < 100% FPL | 2099 | 36.64 | 34.13–39.14 |
| 100% FPL ≤ PL < 300% FPL | 4194 | 30.57 | 28.77–32.37 |
| Education | |||
| > 12 years | 5620 | 18.49 | 17.26–19.73 |
| 12 years | 2283 | 32 | 29.51–34.49 |
| < 12 years | 2506 | 40.17 | 37.70–42.64 |
| Health insurance | |||
| Yes | 8210 | 22.44 | 21.33–23.56 |
| No | 2199 | 36.57 | 33.96–39.19 |
| BMI (kg/m2) | |||
| < 30 | 6379 | 24.98 | 23.66–26.30 |
| ≥ 30 | 4030 | 24.6 | 22.91–26.29 |
| Mouthwash use | |||
| Yes | 5932 | 26.3 | 24.87–27.72 |
| No | 4477 | 23.15 | 21.63–24.67 |
| Dental flossing | |||
| Yes | 6629 | 22.79 | 21.54–24.05 |
| No | 3780 | 29.31 | 27.46–31.16 |
BMI, body mass index; CI, confidence interval; FPL, federal poverty level; PL, poverty level.
Bivariate association analyses indicated all explanatory variables except BMI were significantly associated with moderate–severe periodontitis (all P < 0.0001) using weighted simple logistic regression (Table 2). Type 2 diabetes was significantly associated with moderate–severe periodontitis, OR (OR = 1.45, 95% CI: 1.17–1.79) among males even after adjusting for SES and insurance and BMI (Table 3). On the contrary, no significant association was found between type 2 diabetes and moderate–severe periodontitis among females. Gender differences were also seen in BMI and mouthwash use. BMI was negatively associated with moderate–severe periodontitis in females (OR = 0.81, 95% CI: 0.65–0.94), and mouthwash use was significant in males (OR = 0.82, 95% CI: 0.69–0.97). Both C-statistics (C > 0.7) indicated that final models for males and females were good for predicting the odds of moderate–severe periodontitis.
Table 2.
Bivariate association between moderate–severe periodontitis in US adults from 2009 to 2014 and exploratory variables
| OR | 95% CI | P-value | |
|---|---|---|---|
| Total | |||
| Type 2 diabetes | 1 | ||
| No | |||
| Yes | 1.98 | 1.72–2.27 | < 0.0001 |
| Age (years) | |||
| 30–44 | 1 | ||
| 45–60 | 2.55 | 2.2–2.96 | < 0.0001 |
| ≥ 60 | 3.36 | 2.91–3.88 | < 0.0001 |
| Gender | |||
| Male | 1 | ||
| Female | 0.52 | 0.46–0.58 | < 0.0001 |
| Race/ethnicity | |||
| White | 1 | ||
| Black | 1.93 | 1.71–2.18 | < 0.0001 |
| Hispanic | 1.69 | 1.49–1.92 | < 0.0001 |
| Other | 1.6 | 1.33–1.92 | < 0.0001 |
| PL | |||
| ≥ 300% FPL | 1 | ||
| < 100% FPL | 2.59 | 1.74–2.24 | < 0.0001 |
| 100% FPL ≤ PL < 300% FPL | 1.97 | 1.72–2.22 | < 0.0001 |
| Education | |||
| > 12 years | 1 | ||
| < 12 years | 2.907 | 2.55–3.31 | < 0.0001 |
| 12 years | 2.07 | 1.80–2.38 | < 0.0001 |
| Insurance | |||
| Yes | 1 | ||
| No | 1.99 | 1.75–2.27 | < 0.0001 |
| BMI (kg/m2) | |||
| < 30 | 1 | ||
| ≥ 30 | 0.98 | 0.87–1.10 | 0.6913 |
| Mouthwash use | |||
| Yes | 1 | ||
| No | 0.84 | 0.75–0.95 | 0.0032 |
| Dental flossing | |||
| Yes | |||
| No | 1.4 | 1.25–1.57 | < 0.0001 |
OR and P-values were obtained from simple weighted logistic regressions for bivariate association.
BMI, body mass index; CI, confidence interval; FPL, federal poverty level; OR, odds ratio; PL, poverty level.
Table 3.
ORs of gender differences in the relationship between type 2 diabetes and moderate–severe periodontitis in US adults from 2009 to 2014
| Model I | Model II | Model III | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Type 2 diabetes | ||||||
| Yes versus No | 1.47 (1.18–1.82)*** | 1.19 (0.96–1.48) | 1.22 (1.09–1.50)* | 0.90 (0.74–1.10) | 1.46 (1.16–1.83)*** | 1.10 (0.88–1.38) |
| Age (years) | ||||||
| 30–44 | ||||||
| 45–60 | 2.68 (2.18–3.29)*** | 3.12 (2.49–3.90)*** | 2.64 (2.18–3.20)*** | 2.78 (2.32–3.33)*** | 3.06 (2.46–3.80)*** | 3.53 (2.78–4.46)*** |
| ≥ 60 | 3.616 (2.91–4.86)*** | 4.58 (3.65–5.74)*** | 3.21 (2.61–3.95)*** | 3.91 (3.24–4.71)*** | 4.57 (3.60–5.80)*** | 5.11 (3.99–6.54)*** |
| Race/ethnicity | ||||||
| White | ||||||
| Black | 2.48 (2.07–2.97)*** | 2.11 (1.74–2.56)*** | 1.99 (1.66–2.38)*** | 1.82 (1.53–2.16)*** | 1.79 (1.48–2.17)*** | 1.71 (1.41–2.10)*** |
| Hispanic | 2.40 (1.99–2.90)*** | 2.18 (1.80–2.66)*** | 2.04 (1.68–2.49)*** | 2.08 (1.73–2.49)*** | 1.38 (1.12–1.70)*** | 1.37 (1.10–1.69)** |
| Other | 1.85 (1.42–2.40)*** | 2.11 (1.59–2.80)*** | 1.77 (1.36–2.29)*** | 2.30 (1.81–2.90)*** | 1.77 (1.34–2.33)*** | 1.99 (1.49–2.66)*** |
| PL | ||||||
| ≥ 300% FPL | ||||||
| < 100% FPL | 1.97 (1.56–2.51)*** | 1.71 (1.38–2.11)*** | 1.72 (1.32–2.23)*** | 2.16 (1.66–2.80)*** | ||
| 100% FPL ≤ PL < 300% FPL | 1.50 (1.25–1.79)*** | 1.52 (1.28–1.81)*** | 1.41 (1.14–1.73)*** | 1.85 (1.47–2.33)*** | ||
| Education | ||||||
| > 12 years | ||||||
| < 12 years | 1.45 (1.16–1.82)*** | 1.23 (0.99–1.50) | 2.07 (1.64–2.62)*** | 1.64 (1.30–2.08)*** | ||
| 12 years | 1.32 (1.08–1.63)* | 1.37 (1.12–1.67)* | 1.78 (1.43–2.22)*** | 1.56 (1.24–1.97)*** | ||
| Health insurance | ||||||
| No versus Yes | 1.99 (1.57–251)*** | 1.84 (1.46–2.33)*** | ||||
| BMI (kg/m2) | ||||||
| ≥ 30 versus < 30 | 1.01 (0.85–1.21) | 0.82 (0.68–0.98)* | ||||
| Mouthwash use | ||||||
| No versus Yes | 0.82 (0.69–0.97)* | 0.99 (0.83–1.20) | ||||
| Dental flossing | ||||||
| No versus Yes | 0.89 (0.74–1.06) | 0.87 (0.71–1.06) | ||||
| C-statistic | 0.676 | 0.667 | 0.673 | 0.671 | 0.713 | 0.71 |
Model I included diabetes status and adjusted for demographics (age, race/ethnicity).
Model II contained model I and additionally adjusted for SES (education and PL).
Model III contained model II and additionally adjusted for BMI, health insurance and oral health behaviours (mouthwash use and dental flossing).
P < 0.05; **P < 0.001; ***P < 0.0001.
BMI, body mass index; FPL, federal poverty level; PL, poverty level.
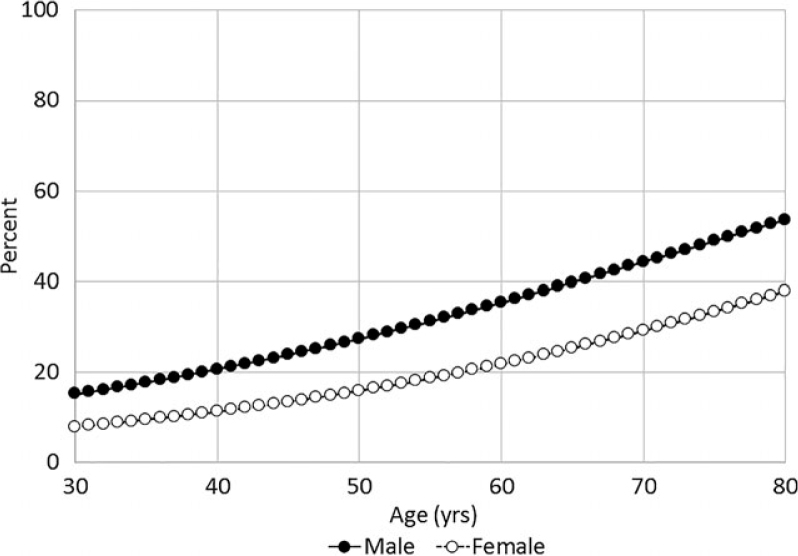
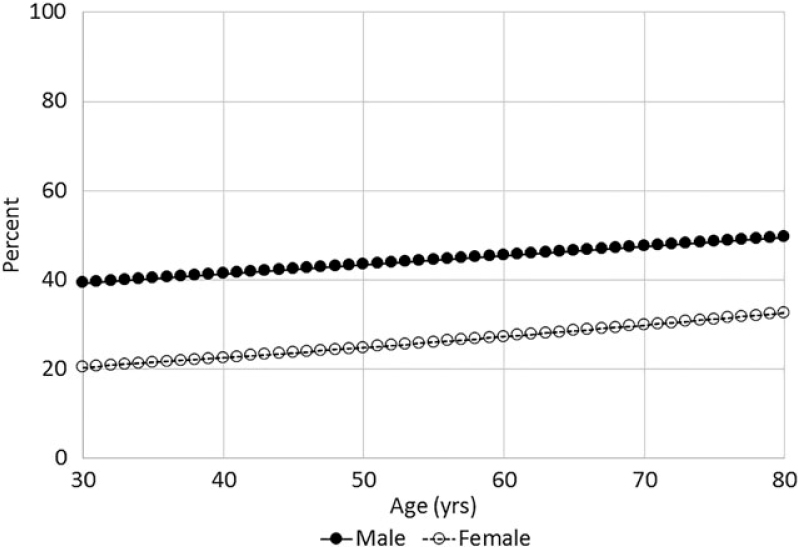
The estimated probability of having moderate–severe periodontitis was calculated from weighted logistic regressions. A larger increasing trend for estimation proportions of moderate–severe periodontitis was seen among adults who had no diabetes compared with those with diabetes (Figures 1 and 2). The prevalence of moderate–severe periodontitis was higher among males compared with their female peers regardless of diabetes status and age (Figures 1 and 2).
Figure 1.
Estimated probability of moderate–severe periodontitis among non-diabetic individuals by gender over age (years).
Figure 2.
Estimated probability of moderate–severe periodontitis among diabetic individuals by gender over age (years).
Discussion
In this study, the disparities in rates of moderate–severe periodontitis exist when people were classified by diabetes status, age, gender, SES and health insurance in the representative sample of US adults used here. More specifically, higher prevalence rates of moderate–severe periodontitis existed in diabetic (36.39%), older (33.93%) and male individuals (30.83%), and those with low income (PL < 100 FPL: 36.41%), less education (< 12 years: 39.62%) and without health insurance (36.45%). All exploratory variables except BMI status were significantly associated with moderate–severe periodontitis in the bivariate analyses (all P < 0.0001).
Previous studies in US adults have reported that the prevalence of total diabetes is higher in males than in females by only 1.6% in NHANES 2011–2012. However, the prevalence of moderate–severe periodontitis in US adults is much higher in males than in females, for example, 11.95% higher in NHANES 2009–2014. Weighted logistic regression analyses, stratified by gender, identified the gender-dependent association between type 2 diabetes and moderate–severe periodontitis. That is, the above-mentioned association only existed in males and not in females after adjustments for demographics, SES status and dental behaviours.
Like previous studies1., 17., the current study confirmed that demographics (age and race/ethnicity) and SES status (education, PL and health insurance) were significantly associated with moderate–severe periodontitis. Interestingly, mouthwash use was negatively associated with moderate–severe periodontitis in males. Possible explanations for this are that male individuals with periodontitis are more likely to use mouthwash than those without periodontitis, and the prevalence for moderate–severe periodontitis is higher in males than females. It is worth noting that mouthwash may reduce surface inflammation but is not an influential factor for periodontitis, as it does not reach the bottom of the pocket.
Obesity is associated with periodontal disease18., 19. and systemic inflammation20. Obese individuals were more likely to have type 2 diabetes, even though some of them were metabolically healthy21. Previous studies have indicated that obesity and type 2 diabetes are highly correlated, and it is usually accepted that both of these conditions contribute to the occurrence and progression of periodontitis22. Interestingly, in the current study, almost the same prevalence of moderate–severe periodontitis was found in individuals with BMI ≥ 30 (24.89%, 95% CI: 23.58–26.20) and individuals with BMI < 30 (24.45%, 95% CI: 22.79–26.13), and the bivariate association test indicated no significant association between BMI and moderate–severe periodontitis (P = 0.6913). Furthermore, weighted multiple logistic regression analysis confirmed that this non-significant association only existed in males with adjustments for diabetes, demographics and SES. However, a negative association between BMI and moderate–severe periodontitis was found in females with OR 0.82 (95% CI: 0.68–0.98). The periodontal literature reports that female sex hormones exaggerate the inflammatory response to plaque, which would not explain the findings in this study23., 24.. In short, the factors that influence the relationship between moderate–severe periodontitis and type 2 diabetes need further exploration, and the underlying mechanisms that potentially link periodontitis, obesity and diabetes merit further investigation and could reveal other clinically useful metabolic measures22.
It is known that periodontitis is not uniformly distributed in the mouth. FMPEs are the gold-standard and provide optimised clinical measures at six sites per tooth in NHANES 2009–2014. Therefore, we expected that data from FMPEs used in the current study provided a more accurate estimation of periodontitis prevalence compared with partial-mouth periodontal examinations used in previous NHANES. As the CDC had applied FMPE beginning in 20091, the gender-based association between diabetes and moderate–severe periodontitis was initially assessed with a nationwide survey using FMPE. Another important advantage of this current study is that NHANES 2009–2014 collected data on dental hygiene behaviours, including mouthwash use and dental flossing, which are influential factors for periodontitis and should be considered by all investigations of the relationship between periodontitis and diabetes. Due to the limitation of this survey, this study could not identify the brand or type of mouthwashs even though there are a variety of them on market. Stratified analyses by gender overcome the shortcoming of overall analysis by making the actual gender difference more salient and accurate. Having used combined data from three biennial NHANES surveys (2009–2010, 2011–2012 and 2013–2014), we expect that our results are more robust in representing the true status of the US population, especially for small subgroups.
Even though this study used a nationally representative health survey to assess gender-based differences in the links between diabetes and moderate–severe periodontitis, the nature of any cross-sectional study limited us to only provide association evidence rather than causal inferences. FMPEs excluded thirds molars in NHANES 2009–2014; thus, the periodontal status of third molars was unknown in this study. The gender differences in the association of diabetes and periodontitis uncovered here potentially advance the understanding of the importance of dental hygiene and self-management of diabetes, which were not the scope of this study. Repeated measurements are recommended by the American Diabetes Association after one positive test of haemoglobin A1c, FPG or 2-hour PG test is detected, but most participants in NHANES had only one study visit, which could increase the odds of misclassification of type 2 diabetes1., 25.. Missing values created in the processes of home interviews, laboratory tests or examinations, which might have otherwise produced some additional uncertainty in our results, were excluded in the data-cleaning step of the current study for more efficient statistical analyses.
There is ample evidence that individuals with diabetes are at high risk of developing moderate–severe periodontitis, and that the prevalence of periodontitis is much higher in diabetic individuals than those without diabetes (12.5% vs. 6.3%)26., 27.. Clinically, periodontitis can be successfully treated in diabetic patients. However, recurrence of periodontitis is more frequent in diabetic patients than non-diabetic patients due to higher levels of blood sugar in the former compared with the latter group27. Therefore, the self-management of diabetes and healthy dental hygiene habits are essential for diabetic patients to be able to control periodontal disease.
In conclusion, dental providers need not only be aware of whether their patients have diabetes or not, but also need to use gender-based approaches for those patients who are diabetic and obese as females generally have healthier behaviours in oral hygiene compared with their male peers28; therefore, it is reasonable to speculate that females are more likely to comply with the dentist and physician than males. The prevalence of diabetes and prediabetes has increased recently in the USA. The greatest of these increases was the finding that more than one-third of diabetic patients were not previously diagnosed, especially in the Asian and Hispanic populations7. The abovementioned situations highlight the needs and challenges faced by oral health professionals. Findings from the current study support the heightened importance of both self-management of diabetes and home oral hygiene to prevent or slow the progress of periodontal disease, especially in males with diabetes. Also, the findings of this study provide a baseline for further studies focusing on periodontitis among diabetic patients.
Acknowledgements
This study was support by East Tennessee State University Research Development Committee major grant RDC#18-003M. The authors thank the US Centers for Disease Control and Prevention, National Center for Health Statistics, for providing the NHANES 2009–2014 data.
Conflict of interest
None.
References
- 1.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Healthy people 2020; 2010. Available from: http://www.healthypeople.gov/2020/about/. Accessed 18 August 2017.
- 4.CDC. CDC’s oral health programs: strategic planning for 2011–2014 overview 2011. Available from: https://www.cdc.gov/oralhealth/strategic_planning/toc.htm. Accessed 18 August 2017.
- 5.CDC. At a glance 2016 diabetes working to reverse the US epidemic; 2016. Available from: https://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2016/diabetes-aag.pdf. Accessed 10 August 2017.
- 6.American Diabetes Association. The staggering cost of diabetes; 2014. Available from: http://www.diabetes.org/newsroom/press-releases/2014/. Accessed 18 August 2017.
- 7.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 8.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two way relationship. Br Dent J. 2014;217:433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 9.Löe H. Periondontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 10.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrell LN, Talih M. Examining periodontal disease disparities among U.S. adults 20 years of age and older: NHANES III (1988–1994) and NHANES 1999–2004. Public Health Rep. 2012;127:497–506. doi: 10.1177/003335491212700505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta M, Shimazaki Y, Takeshita T, et al. Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama Study. J Clin Periodontol. 2013;40:743–752. doi: 10.1111/jcpe.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desvarieux M, Schwahn C, Völzke H, et al. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35:2029–2035. doi: 10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wang K, Maisonet M, et al. Associations of lifestyle factors (smoking, alcohol consumption, diet and physical activity) with type 2 diabetes among American adults from National Health and Nutrition Examination Survey (NHANES) 2005–2014. J Diabetes. 2016;9:846–854. doi: 10.1111/1753-0407.12492. [DOI] [PubMed] [Google Scholar]
- 15.Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Malarcher AM, Herman WH, et al. Diabetes mellitus and cigarette smoking. Findings from the 1989 National Health Interview Survey. Diabetes Care. 1994;17:688–692. doi: 10.2337/diacare.17.7.688. [DOI] [PubMed] [Google Scholar]
- 17.Elani HW, Haprer S, Allison PJ, et al. Socio-economic inequalities and oral health in Canada and the United States. J Dent Res. 2012;91:865–870. doi: 10.1177/0022034512455062. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Shimazaki Y, Kiyohara Y, et al. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: the Hisayama study. J Periodontal Res. 2005;40:346–353. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento GG, Peres KG, Mittinty MN, et al. Obesity and periodontal outcomes: a population-based cohort study in Brazil. J Periodontol. 2017;88:50–58. doi: 10.1902/jop.2016.160361. [DOI] [PubMed] [Google Scholar]
- 20.Thorand B, Löwel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003;163:93–99. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Camhi SM, Whitney Evans E, Hayman LL, et al. Healthy eating index and metabolically healthy obesity in U.S. adolescents and adults. Prev Med. 2015;77:23–27. doi: 10.1016/j.ypmed.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhu M, Nikolajczyk BS. Immune cells link obesity-associated type 2 diabetes and periodontitis. J Dent Res. 2014;93:346–352. doi: 10.1177/0022034513518943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mealey BL, Moritz AJ. Hormonal influences: effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol 2000. 2003;32:59–81. doi: 10.1046/j.0906-6713.2002.03206.x. [DOI] [PubMed] [Google Scholar]
- 24.Jafri Z, Bhardwaj A, Sawai M, et al. Influence of female sex hormones on periodontium: a case series. J Nat Sci Biol Med. 2015;6(Suppl 1):S146–S149. doi: 10.4103/0976-9668.166124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophi CA, Resnick HE, Patner RE, et al. Confirming glycemic status in the Diabetes Prevention Program. J Diabetes Complications. 2013;27:150–157. doi: 10.1016/j.jdiacomp.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulze A, Busse M. Gender differences in periodontal status and oral hygiene of non-diabetic and type 2 diabetic patients. Open Dent J. 2016;10:287–297. doi: 10.2174/1874210601610010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llambés F, Arias-Herrera S, Caffesse R. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6:927–935. doi: 10.4239/wjd.v6.i7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kritsotakis G, Psarrou M, Vassilaki M, et al. Gender difference in the prevalence and clustering of multiple health risk behaviors in young adults. J Adv Nurs. 2016;72:2098–2113. doi: 10.1111/jan.12981. [DOI] [PubMed] [Google Scholar]