Abstract
Objective: To evaluate the effectiveness of dental treatment in improving oral health in critical patients. Methods: This randomised clinical trial was conducted in a general intensive care unit (ICU) at a tertiary care public facility from 1 January 2011 to 8 August 2013. Data from 254 adult patients staying in the ICU for 48 hours or more were analysed. The experimental group (n = 127) had access to dental treatment provided by a dentist four to five times a week, in addition to routine oral hygiene, whereas the control group (n = 127) had access only to routine oral hygiene, including topical application of chlorhexidine, provided by the ICU nursing staff. The baseline oral health status of the enrolled patients was poor and included edentulism, caries, gingivitis, periodontitis and residual roots. Dental treatment consisted of toothbrushing, tongue scraping, removal of calculus, scaling and root planing, caries restoration and tooth extraction. Results: The Oral Hygiene Index Simplified (OHI-S) and Gingival Index (GI) scores decreased in the experimental group but did not change significantly in the control group during the ICU stay. Dental treatment prevented most of the episodes of respiratory tract infections, as previously reported. No severe adverse events from the dental treatment were observed. Conclusion: From an interprofessional perspective, our results support the idea of including dentists in the ICU team to improve oral health in critical patients and effectively prevent respiratory tract infections, in addition to the improvement achievable by applying chlorhexidine alone.
Key words: Infection control, oral hygiene, prevention, clinical trials, interprofessional practice
Introduction
As a paradox of the epidemiological transition, the incidence and prevalence of healthcare-associated infections (HAIs) have increased in parallel with the development of healthcare technology over the past century and, to date, HAIs are a major public health concern in high-, middle- and low-income countries1., 2., 3., 4., 5., 6., 7.. Respiratory tract infections (RTIs) are among the most common and life-threatening HAIs and affect primarily critical patients admitted to intensive care units (ICUs)6., 8., 9., 10., 11.. The crude mortality rate associated with ventilator-associated pneumonia, the most frequent respiratory tract infection in the ICU, ranges from 0% to 60%, and the attributable mortality rate is estimated to be ≥13.0%9.
It is assumed that, in most cases, RTIs begin with the colonisation of the lower respiratory tract by pathogenic bacteria from the oral cavity, and an important risk factor for RTIs is poor oral health12., 13., 14.. A pioneering study conducted by our research group demonstrated that dental treatment performed by a dentist in a general ICU was effective in preventing lower RTIs15. The objective of this study was to describe the dental care procedures implemented and oral health outcomes observed in that study to allow replication of this intervention in other ICUs.
Materials and Methods
This study is a secondary analysis of a randomised clinical trial conducted according to the CONSORT (Consolidated Standards of Reporting Trials) statement16 in a nine-bed general ICU at a public tertiary care facility at the University Hospital of the Ribeirão Preto Medical School from 1 January 2011 to 8 August 2013. Any adult patient admitted to the ICU was considered eligible to participate in the study if they were expected to stay in the ICU for at least 2 days. Pregnant women and patients with blood dyscrasias were excluded.
The study protocol was reviewed and approved by the Human Research Ethics Committee, namely ‘Comitê de Ética em Pesquisa em Seres Humanos do Hospital das Clínicas de Ribeirão Preto’, and complied with the ethical principles of the World Medical Association Declaration of Helsinki17. Written consent was obtained from all participating patients or relatives in cases in which the patients presented a reduced level of consciousness. The study protocol was registered in The Brazilian Clinical Trials Registry (RBR-89CP93), which is affiliated to the World Health Organization (WHO) International Clinical Trial Registry Platform (Unified Trial Number U1111-1152-2671).
The dentist randomised the eligible patients by rolling a dice: an even number indicated that the patient should be included in the experimental group, whereas an odd number indicated that the patient should be allocated to the control group. The patients and dentist were not blinded to the study allocation because of the intrinsic nature of the intervention. However, a nurse from the infection control service was blinded to the patients’ allocation in order to collect clinical outcomes other than those related to the oral cavity.
The intervention group had access to dental treatment that was provided by a single dentist upon admission of patients to the ICU and four to five times a week thereafter until death or ICU discharge, in addition to access to the usual oral hygiene protocol, which was provided by the ICU nursing staff three times a day. The intervention consisted of toothbrushing with a child toothbrush (Baby 2™; Dental Line Robodente, Ribeirão Preto, Brazil), tongue scraping, removal of calculus, scaling and root planing, atraumatic restorative treatment (ART) of caries18., 19. and tooth extraction, according to the patient’s need. Toothbrushing was performed using a toothpaste without sodium lauryl sulphate because this ingredient might affect the antimicrobial activity of chlorhexidine20. Microaspiration was avoided by assessing the endotracheal tube cuff pressure before dental treatment sessions, and adjusting the pressure to 20–30 cmH2O if necessary.
The control group had access only to the routine oral hygiene protocol, consisting of mechanical cleansing of the oral cavity with a spatula wrapped in gauze, followed by topical application of 0.12% chlorhexidine solution or 2.0% chlorhexidine gel, according to the level of consciousness of the patient. A 2.0% chlorhexidine gel was used for unconscious patients; however, its bitter taste limited application of this gel to fully conscious patients and therefore 0.12% chlorhexidine solution was used in such patients. These products were formulated in-house by pharmacists. The procedures were repeated daily, three times a day, for all patients included in the study.
Data were collected prospectively from the medical records or directly from the clinical examination of patients by the dentist. The study groups were compared at baseline by evaluating demographic and clinical characteristics, including oral health status, and this variable was scored according to the Oral Hygiene Index Simplified (OHI-S) and Gingival Index (GI)21., 22.. The primary study outcome was the incidence of lower RTI, and the results were reported elsewhere15. The secondary outcomes presented in this study were OHI-S and GI and were evaluated on days 4, 7, 14, 21 and 28 of ICU admission.
The software Stata version 9.0 (StataCorp., College Station, TX, USA) and R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) were used for data analysis. The Mann–Whitney test with Bonferroni correction for multiple comparisons was used to compare OHI-S and GI scores between the study groups during the ICU stay. The non-parametric analysis of variance (ANOVA) for repeated measures was used to analyse the presence of an interaction between time and group allocation for the changes observed in OHI-S and GI scores23. A two-tailed Fisher’s exact test was used to compare the incidence of adverse events between the study groups. Sample size was calculated based on the rate of lower RTI in the ICU (20.0%) using an α of 5% and study power (1 − β) of 80%. The number of patients required in each group was estimated to be 147, considering a 60% decrease in the rate of lower RTI.
Results
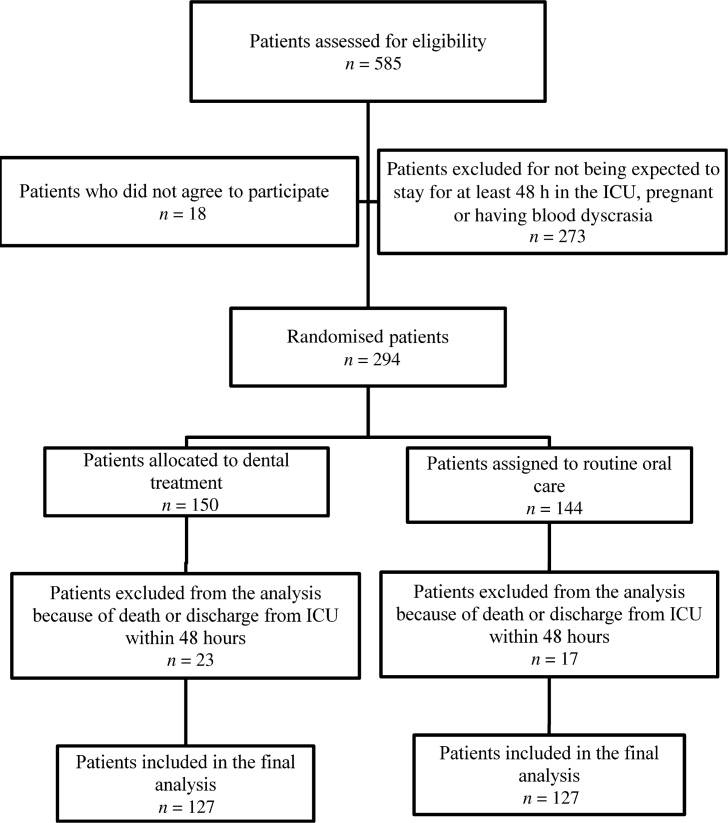
The flowchart of patient recruitment and inclusion is shown in Figure 1. Of the 585 patients initially screened, 294 were enrolled, including 150 in the experimental arm and 144 in the control arm. Patients who died or were discharged within the first 48 hours of ICU stay were not included in the final analysis. Therefore, the per-protocol population included the enrolled patients who remained in the ICU for at least 2 days.
Figure 1.
Flowchart of the inclusion criteria. ICU, intensive care unit.
The analysis of clinical and demographic features of the study populations at baseline indicated that the randomisation strategy adopted yielded appropriate allocation (Table 1). Although the mean age was slightly higher in the control arm than in the experimental arm (mean ages of 60.1 and 53.4 years, respectively), these two groups were severely ill to a similar extent, with a mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score of 23.3 and 21.7, respectively24.
Table 1.
Baseline clinical and demographic characteristics of patients subjected to routine oral care or dental treatment when admitted to the intensive care unit (ICU)
| Baseline clinical and demographic characteristics | Routine oral care*n = 127 | Dental treatment*n = 127 | |
|---|---|---|---|
| Demographic characteristics | Male sex | 66 (52.0) | 67 (52.8) |
| Mean age (years) | 60.1 ± 17.5 | 53.4 ± 18.3 | |
| Clinical characteristics | LOS prior to ICU admission (days) | 11.7 ± 13.3 | 13.2 ± 17.5 |
| Diabetes mellitus | 33 (26.0) | 42 (33.0) | |
| Hypertension | 68 (53.5) | 57 (45.0) | |
| Renal failure | 67 (52.8) | 53 (41.7) | |
| Hepatic failure | 15 (11.8) | 15 (11.8) | |
| Heart failure | 20 (15.7) | 21 (16.5) | |
| Cerebral vascular disease | 14 (11.0) | 14 (11.0) | |
| Respiratory infections | 38 (29.9) | 46 (36.2) | |
| HIV/AIDS | 5 (3.9) | 3 (2.4) | |
| Malignancy | 44 (34.6) | 38 (29.9) | |
| Coronary disease | 15 (11.8) | 10 (7.9) | |
| COPD | 20 (15.7) | 20 (15.7) | |
| Autoimmune disease | 19 (15.0) | 18 (14.2) | |
| Obesity | 36 (28.3) | 90 (70.9) | |
| Malnutrition | 26 (20.5) | 15 (11.8) | |
| APACHE II score | 23.3 ± 7.7 | 21.7 ± 8.0 | |
| Estimated risk of death | 47.3 ± 26.1 | 44.4 ± 26.1 | |
| Reasons for ICU admission | Respiratory failure | 91 (71.6) | 101 (79.5) |
| Shock | 72 (56.7) | 66 (51.2) | |
| Compromised mental status | 44 (34.6) | 37 (29.1) | |
| Major surgery, postoperative | 26 (20.5) | 23 (18.1) | |
APACHE II, Acute Physiology and Chronic Health Disease Classification System II; AIDS, acquired immune-deficiency syndrome; COPD, chronic obstructive pulmonary disease; HIV human immunodeficiency virus; LOS, length of stay.
Data are presented as n (%) of patients for categorical variables and as mean ± standard deviation for continuous variables.
Examination of the oral cavity at ICU admission revealed that both study populations had poor oral health status (Table 2). In this respect, the rate of complete edentulism was 44.9% (57/127) and 31.5% (40/127) in the control and experimental groups, respectively. Caries, gingivitis, residual roots and periodontitis were also common in these groups. The median baseline OHI-S was 2.3 (interquartile range: 1.7–3.0) for the control group and 2.0 (interquartile range: 1.5–2.5) for the experimental group.
Table 2.
Oral health status of patients subjected to a routine oral care protocol or dental treatment when admitted to the intensive care unit (ICU)
| Characteristics | Routine oral care*n = 127 | Dental treatment*n = 127 |
|---|---|---|
| Complete edentulism | 57 (44.9) | 40 (31.5) |
| Caries | 38 (29.9) | 36 (28.3) |
| Residual roots | 25 (19.7) | 18 (14.2) |
| Gingivitis | 65 (51.2) | 74 (58.3) |
| Periodontitis | 44 (34.6) | 31 (24.4) |
| Intra-oral abscess | 2 (1.6) | 0 (0) |
| Mucositis | 8 (6.3) | 8 (6.3) |
| Intra-oral candidiasis | 3 (2.4) | 1 (0.8) |
| OHI-S† | 2.3 (1.7–3.0) | 2.0 (1.5–2.5) |
Data are presented as n (%) of patients for categorical variables and as median (interquartile range) for Oral Hygiene Index Simplified (OHI-S).
OHI-S classification: 0–1.0, satisfactory; 1.1–2.0, regular; 2.1–3.0, deficient; 3.1–6.0, poor.
The proportion of patients in the experimental group who received dental treatment as needed is shown in Table 3. Except for tongue scraping and topical application of chlorhexidine, which were performed in all experimental patients (n = 127), other procedures were considered applicable only for patients presenting with at least one tooth or one residual root (n = 87).
Table 3.
Percentage of critical patients from the experimental group subjected to different dental procedures as needed
| Dental procedure | Proportion of patients | |
|---|---|---|
| Tongue scraping | 127/127 | 100% |
| Chlorhexidine application | 127/127 | 100% |
| Teeth brushing | 87/87 | 100% |
| Removal of calculus | 63/87 | 72.4% |
| Scaling and root planing | 31/87 | 35.6% |
| Atraumatic restorative treatment of caries | 8/87 | 9.2% |
| Tooth extraction | 1/87 | 1.2% |
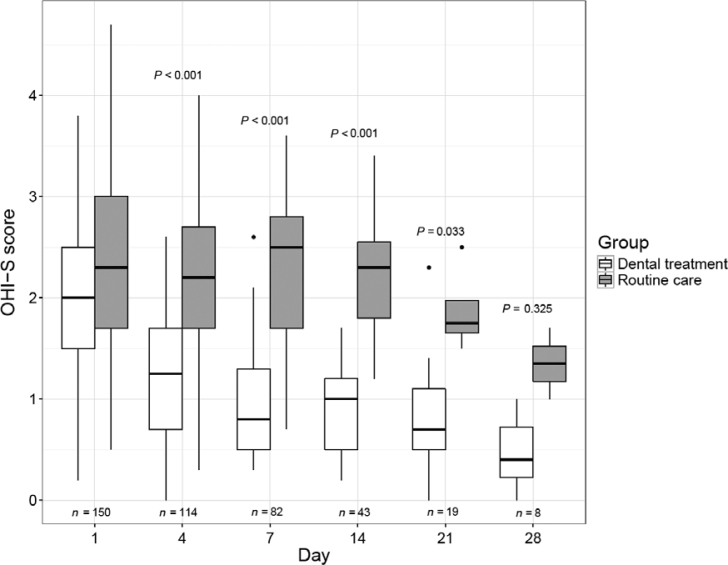
The OHI-S scores in the two study groups during the ICU stay are shown in Figure 2. The median scores were similar between the study groups but were decreased more strongly in the experimental group after day 4 and remained significantly lower than those in the control group until day 21 of ICU admission. Moreover, there was a significant interaction between time and group allocation as a determinant of OHI-S evolution during ICU stay (P = 0.021).
Figure 2.
Box plot of the evolution of the Oral Hygiene Index Simplified (OHI-S) scores in both study groups from day 1 (baseline) to day 28 of intensive care unit (ICU) admission. OHI-S classification: 0–1.0, satisfactory; 1.1–2.0, regular; 2.1–3.0, deficient; 3.1–6.0, poor. P-values refer to the comparison of OHI-S scores between the study groups evaluated on the same day (Mann–Whitney test with Bonferroni correction). Interaction between time and group allocation as a determinant of OHI-S evolution during the ICU stay (P = 0.021) was evaluated using non-parametric analysis of variance (ANOVA) for repeated measures.
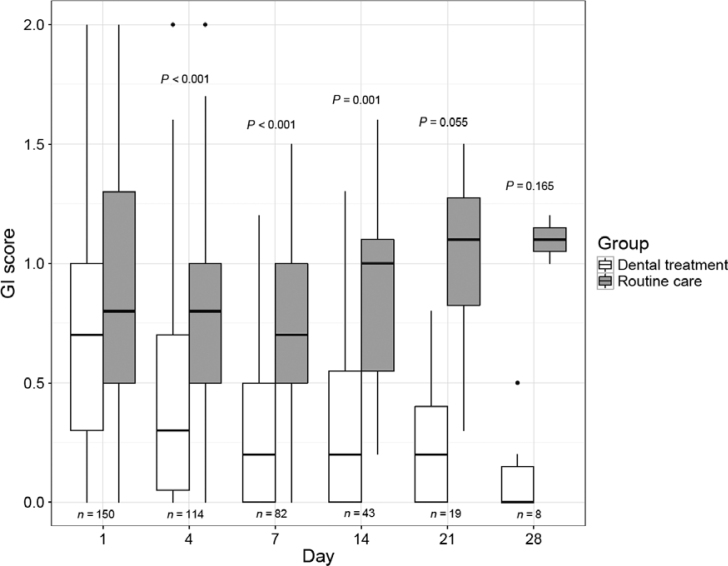
The GI scores in the two study groups during the ICU stay are shown in Figure 3. The median scores were initially similar between the study groups; however, over time, the scores became lower in the experimental group and higher in the control group. Differences between the groups were statistically significant on days 4, 7, 14 and 21 after admission to the ICU. Furthermore, there was a significant interaction between time and group allocation as a determinant of evolution of the GI during the stay in the ICU (P < 0.001).
Figure 3.
Box plot of the evolution of the Gingival Index (GI) scores in both study groups from day 1 (baseline) to day 28 of intensive care unit (ICU) admission. GI classification: 0–1.0, light gingivitis; 1.1–2.0, moderate gingivitis; 2.1–3.0, severe gingivitis. P values refer to the comparison of GI scores between the study groups evaluated on the same day (Mann–Whitney test with Bonferroni correction). Interaction between time and group allocation as a determinant of the GI evolution during the ICU stay (P < 0.001) was evaluated using non-parametric analysis of variance (ANOVA) for repeated measures.
The adverse events possibly caused by dental treatment or general oral care in the study period are shown in Table 4. The most common side effects were minor intra-oral bleeding and mucosal irritation and were more common in patients in the experimental group; however, these side effects did not prevent patients from continuing treatment or enrolling in the study. No severe adverse events potentially related to oral care were observed in the study period.
Table 4.
Comparative incidence of adverse events related to oral-care procedures according to patient allocation
| Adverse event | Routine oral care n = 127 | Dental treatment n = 127 | Relative risk (95% CI) | P* | ||
|---|---|---|---|---|---|---|
| Mucosal irritation | 3 | 2.4% | 15 | 11.8% | 5.0 (1.48–16.85) | 0.006 |
| Intra-oral bleeding | 5 | 3.9% | 4 | 3.2% | 0.80 (0.22–2.91) | 1.000 |
| All adverse events | 8 | 6.3 | 17 | 13.4% | 2.12 (0.95–4.74) | 0.090 |
95% CI, 95% confidence interval.
Two-tailed Fisher’s exact test.
Discussion
Various studies evaluated the topical application of oral antiseptics for preventing lower RTIs in critical patients, but the results were ambiguous. Some studies found that this procedure prevented such infections25., 26., 27., 28., 29., 30., 31., whereas other studies reported that this procedure had no clinical impact32., 33., 34., 35.. Our first hypothesis is that oral antisepsis may be effective only if used by patients with good oral health status because large microbial populations present in dental plaque and periodontal pockets are inaccessible to topical antiseptics36., 37., and this explains why chlorhexidine was more effective in patients undergoing elective cardiac surgery and practicing meticulous oral hygiene than in critical patients, who are usually intubated in an emergency situation and have poor oral health status32., 38., 39., 40.. Therefore, we believe that good oral hygiene should be practiced by critical patients to promote the microbicidal activity of the antiseptic applied to the oral cavity13. In this clinical trial, dental treatment prevented approximately 56% of lower RTIs compared with the control group (adjusted relative risk, 0.44; 95% confidence interval, 0.20–0.96; P = 0.04), as previously reported15.
Our second hypothesis is that dentists are needed in the intensive care team because of their training and skills in performing procedures required by critical patients during their ICU stay, including restoration of caries, scaling and root planing, removal of calculus, draining of intra-oral abscesses and tooth extraction41., 42.. Although the ICU nursing staff plays an important role in promoting oral hygiene, studies have demonstrated that toothbrushing is insufficient to prevent RTIs43., 44., 45.. Our results indicated that patients treated by dentists had better OHI-S and GI scores than patients treated exclusively by the nursing staff during the ICU stay. A dental hygienist may be able to perform some of the described procedures46, including removal of calculus and tongue scraping, leaving only the most specific procedures (such as tooth restoration and extraction) to be performed by a dentist. However, more studies are needed to confirm this assumption. Notwithstanding, our results demonstrate the clinical benefits of interprofessional and coordinated care of critical patients.
Although cost–benefit and cost-effectiveness analyses were not performed, the number-needed-to-treat was calculated to avoid one episode of lower RTI, which was estimated to be 10.5. This low number suggests that including a dentist in the intensive care team may be cost-effective, taking into account that one single episode of ventilator-associated pneumonia, the most common nosocomial RTI, may result in an extra cost of up to US$ 39,82847., 48., 49..
Although adverse events were more common in patients in the experimental group, these events were mild or moderate and did not affect patient enrollment, confirming the safety of the intervention. However, it should be highlighted that patients with blood dyscrasias were excluded from the trial, and thus safety data were not obtained for this population, and this group might experience major intra-oral bleeding during dental procedures.
Our study presents at least two limitations. First, our results may not be readily generalisable to all critical care patients because the less critical and most critical patients stayed for fewer than 48 hours in the ICU and therefore were excluded from the study. Furthermore, the clinical impact of the intervention may be lower in ICU populations with good oral health status at baseline.
Second, our data may be susceptible to measurement bias because the blinding of patient and dentist was not feasible in view of the intrinsic nature of the intervention. However, this limitation seems unlikely because the primary study outcome (rate of RTIs) was evaluated by a blinded investigator and yielded results consistent with the data presented herein.
Conclusion
From the perspective of interprofessional practice, our results support the idea of including a dentist in the intensive care team to improve the oral health status of critical patients, in addition to the improvement achievable by applying chlorhexidine alone, thus preventing lower RTIs more effectively.
Acknowledgements
The authors are grateful to Teresa M. N. Morais, D.D.S., M.S., a pioneer in implementing dental treatment for intensive care patients in Brazil who served as inspiration for this study, and Edson Antônio Nicolini, who significantly contributed to the development and implementation of the present study.
Conflicts of interest
All authors declare that there are no conflicts of interest associated with this study.
Funding
This study was funded by two non-profit organisations: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) under Process No. 2010/51063-4, and Fundação de Apoio ao Ensino, Pesquisa, e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA).
Trial registration
This trial was registered in The Brazilian Clinical Trials Registry (RBR-89CP93), which is affiliated to the WHO International Clinical Trial Registry Platform (UTN: U1111-1152-2671).
References
- 1.McKeown RE. The epidemiologic transition: changing patterns of mortality and population dynamics. Am J Lifestyle Med. 2009;3(1 Suppl):19S–26S. doi: 10.1177/1559827609335350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet Lond Engl. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 3.Zarb P, Coignard B, Griskeviciene J, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17:1–16. doi: 10.2807/ese.17.46.20316-en. [DOI] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva JM, Rezende E, Guimarães T, et al. Epidemiological and microbiological analysis of ventilator-associated pneumonia patients in a public teaching hospital. Braz J Infect Dis. 2007;11:482–488. doi: 10.1590/s1413-86702007000500009. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50:1–26. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46:322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS, Klompas M, Balk R, et al. Developing a new national approach to surveillance for ventilator-associated events: executive summary. Am J Infect Control. 2013;41:1096–1099. doi: 10.1016/j.ajic.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Melsen WG, Rovers MM, Groenwold RHH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Sen P, Gaind R, et al. Prospective surveillance of device-associated healthcare-associated infection in an intensive care unit of a tertiary care hospital in New Delhi, India. Am J Infect Control. 2018;46:202–206. doi: 10.1016/j.ajic.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Salomao R, Rosenthal VD, Grimberg G, et al. Device-associated infection rates in intensive care units of Brazilian hospitals: findings of the International Nosocomial Infection Control Consortium. Rev Panam Salud Pública. 2008;24:195–202. doi: 10.1590/s1020-49892008000900006. [DOI] [PubMed] [Google Scholar]
- 12.Heo S-M, Haase EM, Lesse AJ, et al. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 2008;47:1562–1570. doi: 10.1086/593193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT. Ventilator-associated pneumonia and oral health. Rev Soc Bras Med Trop. 2012;45:543–544. doi: 10.1590/s0037-86822012000500001. [DOI] [PubMed] [Google Scholar]
- 14.Kallet RH. The vexing problem of ventilator-associated pneumonia: observations on pathophysiology, public policy, and clinical science. Respir Care. 2015;60:1495–1508. doi: 10.4187/respcare.03774. [DOI] [PubMed] [Google Scholar]
- 15.Bellissimo-Rodrigues WT, Menegueti MG, Gaspar GG, et al. Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: a randomized clinical trial. Infect Control Hosp Epidemiol. 2014;35:1342–1348. doi: 10.1086/678427. [DOI] [PubMed] [Google Scholar]
- 16.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.Frencken JE, Songpaisan Y, Phantumvanit P, et al. An atraumatic restorative treatment (ART) technique: evaluation after one year. Int Dent J. 1994;44:460–464. [PubMed] [Google Scholar]
- 19.Frencken JE, Pilot T, Songpaisan Y, et al. Atraumatic restorative treatment (ART): rationale, technique, and development. J Public Health Dent. 1996;56:135–140. doi: 10.1111/j.1752-7325.1996.tb02423.x. discussion 161–163. [DOI] [PubMed] [Google Scholar]
- 20.de Sousa AM, Pochapski MT, dos Santos FA, et al. Estudo clínico sobre a influência do dentifrício na efetividade da clorexidina no controle do biofilme dental. Periodontia. 2009;19:71–75. [Google Scholar]
- 21.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;1939:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 22.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 23.Brunner E, Domhof S, Langer F. Wiley; New York, NY: 2002. Nonparametric analysis of longitudinal data in factorial experiments; p. 296. [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 25.Hua F, Xie H, Worthington HV, et al. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10:CD008367. doi: 10.1002/14651858.CD008367.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klompas M, Speck K, Howell MD, et al. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174:751–761. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 27.Labeau SO, Van de Vyver K, Brusselaers N, et al. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:845–854. doi: 10.1016/S1473-3099(11)70127-X. [DOI] [PubMed] [Google Scholar]
- 28.Özçaka Ö, Başoğlu OK, Buduneli N, et al. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res. 2012;47:584–592. doi: 10.1111/j.1600-0765.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 29.Villar CC, Pannuti CM, Nery DM, et al. Effectiveness of intraoral chlorhexidine protocols in the prevention of ventilator-associated pneumonia: meta-analysis and systematic review. Respir Care. 2016;61:1245–1259. doi: 10.4187/respcare.04610. [DOI] [PubMed] [Google Scholar]
- 30.Shi Z, Xie H, Wang P, et al. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2013;8(8):CD008367. doi: 10.1002/14651858.CD008367.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Xie D, Li A, et al. Oral topical decontamination for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. J Hosp Infect. 2013;84:283–293. doi: 10.1016/j.jhin.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT, Viana JM, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect Control Hosp Epidemiol. 2009;30:952–958. doi: 10.1086/605722. [DOI] [PubMed] [Google Scholar]
- 33.Mohr NM, Pelaez Gil CA, Harland KK, et al. Prehospital oral chlorhexidine does not reduce the rate of ventilator-associated pneumonia among critically ill trauma patients: a prospective concurrent-control study. J Crit Care. 2015;30:787–792. doi: 10.1016/j.jcrc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Munro CL, Grap MJ, Sessler CN, et al. Preintubation application of oral chlorhexidine does not provide additional benefit in prevention of early-onset ventilator-associated pneumonia. Chest. 2015;147:328–334. doi: 10.1378/chest.14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haghighi A, Shafipour V, Bagheri-Nesami M, et al. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust Crit Care. 2017;30:69–73. doi: 10.1016/j.aucc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto H, Urade M. Mechanical tooth cleaning before chlorhexidine application. Am J Respir Crit Care Med. 2007;175:418. doi: 10.1164/ajrccm.175.4.418a. [DOI] [PubMed] [Google Scholar]
- 37.Moraes LC, Fatturi-Parolo CC, Ferreira MBC, et al. Saliva, supragingival biofilm and root canals can harbor gene associated with resistance to lactamic agents. Braz Oral Res. 2015;29:52. doi: 10.1590/1807-3107BOR-2015.vol29.0052. [DOI] [PubMed] [Google Scholar]
- 38.DeRiso AJ, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–1561. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 39.Segers P, Speekenbrink RGH, Ubbink DT, et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296:2460–2466. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 40.Chan EY, Ruest A, Meade MO, et al. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ. 2007;334:889. doi: 10.1136/bmj.39136.528160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz MK, Morais TMN, Trevisani DM. Clinical assessment of the oral cavity of patients hospitalized in an intensive care unit of an emergency hospital. Rev Bras Ter Intensiva [Internet] 2014;26:379–383. doi: 10.5935/0103-507X.20140058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morais TM, Silva A, Avi AL, et al. [Importance of dental work in patients under intensive care unit] Rev Bras Ter Intensiva. 2006;18:412–417. [PubMed] [Google Scholar]
- 43.Alhazzani W, Smith O, Muscedere J, et al. Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta-analysis of randomized trials evaluating ventilator-associated pneumonia. Crit Care Med. 2013;41:646–655. doi: 10.1097/CCM.0b013e3182742d45. [DOI] [PubMed] [Google Scholar]
- 44.Lorente L, Lecuona M, Jiménez A, et al. Ventilator-associated pneumonia with or without toothbrushing: a randomized controlled trial. Eur J Clin Microbiol Infect Dis. 2012;31:2621–2629. doi: 10.1007/s10096-012-1605-y. [DOI] [PubMed] [Google Scholar]
- 45.Chacko R, Rajan A, Lionel P, et al. Oral decontamination techniques and ventilator-associated pneumonia. Br J Nurs. 2017;26:594–599. doi: 10.12968/bjon.2017.26.11.594. [DOI] [PubMed] [Google Scholar]
- 46.Barnes CM. Dental hygiene intervention to prevent nosocomial pneumonias. J Evid-Based Dent Pract. 2014;14(Suppl):103–114. doi: 10.1016/j.jebdp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Anderson DJ, Kirkland KB, Kaye KS, et al. Underresourced hospital infection control and prevention programs: penny wise, pound foolish? Infect Control Hosp Epidemiol. 2007;28:767–773. doi: 10.1086/518518. [DOI] [PubMed] [Google Scholar]
- 48.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33:250–256. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 49.Restrepo MI, Anzueto A, Arroliga AC, et al. Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect Control Hosp Epidemiol. 2010;31:509–515. doi: 10.1086/651669. [DOI] [PubMed] [Google Scholar]