Abstract
Objectives: To determine the association of overweight/obesity, dental caries and dietary sugars in Australian adults. Materials and methods: The National Survey of Adult Oral Health (NSAOH) 2004–2006 provided data for analysis of dental caries experience. Self-reported body weight and height were used to calculate body mass index (BMI) for a subsample (n = 3,745, 89.8%) of the NSAOH data. A self-report questionnaire of 13 food items estimated the daily intake of added sugar, total sugars and total carbohydrate, using food composition estimates from the AUSNUT2011–2013. Bivariate analyses (Pearson’s Chi-square with Rao–Scott adjustment and Student’s t-tests) were used to determine the association of overweight/obesity, dental caries, sugar variables and putative confounders. Poisson regression models for the Decayed, Missing and Filled Teeth Index and individual measures of decayed, missing and filled teeth were constructed, with models containing BMI, dietary added sugar, total sugar and total carbohydrate, controlling for putative confounders. Results: There was a positive association between dental caries experience and being overweight or obese compared with having normal weight or being underweight as well as between sugar consumption with all four dental caries outcome measures. When controlled for putative confounders where sugar consumption was identified as a key determinant, the statistical significance between dental caries experience and being overweight or obese disappeared. The demographic and socioeconomic factors associated with dental caries experience were age, sex, education, smoking status and usual reason for dental visit. Conclusion: Analysis of the relationship between dental caries and obesity must include data about sugar and carbohydrate consumption.
Key words: Dental caries, dietary sugars, obesity, public health, body mass index
INTRODUCTION
Dental caries is a reversible, biological process that initiates as a localised demineralisation of hard tissues of the teeth, under an influence of acid derived from food debris or sugar1. It is a leading oral chronic condition2, affecting the permanent dentition of 2.4 billion people (35.3% of the total population) worldwide3. The burden of dental caries is also high in Australian adults (25.5%)4 and children (18.3%–44.0%)5.
Obesity results from a complex interplay of diet, exercise and other systems6, leading to an imbalance between energy intake and expenditure during an extended period7. An increasing proportion of Australian adults are overweight or obese (63.4%)8, which significantly contributes to the burden of disease, and is associated with increased rates of cardiovascular diseases, type 2 diabetes, musculoskeletal disorders, cancers and oral diseases9., 10..
Obesity and dental caries are prominent public health problems in Australia, they occur in complex aetiological environments, and are associated with compromised social and physical function, and reduced quality of life11. Age, sex, social determinants (income and education), health-related behaviours (smoking, diet, alcohol consumption and healthcare attendance) and water fluoridation are significant moderators of both dental caries and obesity12., 13.. Dietary sugar intake is a common risk factor for dental caries and obesity14. A systematic review of the effect of dietary sugars on body weight found that a reduction in body weight was associated with reduced sugar intake, and prospective cohort studies in children found that individuals with increased sugar intake were likely to become overweight or obese14. Restricting sugar intake in adults and children also results in a reduction in dental caries experience15.
Evidence for the association between diet, dental caries and obesity is limited. A systematic review and meta-analysis of dental caries and obesity in children and adolescents reported higher dental caries experience with increase in body weight12. However, there remains insufficient evidence to support this association, due to a high degree of heterogeneity between studies and the need for further investigation of the association16., 17.. Considering Australia’s increasing levels of obesity and availability of national oral health survey data, opportunity exists to further investigate this interaction.
This study investigated the association of overweight/obesity, dental caries experience and diet in a nationally representative sample of Australian adults using the National Survey of Adult Oral Health (NSAOH) 2004–2006.
MATERIALS AND METHODS
The NSAOH 2004–2006 employed a three-stage, stratified, clustered sampling design to draw a representative sample of Australian population aged 15 years and older4. Full details of sampling, examination protocol and survey participation have been described previously4. The ethics approval was obtained from University of Adelaide Human Research Ethics Committee, and the study was conducted according to the World Medical Association Declaration of Helsinki (version, 2008). All examined subjects provided a signed, informed consent to participate in the study. The consent procedures were approved by the University of Adelaide Human Research Ethics Committee.
Data extracted from the NSAOH 2004–2006 included the dental caries experience data measured using the Decayed Missing Filled Teeth (DMFT) index. This is a cumulative measure comprising a count of the number of decayed (D), missing (M) and filled teeth (F) and its individual components, more details of the examination protocol have been published previously4. Self-reported body weight (kg) and body height (cm) were obtained using a questionnaire completed by survey participant following the oral health assessment. Categorical variables for body mass index (BMI) were created using the World Health Organisation (WHO) BMI classifications for adults8, specifying underweight/normal BMI as < 25 kg/m2, overweight/obese ≥ 25 kg/m2.
The self-reported habitual dietary intake of 13 food items was recorded (Appendix A), with participants reporting the total number of servings consumed on a usual day and in the last hour before bed for each food item. In our study, a nutritionist (KK) calculated added sugar, total sugar and total carbohydrate levels for each food item using the FoodWorks version 8 dietary analysis package18, with the Australian Food, Supplement and Nutrient Database (AUSNUT) 2011–2013 food composition database19. AUSNUT is a set of files that enables food, dietary supplement and nutrient intake estimates to be made from dietary data. Questionnaire and database foods were matched using the most similar generic food item. Serving sizes were generated initially by consulting the NSAOH questionnaire; where these were unclear, a serving size for the selected food or beverage was obtained through the standard portion sizes outlined in the Australian Guide to Healthy Eating (AGHE)20. Where foods were not available from the AGHE (e.g. discretionary foods), a value of ~600 kJ was attributed21.
The estimated consumption of added sugar, total sugar and total carbohydrate from 13 food items for all individuals was calculated by multiplying the number of servings reported by the added sugar, total sugar and total carbohydrate values for each food item. New variables were created for these cumulative totals by calculating the sum of each variable from all 13 food items. Total values of recorded added sugar, total sugar and total carbohydrate were used in our analysis as primary exposure variables.
Variables from the NSAOH data set were selected a priori for analysis as potential confounders. These included: socioeconomic factors (income and education); health and lifestyle factors (diabetes and smoking status); alcohol consumption; lifetime fluoridation exposure and oral health behaviours (usual reason for dental visit, time since last visit, mouth rinsing, tooth brushing and flossing). Variables associated with both dental caries and obesity were defined as confounders.
Categorical variables were designated for sex (male, female), diabetes (yes, no), alcohol consumption (≤ 2, > 2 standard drinks per day), usual reason for dental visit (check-up, problem), time since last dental visit (< 12, ≥ 12 months), mouth rinsing (yes, no), tooth brushing (< twice a day, ≥ twice a day), flossing (yes, no), age (15–44, 45–59, ≥ 60 years), income (< $30k, $30k–$60k, ≥ $60k), education (high-school or less, trade certificate/diploma, and degree or higher) and smoking (current, previous, never). A standard drink contains 10 g of alcohol is an established metric for estimating alcohol consumption across drink types22.
The Australian Research Centre for Population Oral Health (ARCPOH) maintains a database of fluoride concentrations in drinking water for 99.4% of the Australian population23. Fluoride concentrations were recorded and matched to geographical location by postcode24. A record of city or area of residence collected for each individual for each year post-1964 was collected and matched to the ARCPOH water fluoridation database. Fluoride concentrations for each year at each location were summed and divided by the participant age, then multiplied by 100 to give percentage of lifetime water fluoridation exposure24.
Statistical analysis was undertaken using R version 3.4.125. The complex survey design of the NSAOH was accounted for; data were stratified by metropolitan and rural regions, clustered by post code and weighted for the probability of participants being selected for inclusion in the questionnaire.
Weighted-percentages, means and confidence intervals for population descriptive statistics were calculated. Bivariate analysis was conducted to explore the associations of BMI category, dental caries experience, potential confounders and sugar variables. Pearson’s Chi-square with Rao–Scott adjustment and Student’s t-tests were used to evaluate differences in levels of categorical and continuous variables, respectively. Linear regression was used to examine associations between dental outcomes and the continuous variables for dietary sugars and water fluoridation.
Multiple variable regression models were prepared using the generalised linear modelling function within the SURVEY package26, accounting for the complex survey design and employing the quasi-Poisson regression family. Dependent variables for models were dental caries experience (DMFT), decayed teeth (D), missing teeth (M) and filled teeth (F), respectively. Models were prepared for each dependent variable by BMI and dietary added sugar (Model 1), total carbohydrate (Model 2) and total sugar (Model 3). Potential confounders that were found to be associated with both dental outcomes and BMI were included in the analysis.
RESULTS
The NSAOH 2004–2006 contained data from 14,123 people interviewed with a computer-assisted telephone interview (CATI). Of these, 5,507 participants underwent clinical examination and 4,170 participants completed the questionnaire4. Due to varying response rates between the clinical examination and questionnaire, height and weight data available for the BMI could only be calculated for 3,745 (89.9%) individuals who completed the questionnaire.
The study population was aged between 15 and 91 years, with equal distribution of sexes post-application of population weighting. Most participants were 15–44 years (57%) old. Socioeconomic status was high, with 46.5% having an annual income over $60k and 67% higher than secondary education level. Diabetes (4.3%) and current smoking (15.2%) were uncommon characteristics of the population. Over half of participants (57.7%) reported drinking less than two standard drinks per day. Slightly more than 50% of the population was overweight/obese, and the average lifetime spent with a fluoride concentration of 1 ppm was 53.7%. More than half of the participants had visited their dentist in the last 12 months and had a check-up-based dental appointment (55.7% and 59.7%) at their last dental visit. Mouth rinsing, tooth brushing ≥ 2 times and flossing were reported at 58.1%, 55.5% and 48.5% of the population, respectively (Table 1). Mean intake dietary added sugar, total sugar and total carbohydrates were estimated at 71.70, 125.08 and 171.50 g/day, respectively.
Table 1.
Characteristics of the study population
| Characteristic | Level | n | Weighted percentage % | 95% CI |
|---|---|---|---|---|
| Age | 15–44 | 1,591 | 56.7 | 54.2–59.3 |
| 45–59 | 1,333 | 25.2 | 23.3–27.1 | |
| 60+ | 1,246 | 18.1 | 16.4–19.7 | |
| Sex | Male | 1,604 | 50.0 | 47.4–52.6 |
| Female | 2,566 | 50.0 | 47.4–52.6 | |
| Income | < 30k | 1,253 | 24.1 | 21.7–26.6 |
| 30k to < 60k | 567 | 29.4 | 26.9–31.9 | |
| 60k+ | 2,093 | 46.5 | 43.3–49.8 | |
| Education | High-school or less | 1,349 | 32.7 | 30.1–35.3 |
| Trade/dip/cert | 1,351 | 32.5 | 29.8–35.1 | |
| Degree/teaching/nursing | 1,372 | 34.9 | 31.9–37.8 | |
| Diabetes | Yes | 213 | 4.3 | 3.2–5.5 |
| No | 3,956 | 95.7 | 94.5–96.8 | |
| Smoking | Current | 578 | 15.2 | 13.2–17.1 |
| Previous | 1,315 | 27.3 | 25.1–29.5 | |
| Never | 2,277 | 57.5 | 55.0–60.1 | |
| Alcohol | ≤ 2 drinks | 2,191 | 57.7 | 54.5–60.8 |
| > 2 drinks | 1,152 | 42.3 | 39.2–45.5 | |
| Usual reason for dental visit | Check-up | 1,712 | 55.7 | 52.6–58.8 |
| Problem | 1,602 | 44.3 | 41.2–47.4 | |
| Time since last visit | < 12 months | 2,630 | 59.7 | 57.1–62.4 |
| > 12 months | 1,537 | 40.3 | 37.6–42.9 | |
| Mouth rinsing | No | 2,821 | 41.9 | 39.3–44.6 |
| Yes | 1,347 | 58.1 | 55.4–60.7 | |
| Tooth brushing | < 2 day | 1,612 | 44.5 | 41.8–47.2 |
| ≥ 2 day | 2,535 | 55.5 | 52.8–58.2 | |
| Flossing | No | 1,952 | 51.5 | 48.6–54.3 |
| Yes | 2,217 | 48.5 | 45.7–51.4 | |
| BMI | Underweight/normal | 1,685 | 48.9 | 45.9–51.9 |
| Overweight/obese | 2,060 | 51.1 | 48.1–54.1 | |
| Fluoride | % Lifetime > 1 ppm | 3,770 | 53.7 | 51.2–56.1 |
BMI, body mass index; CI, confidence interval.
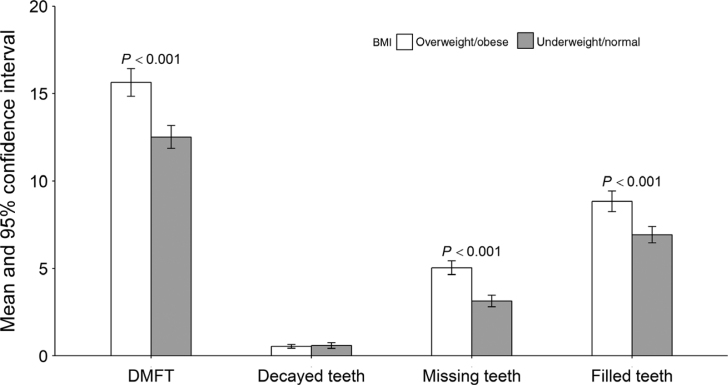
Table 2 illustrates the bivariate analysis of BMI and putative confounders. The analysis suggested that overweight/obesity was significantly associated with people aged 60 years and older (< 0.001), males (< 0.001), former smokers (P < 0.001) and problem-based dental visit behaviour (P < 0.001). Lower total carbohydrate intake was observed in overweight/obese people compared with underweight/normal weight people (P < 0.05). Mean DMFT, missing teeth, filled teeth, but not decayed teeth, were significantly higher among overweight/obese individuals than those of underweight/normal weight. No significant differences were observed in the mean values of decayed teeth between the two weight groups (Figure 1; Table 3).
Table 2.
Bivariate analysis of BMI (underweight/normal BMI ≤ 25 kg/m2, overweight/obese BMI as > 25 kg/m2), and covariates
| Characteristic | Level | Underweight/normal % | 95% CI | Overweight/obese % | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Age | 15–44 | 57.5 | 52.7–62.3 | 42.5 | 37.7–47.3 | |
| 45–59 | 37.8 | 33.9–41.6 | 62.2 | 58.4–66.1 | ||
| 60+ | 37.1 | 33.3–40.9 | 62.9 | 59.1–66.7 | < 0.001 | |
| Sex | Male | 43.2 | 38.1–48.2 | 56.8 | 51.8–61.9 | |
| Female | 54.9 | 51.7–58.1 | 45.1 | 41.9–48.3 | < 0.001 | |
| Income | < 30k | 44.0 | 37.9–50.2 | 56.0 | 49.8–62.1 | |
| 30k to < 60k | 47.1 | 42.7–51.5 | 52.9 | 48.5–57.3 | ||
| 60k+ | 49.4 | 44.3–54.5 | 50.6 | 45.5–55.7 | 0.3686 | |
| Education | High-school or less | 47.4 | 42.2–52.7 | 52.6 | 47.3–57.8 | |
| Trade | 41.9 | 36.4–47.3 | 58.1 | 52.7–63.6 | ||
| Degree | 51.4 | 46.0–56.8 | 48.6 | 43.2–54.0 | 0.0554 | |
| Diabetes | Yes | 47.4 | 42.2–52.7 | 52.6 | 46.0–56.8 | |
| No | 41.9 | 36.4–47.3 | 58.1 | 47.3–57.8 | 0.1222 | |
| Smoking | Current | 50.2 | 43.0–57.5 | 49.8 | 42.5–57.0 | |
| Previous | 37.1 | 32.3–41.8 | 62.9 | 58.2–67.7 | ||
| Never | 54.1 | 50.2–57.9 | 45.9 | 42.1–49.8 | < 0.001 | |
| Alcohol | ≤ 2 drinks | 45.9 | 42.0–49.8 | 54.1 | 50.2–58.0 | |
| > 2 drinks | 48.5 | 42.7–54.3 | 51.5 | 45.7–57.3 | 0.4571 | |
| Usual reason for dental visit | Check-up | 54.3 | 50.1–58.5 | 45.7 | 41.5–49.9 | |
| Problem | 40.9 | 36.4–45.4 | 59.1 | 54.6–63.6 | < 0.001 | |
| Time since last visit | < 12 months | 47.2 | 43.6–50.8 | 52.8 | 49.2–56.4 | |
| > 12 months | 51.5 | 46.8–56.2 | 48.5 | 43.8–53.2 | 0.1355 | |
| Mouth rinsing | No | 47.6 | 42.9–52.2 | 52.4 | 47.8–57.1 | |
| Yes | 49.9 | 46.1–53.6 | 50.1 | 46.4–53.9 | 0.4447 | |
| Tooth brushing | < 2 day | 45.4 | 40.1–50.7 | 54.6 | 49.3–59.9 | |
| ≥ 2 day | 51.6 | 48.1–55.0 | 48.4 | 45.0–51.9 | 0.0546 | |
| Flossing | No | 47.2 | 42.5–51.8 | 52.8 | 48.2–57.5 | |
| Yes | 50.7 | 47.3–54.2 | 49.3 | 45.8–52.7 | 0.2129 | |
| Underweight/normal mean | Overweight/obese mean | P-value | ||||
| Added sugar | g/day | 82.2 | 74.1–90.3 | 78.5 | 73.5–83.4 | 0.4552 |
| Carbohydrates | g/day | 190.4 | 179.3–201.5 | 176.7 | 170.2–183.2 | < 0.05 |
| Total sugars | g/day | 139.4 | 130.3–148.6 | 131.3 | 125.8–136.8 | 0.1487 |
| Fluoride | % Lifetime 1 ppm | 55.5 | 51.8–59.2 | 52.7 | 49.3–56.0 | 0.2562 |
Chi-square with Rao–Scott correction test used for categorical predictors, Student’s t-test for continuous predictors. Significance at P < 0.05.CI, confidence interval.
Figure 1.
Display of dental outcomes by body mass index (BMI) category, error bars indicating 95% confidence interval. DMFT, Decayed Missing Filled Teeth.
Table 3.
Summary measures of dental caries experience by BMI category (underweight/normal BMI ≤ 25 kg/m2, overweight/obese BMI as > 25 kg/m2)
| Underweight/normal | 95% CI | Overweight/obese | 95% CI | t-test | |
|---|---|---|---|---|---|
| Dental caries experience (DMFT) | 12.52 | 11.87–13.17 | 15.64 | 14.85–16.42 | < 0.001 |
| Decayed teeth (D) | 0.59 | 0.43–0.76 | 0.54 | 0.44–0.65 | 0.609 |
| Missing teeth (M) | 3.14 | 2.81–3.47 | 5.04 | 4.65–5.44 | < 0.001 |
| Filled teeth (F) | 6.93 | 6.47–7.40 | 8.84 | 8.25–9.43 | < 0.001 |
CI, confidence interval; DMFT, Decayed Missing Filled Teeth.
Table 4 demonstrates a significant association between dental caries experience measures (DMFT, decayed teeth, missing teeth and filled teeth) with smoking (P < 0.05), alcohol consumption (P < 0.05), usual reason for dental visit (P < 0.05), time since last visit (P < 0.05), flossing (P < 0.05), and all three measures of dietary sugars (P < 0.05).
Table 4.
Bivariate analysis of putative confounders and dental outcomes DMFT
| Characteristic | Level | DMFT | 95% CI | P-value | Decayed teeth | 95% CI | P-value | Missing teeth | 95% CI | P-value | Filled teeth | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 15–44 | 9.13 | 8.63–9.62 | 0.65 | 0.51–0.79 | 1.02 | 0.87–1.18 | 4.73 | 4.36–5.09 | ||||
| 45–59 | 19.29 | 18.82–19.77 | 0.52 | 0.39–0.65 | 6.21 | 5.79–6.63 | 12.56 | 12.13–12.99 | |||||
| 60+ | 23.18 | 22.79–23.56 | < 0.001 | 0.42 | 0.33–0.50 | 0.2016 | 11.65 | 11.1–12.2 | < 0.001 | 11.11 | 10.59–11.64 | < 0.001 | |
| Sex | Male | 13.56 | 12.79–14.34 | 0.68 | 0.54–0.82 | 4.08 | 3.73–4.43 | 7.45 | 6.88–8.01 | ||||
| Female | 14.89 | 14.34–15.44 | < 0.05 | 0.48 | 0.37–0.58 | < 0.05 | 4.42 | 4.07–4.78 | 0.159 | 8.27 | 7.83–8.69 | < 0.05 | |
| Income | < 30k | 18.12 | 16.79–19.46 | 0.71 | 0.56–0.87 | 8.22 | 7.39–9.05 | 8.41 | 7.69–9.11 | ||||
| 30k to < 60k | 15.22 | 14.53–15.91 | 0.71 | 0.52–0.89 | 4.37 | 3.92–4.82 | 8.68 | 8.12–9.25 | |||||
| 60k+ | 12.37 | 11.67–13.07 | < 0.001 | 0.42 | 0.29–0.54 | 0.9545 | 2.56 | 2.28–2.84 | < 0.001 | 7.69 | 7.09–8.29 | 0.5381 | |
| Education | High-school or less | 15.09 | 14.34–15.85 | 0.84 | 0.65–1.02 | 5.53 | 5.03–6.02 | 7.36 | 6.84–7.87 | ||||
| Trade | 15.37 | 14.49–16.26 | 0.68 | 0.48–0.89 | 4.82 | 4.35–5.28 | 8.60 | 8.01–9.19 | |||||
| Degree | 13.91 | 13.0–14.81 | 0.6421 | 0.29 | 0.22–0.35 | 0.3036 | 3.36 | 2.99–3.73 | < 0.05 | 8.75 | 8.04–9.46 | < 0.05 | |
| Diabetes | Yes | 18.12 | 14.77–21.48 | 0.63 | 0.35–0.91 | 8.02 | 5.99–10.06 | 8.74 | 6.98–10.49 | ||||
| No | 14.05 | 13.57–14.53 | < 0.05 | 0.57 | 0.48–0.67 | 0.7079 | 4.08 | 3.83–4.33 | < 0.001 | 7.81 | 7.45–8.18 | 0.3128 | |
| Smoking | Current | 14.35 | 13.34–15.37 | 1.43 | 1.04–1.83 | 4.14 | 3.47–4.81 | 6.96 | 6.26–7.65 | ||||
| Previous | 16.44 | 15.59–17.29 | 0.53 | 0.38–0.67 | 5.66 | 5.16–6.17 | 9 | 8.35–9.64 | |||||
| Never | 13.14 | 12.51–13.78 | < 0.05 | 0.37 | 0.31–0.44 | < 0.001 | 3.61 | 3.29–3.93 | < 0.001 | 7.55 | 7.07–8.04 | < 0.001 | |
| Alcohol | ≤ 2 drinks | 15.68 | 15.07–16.29 | 0.44 | 0.34–0.53 | 4.83 | 4.47–5.18 | 9.06 | 8.58–9.55 | ||||
| > 2 drinks | 12.58 | 11.80–13.37 | < 0.001 | 0.83 | 0.62–1.04 | < 0.05 | 3.14 | 2.77–3.51 | < 0.001 | 6.89 | 6.31–7.47 | < 0.001 | |
| Usual reason for dental visit | Check-up | 13.56 | 12.87–14.25 | 0.27 | 0.19–0.34 | 3.66 | 3.27–4.05 | 7.92 | 7.38–8.45 | ||||
| Problem | 17.13 | 16.4–17.87 | < 0.001 | 0.68 | 0.51–0.85 | < 0.001 | 5.36 | 4.92–5.79 | < 0.001 | 9.81 | 9.27–10.35 | < 0.001 | |
| Time since last visit | < 12 months | 15.72 | 15.15–16.29 | 0.44 | 0.34–0.55 | 4.62 | 4.31–4.93 | 9.23 | 8.80–9.66 | ||||
| > 12 months | 12.01 | 11.29–12.73 | < 0.001 | 0.77 | 0.62–0.93 | < 0.001 | 3.70 | 3.33–4.08 | < 0.001 | 5.81 | 5.32–6.31 | < 0.001 | |
| Mouth rinsing | No | 14.94 | 14.17–15.70 | 0.48 | 0.37–0.59 | 4.62 | 4.20–5.03 | 8.41 | 7.84–8.98 | ||||
| Yes | 13.69 | 13.09–14.28 | < 0.05 | 0.64 | 0.52–0.77 | 0.0688 | 3.96 | 3.65–4.27 | < 0.05 | 7.46 | 7.04–7.88 | < 0.05 | |
| Tooth brushing | < 2 day | 13.33 | 12.62–14.05 | 0.79 | 0.62–0.97 | 4.01 | 3.64–4.39 | 6.78 | 6.29–7.27 | ||||
| ≥ 2 day | 14.92 | 14.35–15.49 | < 0.001 | 0.39 | 0.33–0.47 | < 0.001 | 4.42 | 4.10–4.74 | 0.0855 | 8.72 | 8.28–9.16 | < 0.001 | |
| Flossing | No | 13.46 | 12.73–14.18 | 0.77 | 0.61–0.92 | 4.54 | 4.16–4.92 | 6.68 | 6.20–7.17 | ||||
| Yes | 15.04 | 14.45–15.63 | < 0.001 | 0.38 | 0.28–0.47 | < 0.001 | 3.94 | 3.62–4.26 | < 0.05 | 9.09 | 8.63–9.56 | < 0.001 | |
| Linear regression | Linear regression | Linear regression | Linear regression | ||||||||||
| Added sugar | Intercept | 14.854 | 14.08–15.63 | 0.199 | −0.01 to 0.41 | 4.319 | 3.96–4.68 | 9.162 | 8.54–9.79 | ||||
| Coef | −0.012 | −0.021 to −0.003 | < 0.05 | 0.005 | 0.002–0.01 | < 0.05 | −0.005 | −0.008 to −0.001 | < 0.05 | −0.017 | −0.024 to −0.01 | < 0.001 | |
| Carbohydrate | Intercept | 15.139 | 14.01–16.27 | 0.09 | −0.26 to 0.44 | 4.521 | 4.06–4.98 | 9.433 | 8.62–10.24 | ||||
| Coef | −0.007 | −0.013 to −0.001 | < 0.05 | 0.003 | 0.001–0.005 | < 0.05 | −0.003 | −0.01 to −0.001 | < 0.05 | −0.009 | −0.01 to −0.005 | < 0.001 | |
| Total sugars | Intercept | 15.37 | 14.32–16.42 | 0.076 | −0.23 to 0.38 | 4.579 | 4.15–5.01 | 9.66 | 8.87–10.44 | ||||
| Coef | −0.011 | −0.019 to −0.004 | < 0.05 | 0.004 | 0.001–0.006 | < 0.05 | −0.005 | −0.01 to −0.002 | < 0.001 | −0.014 | −0.02 to −0.01 | < 0.001 | |
| Fluoride | Intercept | 18.623 | 17.56–19.69 | 0.49 | 0.37–0.61 | 6.483 | 5.91–7.06 | 10.86 | 10.10–11.63 | ||||
| Coef | −0.081 | −0.098 to −0.06 | < 0.001 | 0.00 | −0.002 to 0.002 | 0.9636 | −0.043 | −0.05 to −0.03 | < 0.001 | −0.052 | −0.064 to −0.04 | < 0.001 | |
Student’s t-test for difference in means by level of categorical variable, linear regression for continuous variables.
CI, confidence interval; DMFT, Decayed Missing Filled Teeth.
Table 5 provides each of the three multiple variable models of added sugars (Model 1), total carbohydrates (Model 2) and total sugars (Model 3), the rate ratios for DMFT were significantly higher in individuals aged 45 years and older, females and participants with problem-based dental visiting behaviour. The number of decayed teeth was significantly associated with added sugars, total carbohydrates and total sugars, with smoking and problem-based dental visits significant across all models. A biological gradient was observed between decayed teeth and smoking status across all three models, with higher rates of decayed teeth observed in current smokers compared with previous smokers, and the lowest rate in those who had never smoked. The number of missing teeth was significantly associated with age, higher education, problem-based dental visits and sugar intake in all models. The number of filled teeth was significantly higher with age, level of education and reason for dental visit in all models.
Table 5.
Multiple variable regression models for DMFT, decayed teeth, missing teeth and filled teeth with putative confounding variables, added sugar, total carbohydrate and total sugars
| Covariate | DMFT | 95% CI | P-value | Decayed teeth | 95% CI | P-value | Missing teeth | 95% CI | P-value | Filled teeth | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1* | ||||||||||||
| BMI overweight/obese | 1.02 | 0.97–1.07 | 0.474 | 1.01 | 0.67–1.54 | 0.949 | 1.03 | 0.94–1.13 | 0.536 | 1.014 | 0.94–1.09 | 0.714 |
| Added sugar | 1.0002 | 0.999–1.004 | 0.145 | 1.01 | 1.00–1.02 | < 0.05 | 1.01 | 1.00–1.01 | < 0.001 | 0.999 | 0.996–1.003 | 0.744 |
| Age | ||||||||||||
| 45–60 | 1.91 | 1.78–2.05 | < 0.001 | 0.7 | 0.48–1.03 | 0.076 | 4.84 | 4.00–5.85 | < 0.001 | 2.26 | 2.05–2.49 | < 0.001 |
| > 60 | 2.27 | 2.13–2.42 | < 0.001 | 0.77 | 0.5–1.18 | 0.228 | 8.46 | 6.95–10.31 | < 0.001 | 2.11 | 1.91–2.34 | < 0.001 |
| Sex | ||||||||||||
| Female | 1.08 | 1.03–1.14 | < 0.05 | 0.97 | 0.71–1.34 | 0.868 | 1.11 | 1.01–1.22 | < 0.05 | 1.05 | 0.97–1.14 | 0.169 |
| Education | ||||||||||||
| Trade | 1.04 | 0.98–1.11 | 0.189 | 1.01 | 0.68–1.5 | 0.957 | 0.93 | 0.84–1.02 | 0.142 | 1.12 | 1.03–1.23 | < 0.05 |
| Degree | 1.01 | 0.96–1.07 | 0.71 | 0.67 | 0.44–1.03 | 0.068 | 0.74 | 0.66–0.83 | < 0.001 | 1.15 | 1.06–1.25 | < 0.001 |
| Smoker | ||||||||||||
| Previous | 0.98 | 0.9–1.06 | 0.617 | 0.52 | 0.32–0.86 | < 0.05 | 0.99 | 0.84–1.16 | 0.88 | 1.083 | 0.96–1.22 | 0.1821 |
| Never | 0.92 | 0.85–1 | 0.062 | 0.37 | 0.25–0.54 | < 0.001 | 0.89 | 0.76–1.04 | 0.151 | 1.055 | 0.94–1.19 | 0.3765 |
| Visit for problem | 1.13 | 1.07–1.19 | < 0.001 | 2.06 | 1.46–2.91 | < 0.001 | 1.15 | 1.05–1.26 | < 0.05 | 1.114 | 1.03–1.20 | < 0.05 |
| Model 2† | ||||||||||||
| BMI overweight/obese | 1.02 | 0.97–1.08 | 0.371 | 1.07 | 0.72–1.58 | 0.747 | 1.04 | 0.94–1.14 | 0.479 | 1.00 | 0.94–1.09 | 0.641 |
| Total carbohydrate | 1.0002 | 0.999–1.001 | 0.216 | 1.002 | 1.001–1.003 | < 0.001 | 1.001 | 1.0003–1.001 | < 0.05 | 1.000 | 0.999–1.001 | 0.865 |
| Age | ||||||||||||
| 45–60 | 1.91 | 1.78–2.05 | < 0.001 | 0.72 | 0.49–1.06 | 0.099 | 4.80 | 3.96–5.82 | < 0.001 | 2.268 | 2.05–2.50 | < 0.001 |
| > 60 | 2.26 | 2.12–2.41 | < 0.001 | 0.77 | 0.50–1.19 | 0.238 | 8.32 | 6.83–10.15 | < 0.001 | 2.123 | 1.92–2.35 | < 0.001 |
| Sex | ||||||||||||
| Female | 1.09 | 1.03–1.14 | < 0.05 | 1.00 | 0.72–1.37 | 0.978 | 1.10 | 1.00–1.21 | < 0.05 | 1.06 | 0.98–1.14 | 0.147 |
| Education | ||||||||||||
| Trade | 1.04 | 0.98–1.11 | 0.168 | 0.97 | 0.66–1.43 | 0.867 | 0.92 | 0.84–1.02 | 0.13 | 1.12 | 1.03–1.23 | < 0.05 |
| Degree | 1.01 | 0.95–1.06 | 0.803 | 0.67 | 0.44–1.03 | 0.07 | 0.74 | 0.66–0.83 | < 0.001 | 1.15 | 1.06–1.25 | < 0.001 |
| Smoker | ||||||||||||
| Previous | 0.98 | 0.9–1.06 | 0.568 | 0.53 | 0.34–0.85 | < 0.05 | 0.99 | 0.84–1.16 | 0.874 | 1.079 | 0.96–1.22 | 0.210 |
| Never | 0.92 | 0.84–1.00 | 0.064 | 0.37 | 0.25–0.53 | < 0.001 | 0.88 | 0.75–1.04 | 0.138 | 1.053 | 0.93–1.19 | 0.410 |
| Visit for problem | 1.13 | 1.07–1.19 | < 0.001 | 2.04 | 1.44–2.90 | < 0.001 | 1.15 | 1.05–1.26 | < 0.05 | 1.11 | 1.03–1.19 | < 0.05 |
| Model 3‡ | ||||||||||||
| BMI overweight/obese | 1.02 | 0.97–1.08 | 0.398 | 1.05 | 0.70–1.56 | 0.828 | 1.03 | 0.94–1.14 | 0.504 | 1.02 | 0.94–1.09 | 0.646 |
| Total sugar | 1.0003 | 0.99–1.00 | 0.254 | 1.003 | 1.001–1.003 | < 0.001 | 1.001 | 1.0004–1.002 | < 0.05 | 0.999 | 0.999–1.001 | 0.659 |
| Age | ||||||||||||
| 45–60 | 1.91 | 1.78–2.05 | < 0.001 | 0.72 | 0.49–1.06 | 0.097 | 4.82 | 3.98–5.85 | < 0.001 | 2.26 | 2.05–2.50 | < 0.001 |
| > 60 | 2.26 | 2.12–2.41 | < 0.001 | 0.78 | 0.51–1.20 | 0.263 | 8.37 | 6.87–10.21 | < 0.001 | 2.12 | 1.91–2.35 | < 0.001 |
| Sex | ||||||||||||
| Female | 1.09 | 1.03–1.14 | < 0.05 | 0.99 | 0.72–1.36 | 0.934 | 1.10 | 1.00–1.21 | < 0.05 | 1.06 | 0.98–1.14 | 0.149 |
| Education | ||||||||||||
| Trade | 1.05 | 0.98–1.11 | 0.161 | 0.99 | 0.67–1.46 | 0.946 | 0.93 | 0.84–1.02 | 0.138 | 1.13 | 1.03–1.23 | < 0.05 |
| Degree | 1.01 | 0.95–1.06 | 0.802 | 0.68 | 0.44–1.04 | 0.075 | 0.74 | 0.66–0.83 | < 0.001 | 1.15 | 1.06–1.25 | < 0.05 |
| Smoker | ||||||||||||
| Previous | 0.98 | 0.90–1.06 | 0.597 | 0.53 | 0.33–0.86 | < 0.05 | 0.99 | 0.84–1.17 | 0.949 | 1.08 | 0.96–1.21 | 0.215 |
| Never | 0.92 | 0.85–1.00 | 0.066 | 0.37 | 0.25–0.54 | < 0.001 | 0.89 | 0.76–1.05 | 0.166 | 1.05 | 0.93–1.19 | 0.411 |
| Visit for problem | 1.13 | 1.07–1.19 | < 0.001 | 2.05 | 1.45–2.9 | < 0.001 | 1.15 | 1.05–1.26 | < 0.05 | 1.11 | 1.03–1.20 | < 0.05 |
Model 1: the association of added sugar, putative confounders in relation to DMFT and decayed, missing and filled teeth.
Model 2: the association of total carbohydrate, putative confounders in relation to DMFT and decayed, missing and filled teeth.
Model 3: the association of total sugar, putative confounders in relation to DMFT and decayed, missing and filled teeth.
BMI, body mass index; CI, confidence interval; DMFT, Decayed Missing Filled Teeth.
DISCUSSION
In this study, we determined that there was a positive association between dental caries experience (DMFT), missing (M) and filled teeth (F), and being overweight or obese compared with having normal weight or being underweight as well as between sugar consumption with all four dental caries outcome measures. However, when controlled for putative confounders where sugar consumption was identified as a key determinant, the statistical significance between dental caries experience and being overweight or obese disappeared. The literature on the relationship between dental caries and obesity is unclear, with reports demonstrating increased, decreased and no association as weight increases. This study has demonstrated that any such analysis must include data about sugar and carbohydrate consumption. Similar results were observed in Brazilian adolescents and Korean adults27., 28..
In this study, the dental caries experience was significantly higher in people aged 45 years and older; females; and people with problem-based dental visiting behaviour. Dental caries and obesity share common risk factors and occur in complex socioeconomic and socio-behavioural environments. No association between obesity and dental caries could be a result of influences of other factors on dental caries occurrence and progression. These factors include fluoridated water and beverage consumption, low socioeconomic status, high cost of dental services, having no dental insurance, poorer living conditions, poor health literacy, and elevated psychological stress29., 30..
In this study, dietary sugar intake (added sugars, total sugars and total carbohydrates) was significantly associated with decayed and missing teeth in adults. Supporting this finding, a dose–response relationship was reported between sugar consumption and dental caries experience in Australian adolescents31. Sugar intake is an established risk factor for dental caries15., 32.. Population-based approaches targeting reduced sugar consumption, through restricting consumption of sugar-sweetened beverages is required across the life course33.
This study has some caveats. Firstly, self-reported body height and body weight could have introduced a reporting bias, because of underestimation of their body weights and overestimation of their body heights34. While direct measurement of weight and height is preferable when calculating BMI in epidemiological studies, in reality, self-reported weight and height are common substitutes due to the time, cost and data collection constraints35. Secondly, a cross-sectional study design is another limitation because it hinders determination of the temporal relationship between the exposure and the outcome. Thirdly, the food frequency questionnaire (FFQ) employed in this study was a self-completed questionnaire, using predetermined serving sizes. These methods are routinely used to determine nutrient intake in large, epidemiological studies when minimising the burden associated with data collection is important. The intrinsic problems related to reported dietary data collection remain unsolved, and therefore readers should be mindful of the limitations of the FFQ data when interpreting the results of this study.
As total daily energy cannot be calculated from the 13 food items in this questionnaire, the results of this analysis cannot be compared with the WHO recommendations for added sugars, which stipulate that added sugars should comprise less than 10% of daily energy intake36. The NSAOH dietary questions were primarily included as indicators of risk for dental caries rather than broad measures of nutrition or as specific risk indicators for poor nutrition and obesity. As such, some potentially important carbohydrate-rich food items such as breads and foods particularly high in dietary fats were not considered, which may have contributed to our understanding of the role of diet in overweight/obesity. The measure of total carbohydrate returned from this survey is likely to under-represent daily carbohydrate intake due to a lack of reporting high-starch foods. Further, the dietary variables are self-reported, and consist of mainly high sugar and discretionary foods. These foods are especially vulnerable to under reporting, particularly by individuals attempting to control their weight37.
Irrespective of the outlined limitations, profound strengths of this study remain: NSAOH is a nationwide survey; it generated a large sample size; a sophisticated sampling procedure was used; it provided population level prevalence estimates for adult oral health; and there was a negligible non-participation bias4.
CONCLUSION
There was a positive association between being overweight or obese and dental caries, but the statistical significance disappeared in the regression analysis, where sugar consumption was identified as a key determinant. Analysis on the relationship between dental caries and obesity must include data about sugar and carbohydrate consumption.
Acknowledgements
Australian Government Research Training Program Scholarship Department of Education and Training, School of Medicine and Centre of Rural Health, College of Health and Medicine, University of Tasmania, Translating Research into Practice Fellowship. Australian Research Centre for Population Oral Health (ARCPOH), University of Adelaide, Adelaide, Australia for use of the National Survey of Adult Oral Health (NSAOH) 2004–2006 data. NSAOH 2004–2006 supported by National Health and Medical Research Council (Nos. 299060, 349514, 349537), the Australian Government Department of Health and Aging, Population Health Division, the Australian Institute of Health and Welfare, Colgate Oral Care, the Australian Dental Association, and the US Centers for Disease Control and Prevention. The authors are grateful to Professor Marco Peres from the University of Adelaide, Director of ARCPOH for his support and constructive comments.
Conflict of interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Food item, weight, carbohydrate, sugar and added sugars from AUSNUT2013
| Survey descriptor | AUSNUT2013 descriptor | Qty | Data source | Total carbohydrate (g) | Total sugars (g) | Added sugars (g) |
|---|---|---|---|---|---|---|
| Fruit or natural juice | Fruit, fresh, nfs | 150 g | AGHE | 18.15 | 15.60 | 0 |
| Sweetened fruit drinks | Fruit drink, from dry base, regular, recommended dilution | 250 mL | AGHE | 23.36 | 23.36 | 23.35 |
| Sweetened soft drinks | Soft drink, nfs | 1 cup | NSAOH | 17.68 | 17.68 | 17.79 |
| Lo-cal soft drinks | Soft drink, lemonade, intense sweetened or diet | 250 mL | NSAOH | 0 | 0 | 0 |
| Plain milk | Milk, cow, fluid, unflavoured, nfs | 250 mL | NSAOH | 14.16 | 14.16 | 0 |
| Flavoured milk | Milk, cow, fluid, flavoured, chocolate, nfs | 250 mL | NSAOH | 24.38 | 23.10 | 8.57 |
| Sweetened dairy products | Dairy dessert, flavours other than chocolate, regular fat | 1 cup | NSAOH | 40.82 | 31.98 | 20.8 |
| Breakfast cereal | Breakfast cereal, nfs | 1 cup | AGHE | 33.35 | 6.10 | 3.68 |
| Biscuits, cakes | Muffin, cake-style, commercial, nfs | 40 g | AGHE | 19.72 | 10.32 | 9.02 |
| Table sugar | Sugar, white, fruit sugar (fructose), granulated or lump | 1 tsp | NSAOH | 4.15 | 4.15 | 4.15 |
| Chocolate, confectionery | Chocolate, nfs | 30 g | AGHE | 17.25 | 15.93 | 15.92 |
| Syrups, jams | Jam, nfs | 1 tbs | NSAOH | 18.40 | 18.37 | 18.01 |
| Muesli bars | Bar, muesli or snack, nfs | 1 regular bar | AGHE | 22.68 | 8.12 | 7.01 |
nfs, not further specified.
Table A2.
Justification for selecting the quantity
| AusNut2013 descriptor | Qty | Weight/ g | NSAOH | AGHE |
|---|---|---|---|---|
| Fruit, fresh, not further defined | 150 g | 150 | 1 medium piece | 1 medium piece of fruit, ~150 g |
| Juice, fruit, commercial, not further defined | 125 g | 125 | 1 medium glass | 125 mL juice is 1 serve of fruit |
| Soft drink not further defined | 250 mL | 260 | 1 medium glass | |
| Soft drink, lemonade, intense sweetened or diet | 250 mL | 250 | 1 medium glass | |
| Milk, cow, fluid, unflavoured, not further defined | 250 mL | 257.5 | 1 medium glass | |
| Milk, cow, fluid, flavoured, chocolate, not further defined | 250 mL | 265 | 1 medium glass | |
| Dairy dessert, flavours other than chocolate, regular fat | 1 cup | 260 | 1 cup | |
| Breakfast cereal not further defined | 1 cup | 50 | 1 cup | |
| Muffin, cake-style, commercial, not further defined | 40 g | 40 | ~600 kJ | |
| Sugar, white, fruit sugar (fructose), granulated or lump | 1 tsp | 4.15 | 1 teaspoon | |
| Chocolate not further defined | 30 g | 30 | 30 g or ~600 kJ | |
| Jam not further defined | 1 tbs | 28 | 1 tablespoon | |
| Bar, muesli or snack, not further defined | 1 regular bar | 35 | 1 bar | ~600 kJ |
References
- 1.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 2.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 3.Kassebaum N, Bernabé E, Dahiya M, et al. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 4.Slade GD, Spencer AJ, Roberts-Thomson KF. Australian Institute of Health and Welfare; Canberra, ACT: 2007. Australia’s dental generations: The national survey of adult oral health. (Dental Statistics and Research Series No. 34): Cat. no. DEN 165; Contract No.: 2007. Available from: https://www.adelaide.edu.au/arcpoh/downloads/publications/%e2%80%a6/dental%e2%80%a6/nsaoh-report.pdf. Accessed 12 July 2018. [Google Scholar]
- 5.Ha DH, Roberts-Thomson KF, Arrow P, et al. In: Oral Health of Australian Children: The National Child Oral Health Study 2012–14. Do LG, Spencer AJ, editors. University of Adelaide Press; Adelaide, SA: 2016. Children’s oral health status in Australia, 2012–14. [Google Scholar]
- 6.Rutter H. The complex systems challenge of obesity. Clin Chem. 2018;64:44–46. doi: 10.1373/clinchem.2017.272831. [DOI] [PubMed] [Google Scholar]
- 7.Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 8.AIHW . AIHW; Canberra, ACT: 2016. Australia’s Health 2016. Report No.: Australia’s health no. 15. Cat. no. AUS 199. [Google Scholar]
- 9.WHO . World Health Organisation; Geneva: 2016. Overweight and Obesity. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 10.Khan S, Barrington G, Bettiol S, et al. Is overweight/obesity a risk factor for periodontitis in young adults and adolescents?: a systematic review. Obes Rev. 2018;19:852–883. doi: 10.1111/obr.12668. [DOI] [PubMed] [Google Scholar]
- 11.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 12.Hayden C, Bowler JO, Chambers S, et al. Obesity and dental caries in children: a systematic review and meta-analysis. Community Dent Oral Epidemiol. 2013;41:289–308. doi: 10.1111/cdoe.12014. [DOI] [PubMed] [Google Scholar]
- 13.Awan K, Khan S, Abadeen Z, et al. Knowledge, perceptions, and attitudes of dental students towards obesity. Saudi Dent J. 2016;28:44–48. doi: 10.1016/j.sdentj.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 15.Moynihan P, Kelly S. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2014;93:8–18. doi: 10.1177/0022034513508954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantovitz KR, Pascon FM, Rontani RM, et al. Obesity and dental caries – a systematic review. Oral Health Prev Dent. 2006;4:137–144. [PubMed] [Google Scholar]
- 17.Silva AE, Menezes AM, Demarco FF, et al. Obesity and dental caries: systematic review. Rev Saude Publica. 2013;47:799–812. doi: 10.1590/S0034-8910.2013047004608. [DOI] [PubMed] [Google Scholar]
- 18.Xyris software. FoodWorks, Dietary Analysis Package version 5. Highgate Hill, Qld, Australia; 2007.
- 19.FSANZ . FSANZ: Food Standard Australia New Zealand; Canberra, ACT: 2014. AUSNUT 2011–13 Food Nutrient Database. [Google Scholar]
- 20.NHMRC . National Health and Medical Research Council; Canberra, ACT: 2015. Australian Guide to Healthy Eating: Serving Size. [Google Scholar]
- 21.NHMRC. Discretionary food and drink choices: National Health and Medical Research Council; 2017. Available from: https://www.eatforhealth.gov.au/food-essentials/discretionary-food-and-drink-choices. Accessed 15 July 2018
- 22.DOHA . Department of Health and Ageing, Australian Government; Canberra, ACT: 2018. New National Guidelines for Alcohol Consumption: Standard Drink Guide. [Google Scholar]
- 23.Crocombe LA, Brennan D, Slade G. Does lower lifetime fluoridation exposure explain why people outside capital cities have poor clinical oral health? Aust Dent J. 2016;61:93–101. doi: 10.1111/adj.12315. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney G, Slade GD, Kitchener S, et al. Lifetime fluoridation exposure and dental caries experience in a military population. Community Dent Oral Epidemiol. 2008;36:485–492. doi: 10.1111/j.1600-0528.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team . R Foundation for Statistical Computing; Vienna: 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 26.Lumley T. Survey: analysis of complex survey samples. R Package Version 3.322017
- 27.Song I-S, Han K, Ryu J-J, et al. Obesity is inversely related to the risks of dental caries in Korean adults. Oral Dis. 2017;23:1080–1086. doi: 10.1111/odi.12693. [DOI] [PubMed] [Google Scholar]
- 28.Freitas AR, Aznar FDC, Tinós AMFG, et al. Association between dental caries activity, quality of life and obesity in Brazilian adolescents. Int Dent J. 2014;64:318–323. doi: 10.1111/idj.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa S, Martins C, Pinto M, et al. Socioeconomic factors and caries in people between 19 and 60 years of age: an update of a systematic review and meta-analysis of observational studies. Int J Environ Res Public Health. 2018;15:1775. doi: 10.3390/ijerph15081775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hujoel PP, Hujoel MLA, Kotsakis GA. Personal oral hygiene and dental caries: a systematic review of randomised controlled trials. Gerodontology. 2018;35:282–289. doi: 10.1111/ger.12331. [DOI] [PubMed] [Google Scholar]
- 31.Armfield JM, Spencer AJ, Roberts-Thomson KF, et al. Water fluoridation and the association of sugar-sweetened beverage consumption and dental caries in Australian children. Am J Public Health. 2013;103:494–500. doi: 10.2105/AJPH.2012.300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernabé E, Vehkalahti MM, Sheiham A, et al. Sugar-sweetened beverages and dental caries in adults: a 4-year prospective study. J Dent. 2014;42:952–958. doi: 10.1016/j.jdent.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Hopcraft M, Beaumont S. The growing problems of dental caries and obesity: an Australian perspective. Br Dent J. 2016;221:379. doi: 10.1038/sj.bdj.2016.723. [DOI] [PubMed] [Google Scholar]
- 34.Robinson E, Oldham M. Weight status misperceptions among UK adults: the use of self-reported vs. measured BMI. BMC Obes. 2016;3:21. doi: 10.1186/s40608-016-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman E, Strauss RS. Self-reported height and weight and the definition of obesity in epidemiological studies. J Adolesc Health. 2003;33:140–141. doi: 10.1016/s1054-139x(03)00247-7. author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 36.WHO . World Health Organisation; Geneva: 2015. Guideline: Sugars intake for Adults and Children. Available from: http://apps.who.int/iris/bitstream/10665/149782/1/9789241549028_eng.pdf. [PubMed] [Google Scholar]
- 37.Gibson S. Are high-fat, high-sugar foods and diets conducive to obesity? Int J Food Sci Nutr. 1996;47:405–415. doi: 10.3109/09637489609006954. [DOI] [PubMed] [Google Scholar]