Abstract
Recent animal studies demonstrate that compulsive cocaine seeking strongly reduces prelimbic frontal cortex activity, while optogenetic stimulation of this brain area significantly inhibits compulsive cocaine seeking, providing a strong rationale for applying brain stimulation to reduce cocaine consumption. Thus, we employed repetitive transcranial magnetic stimulation (rTMS), to test if dorsolateral prefrontal cortex (DLPFC) stimulation might prevent cocaine use in humans. Thirty-two cocaine-addicted patients were randomly assigned to either the experimental group (rTMS) on the left DLPFC, or to a control group (pharmacological agents) during a 29-day study (Stage 1). This was followed by a 63-day follow-up (Stage 2), during which all participants were offered rTMS treatment. Amongst the patients who completed Stage 1, 16 were in the rTMS group (100%) and 13 in the control group (81%). No significant adverse events were noted. During Stage 1, there were a significantly higher number of cocaine-free urine drug tests in the rTMS group compared to control (p=0.004). Craving for cocaine was also significantly lower in the rTMS group compared to the controls (p=0.038). Out of 13 patients who completed Stage 1 in the control group, 10 patients received rTMS treatment during Stage 2 and showed significant improvement with favorable outcomes becoming comparable to those of the rTMS group. The present preliminary findings support the safety of rTMS in cocaine addicted patients, and suggest its potential therapeutic role for rTMS-driven PFC stimulation in reducing cocaine use, providing a strong rationale for developing larger placebo-controlled studies.
Keywords: Addiction, Cocaine use disorder, Craving, Prefrontal cortex, Repeated transcranial magnetic stimulation, Optogenetics
1. Introduction
Cocaine use disorder (CUD) represents a significant health problem and is very common worldwide, with about 14–21 million users in 2014 (European Monitoring Centre for Drugs and Drug Addiction, 2014). In spite of the significant morbidity associated with cocaine use, no unequivocally effective pharmacological or psychological therapies have been identified to date. Chronic cocaine use causes damage and changes in the prefrontal cortex (PFC) (Volkow et al., 2004a), including significant brain volume reduction (Moreno-López et al., 2012; Matochik et al., 2003), cortical hypoactivity (Goldstein and Volkow, 2002, 2011; Kaufman et al., 2003), impairment in executive functions, and dysregulation of neurotransmitters systems (Volkow et al., 2003; Licata and Renshaw, 2010; Ke et al., 2004).
Physiologically, the PFC is thought to play a critical role in the addictive cycle, including reinforcement learning, craving, and inhibitory control (Koob and Volkow, 2010). Importantly, preclinical studies have also shown that loss of inhibitory control, resulting from damage to the PFC, seems to be crucial in compulsive drug-seeking behaviors (Jasinska et al., 2014; Chen et al., 2013). In particular, recent research employed a rat model in which compulsive cocaine seeking persisted in a subgroup of rats despite delivery of mild foot shocks, and demonstrated that prolonged cocaine self-administration significantly decreased in vivo and ex vivo intrinsic excitability of deep layer pyramidal neurons in the prelimbic cortex (PLC), which was significantly more pronounced in compulsive drug-seeking animals. Furthermore, in vivo optogenetic prelimbic cortex stimulation significantly prevented compulsive cocaine seeking (Chen et al., 2013). These findings created a rationale, and additional data to test the hypothesis that stimulation of functionally equivalent brain regions in humans could reduce cocaine seeking and consumption.
Several studies indicate that the rodent PLC is the closest functional homolog of the dorsolateral PFC (DLPFC) in humans (Papaleo et al., 2012; Balleine and Dickinson, 1998), while others suggest a functional correspondence with the anterior cingulate cortex (ACC) (Gass and Chandler, 2013). Consensus on this matter is still lacking, due to the extraordinarily large anatomical diversity between the rodent and the human frontal/anterior cortices, but both DLPFC and ACC play a major role in top–down inhibitory control and reward mechanisms. They are also linked structurally and functionally (Holroyd and Coles, 2002), as it has been shown that neurostimulation of DLPFC has direct effects over ACC (Conti and Nakamura-Palacios, 2014).
Therefore, a direct clinical translation of the previous preclinical literature (Chen et al., 2013) could be attempted by testing the hypothesis that electrical stimulation of the DLPFC significantly decreases compulsive cocaine seeking behaviors. Operationally, this hypothesis can be tested by using transcranial magnetic stimulation (TMS), a non-invasive, and safe, human brain stimulation technology based on electromagnetic induction (Lefaucheur et al., 2014; Barker et al., 1985). The TMS-induced intracranial electric field can be of sufficient magnitude to depolarize neurons (Terao and Ugawa, 2002; Di Lazzaro et al., 2008). The extent of the induced field depends on the TMS coil geometry and size; figure-of-eight coils allow relatively focal targeting of the brain surface (Cohen et al., 1990). While the effects of an individual TMS pulse lasts only a fraction of a second, when TMS pulses are applied repetitively, they can modulate long-term cortical excitability. Specifically, repetitive TMS (rTMS) at a low frequency (about 1 Hz) is typically considered to have inhibitory effects, (Chen et al., 1997) while high-frequency rTMS (≥5 Hz) is excitatory (Pascual-Leone et al., 1994). Therefore, this pilot study was conducted to test whether excitatory rTMS of the left DLPFC in cocaine-dependent patients is safe and reduces cocaine use.
2. Experimental procedures
2.1. Study design
This was a between-subject open-label randomized clinical trial with rTMS vs. standard treatment (experimental vs. control group, respectively) conducted at the Department of Neuroscience Out-patient Clinic of the teaching hospital affiliated with the medical school of the University of Padua, Italy. The appropriate local Ethics Committee reviewed and approved the study. Patients were individuals seeking outpatient treatment for CUD. For inclusion/exclusion criteria, see Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Diagnoses based on the diagnostic and statistical manual of mental disorders, 5th Edition (DSM-5).
2.2. Treatment conditions
2.2.1. Experimental group:
The experimental group was treated with rTMS. The stimulator device was a MagPro R30 with the Cool-B70 figure-of-eight coil (MagVenture, Falun, Denmark). Resting Motor Threshold (rMT) measurements were performed via visual twitch in the contralateral (right) hand. The coil was positioned over the supposed motor cortex area, then the coil was moved until the location at which a reproducible APB (abductor pollicis brevis) response, elicited at the lowest stimulator intensity could be identified. We determined the lowest device output to produce the thumb movement 50% of the trials (Ziemann and Hallett, 2000), using single-pulse TMS with at least 6 seconds between pulses, and that was set as rMT. To best identify the target of the rTMS stimulation, the left DLPFC, we used a TMS Navigator (LOCALITE, St. Augustin, Germany), using individual level MRIs. The coil was placed as follows. The TMS navigation system was used only during an initial planning session where the subject’s head was fitted with an individual TMS cap. The MRI of the participant was co-registered and stretched using linear methods (9 degrees-of-freedom) to match the MNI template brain with the Localite navigation software. Thereafter, the TMS coil center was placed at MNI coordinates [x=−50, y=30, z=36] with the coil handle pointing 45° relative to the midsagittal line. This coil location was marked on the cap. During the subsequent therapy sessions, the TMS coil was placed in the same position using the cap markings. In order to assure that the cap was placed exactly in the same place at each session, the distance from nasion, inion and set ears was determined for each patient.
The TMS parameters were as follows: 15 Hz frequency, pulse intensity 100% of the rMT, 60 pulses per train, inter train pause of 15 s, 40 stimulation trains, and 2400 total pulses for a total duration of 13 min. These parameters were based on two previous studies where rTMS has been used to treat CUD patients. The first one (Camprodon et al., 2007) used 10 Hz, 90% threshold, and 2000 total pulses, the second one (Politi et al., 2008) used 15 Hz, 100% threshold, and 600 total pulses. The first study included six patients and one treatment session only, whereas the second study had thirty-six patients and ten rTMS sessions. rTMS parameters in these studies seemed quite conservative. Thus, we decided to modify the previous study settings, by trying to set a higher level of stimulation, yet widely respecting the safety guidelines (Rossi et al., 2009).
2.2.2. Control group
The control group was treated with an established protocol used at the Department of Neuroscience of the University of Padua, Italy, where the study was conducted. This protocol included pharmacological treatment with pramipexole 0.35 mg t.i.d., bupropione 150 mg daily, oxazepam 15 mg t.i.d., triazolam 0.25 mg daily, and gamma hydroxybutyrate 1.75 g daily. The rationale for using these medications was that: 1) depressive, anxiety and sleep-related symptoms are often present in CUD patients that stop using cocaine; and 2) these medications used at the doses reported above may improve these symptoms. Specifically, bupropione (Reimherr et al., 1998) and pramipexole (Corrigan et al., 2000; Mah et al., 2011) may improve depressive symptoms, oxazepam (Ansseau et al., 1990) may relief anxiety-related symptoms and triazolam (Pakes et al., 1981), and gamma hydroxybutyrate (Mamelak et al., 1985) may improve sleep problems.
No alcohol use was allowed during the study, as it would be a potential confounder. Considering that social/binge drinkers were allowed to be in the study if they did not satisfy criteria for alcohol use disorder, all patients enrolled in the study were asked to abstain from alcohol use during the duration of the study. In order to assure compliance, disulfiram 400 mg daily was administered during the duration of the study.
2.2.3. Study procedures
The study consisted of three stages: in-person screening (Stage 0), 29-day randomized treatment (Stage 1), and 63-day follow-up (Stage 2).
2.2.3.1. Stage 0 (Screening).
Potential participants were recruited among those referred to the Outpatient Clinic seeking treatment for CUD. Screening included psychological assessments, medical history, physical exam, and urine labs.
2.2.3.2. Stage 1 (Treatment).
Eligible participants were randomized to the rTMS or control group for 29 days. Both groups were seen with the same frequency in the Outpatient Clinic, i.e. once a day during the first five days, and then twice a week for the following three weeks. The control group was treated pharmacologically, as detailed before, while the experimental group received one rTMS session per day during the first five days of treatment, and then once a week for the following three weeks, for a total of 8 rTMS sessions. The length of each visit was approximately the same across both groups (e.g., while patients enrolled in the experimental group were receiving the TMS session, those in the control group received medical management for the pharmacological treatment). At each visit, possible adverse events (AEs) were evaluated, previous cocaine use was determined via a urine drug test and craving for cocaine was assessed using a 0–10 Visual Analog Scale. The urine drug screen panel also included the following: morphine, methadone, THC, phencyclidine, amphetamine, and methamphetamine.
2.2.3.3. Stage 2 (Follow-up).
At the end of the 29-day treatment stage, a 63-day follow-up took place during which participants were offered to continue being followed-up with the same treatment received during the Stage 1, or to switch to the other treatment. For those participants in the control group that agreed to receive rTMS sessions during the Stage 2, a 1 week wash-out window from the pharmacological treatments was applied before starting the first rTMS session. During the Stage 2, rTMS took place (if applicable) once a week for the rTMS group, while the others received a rTMS session per day during the first five days of treatment, and then once a week for the following weeks as in the Stage 1 rTMS group. Adverse events and cocaine use via urine drug test were monitored twice a week.
3. Study outcomes and assessments
3.1. Adverse events (AEs)
Adverse events experienced by the participants in response to treatment was collected during each visit by the study physicians.
3.1.1. Cocaine use outcomes
Primary outcome was the use of cocaine during Stage 1, assessed as either a positive or negative urine drug screen for cocaine in the rTMS vs. control groups. As expected, at the beginning of the treatment, all patients had drug urine screen positive for cocaine. Consistent with the potential positivity of a urine drug screen due to cocaine metabolites, a grace period was applied for the first 8 days of Stage 1. Therefore, except for the baseline comparisons and AE analyzes, all other analyzes were conducted starting on Day 9. Secondary outcomes included cocaine use during Stage 2, including comparisons to Stage 1 (within-subject comparisons), and cocaine craving.
3.1.2. Assessment of depressive symptoms
Given the high comorbidity between CUD and depression (Hatzigiakoumis et al., 2011; Roy et al., 2015), depressive symptoms were assessed. The depression subscale of the SCL-90 (Lipman, 1986) was used; its validity, compared to the standard Hamilton Depression Scale, has been shown (Bech et al., 2014). The SCL-90 was administered at baseline (pre-treatment), and then at Day 5 and Day 29.
4. Statistical analysis
In univariate analysis, for normally distributed variables the Student t-test between groups was used, while for categorical variables the Chi Squared test was performed. In multivariate analysis, the logistic regression was performed. McNemar Chi squared test was used to test for significance in repeated measure for dichotomous variables. Kaplan Meier curves were used to plot the cumulated proportion of event free patients in the groups: significance between pairs of curves was tested by Log-Rank. For craving evaluation, Anova for Repeated Measure was performed to assess the interaction between group and craving decrease. The significance level was set at p<0.05.
5. Results
5.1. Participant characteristics
Of 45 individuals screened in the Clinic, 32 CUD patients were eligible, signed the informed consent and were randomized (rTMS group, n=16; control group, n=16). The consent form included information regarding the experimental nature of the rTMS treatment, as well as the fact that CUD is not an indication for the medications used in the control group. Figure 1 outlines the study flow, including reasons why 13 subjects were excluded. Baseline characteristics of the enrolled patients are shown in Table 2. The two groups were comparable for baseline and demographic characteristics, with the exception of age (Table 2), which was therefore used as a covariate in the analysis.
Figure 1.

Study flow-chart of patient participation comparing control and rTMS treatment groups.
Table 2.
Participant characteristics at baseline [M ± (SD) or percentage (%)].
| rTMS group (n = 16) | Control group (n = 16) | |
|---|---|---|
| Age* | 43.50 (9.75) | 37.06 (5.95) |
| Women (N) | 2 | 0 |
| Race: Caucasians (%) | 100 | 100 |
| Age of first cocaine use | 26.69 (9.34) | 24.06 (6.23) |
| Years of cocaine use | 16.81 (7.95) | 13.00 (5.55) |
| Cocaine use during the last month (days per week) | 4.81 (1.94) | 4.31 (2.02) |
| Cocaine use during the last month (grams per day) | 1.81 (1.11) | 1.75 (0.77) |
| Tobacco smokers (%) | 62.5% | 56.25% |
| Last use | ||
| Less than 24 h | 37.50% | 43.75% |
| Between 24 and 48 h | 18.75% | 25.00% |
| More than 48 h | 43.75% | 31.25% |
p<0.05; unless otherwise noted, there were no significant differences between the groups on these measures [p>0.05]
Twenty-nine participants (rTMS group, n=16; control group, n=13) completed Stage 1. Out of the 16 participants assigned to the experimental group, one subject did not complete the follow-up (Stage 2), dropping out at day 46 of Stage 2, while the other 15 patients continued, therefore receiving weekly rTMS sessions. Out of the 13 participants who completed Stage 1 in the control group, 10 participants switched to weekly rTMS sessions during Stage 2, while 2 wanted to continue the standard treatment (both of them had good response during Stage 1, as also documented by negative urine drug screens) and 1 participant decided to discontinue completely the Stage 2 follow-up (Figure 1).
5.2. Safety
A few participants in the rTMS group reported mild discomfort at the start of stimulation, especially during the first session, but overall there were no significant differences in AEs across groups. rTMS treatment was safe and there were no serious or unexpected adverse events (AEs).
During the duration of the study, 5 patients had a positive urine drug screen for THC (3 in the rTMS group and 2 in the control group). No urine drug screens were positive for the other drugs assessed (morphine, methadone, phencyclidine, amphetamine, and methamphetamine) during the study.
5.3. Cocaine use outcomes
Consistent with the intention to treat analysis, the outcome was defined positive if the patient never relapsed (i.e., urine drug screen was always negative after the grace period) during Stage 1, while it was defined negative if there was at least one relapse (i.e., at least 1 urine drug screen positive for cocaine after the grace period) or if the patient dropped out during Stage 1. Positive outcomes were 11 (69%) in the rTMS group and 3 (19%) in the control group. A univariate analysis demonstrated that, during Stage 1, the difference in the number of positive outcomes between the two groups was statistically significant (Chi square, p=0.004).
Considering the difference in age between the two groups (Table 2), a multivariate analysis was conducted next, using the logistic regression model adjusted for age (a variable for which the two groups showed significant difference). This analysis indicated that the effect of the therapy remained significant (p=0.035) with an OR=6.47 (95% CI 1.14=−36.6), while the effect of age was not statistically significant (p=0.102). We also conducted a Kaplan Meier curve where the event was positive drug screen; this analysis confirmed a significant difference between rTMS and standard treatment (log rank p=0.0013) (Figure 2).
Figure 2.
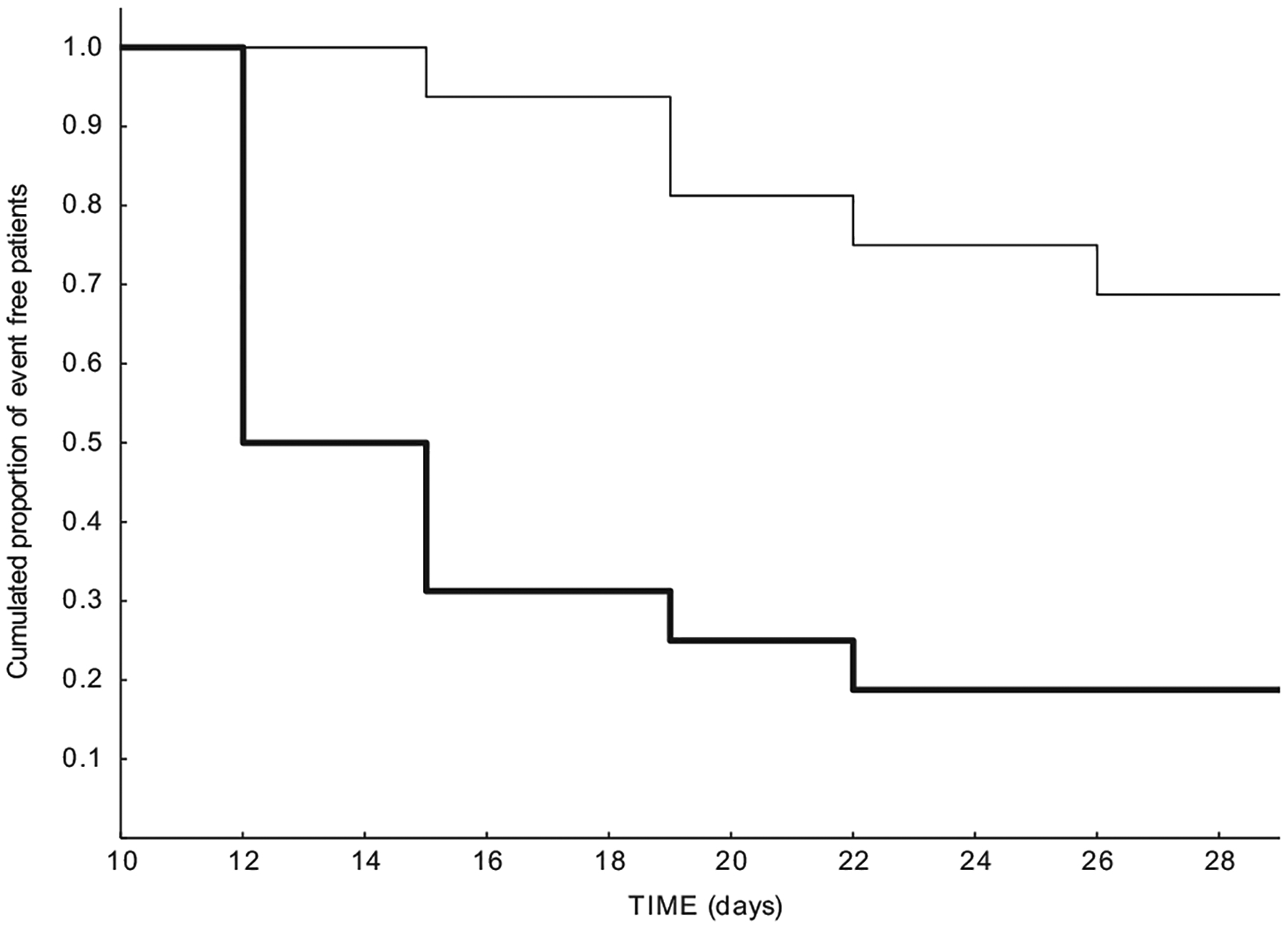
Kaplan Meier curve for comparison between rTMS (thin line) and controls (thick line) during Stage 1. Event is positive drug urine screen (log rank p=0.0013).
5.4. Outcomes during the Stage 2 follow-up
Out of the 10 patients in the control group who switched to the rTMS treatment during Stage 2, three patients presented with a positive drug screen. A within-subject comparison indicated that during Stage 1, eight had a positive drug screen with standard treatment. This difference was significant using the McNemar Chi square (one tail, p=0.037).
Next, we performed a logistic regression model adjusted for age, to compare the results of the rTMS group with the control group that moved to rTMS during Stage 2. Specifically, the comparison was made between the rTMS group in Stage 1 (the period from Day 12 to 29), and the control group in Stage 2 (the period from Day 47 and 64) who shifted to TMS treatment. The regression model showed no significant difference related to the shift (p=0.64) and age (p=0.17).
5.5. Craving
During Stage 1, there was a significant difference in cocaine craving scores, i.e. craving was significantly lower in the rTMS group vs. controls [F(1,27)=4,7379, p=0.038; Figure 3]. During Stage 2, a comparison of the groups (both treated with rTMS during this stage) revealed no significant difference in craving (p>0.05).
Figure 3.
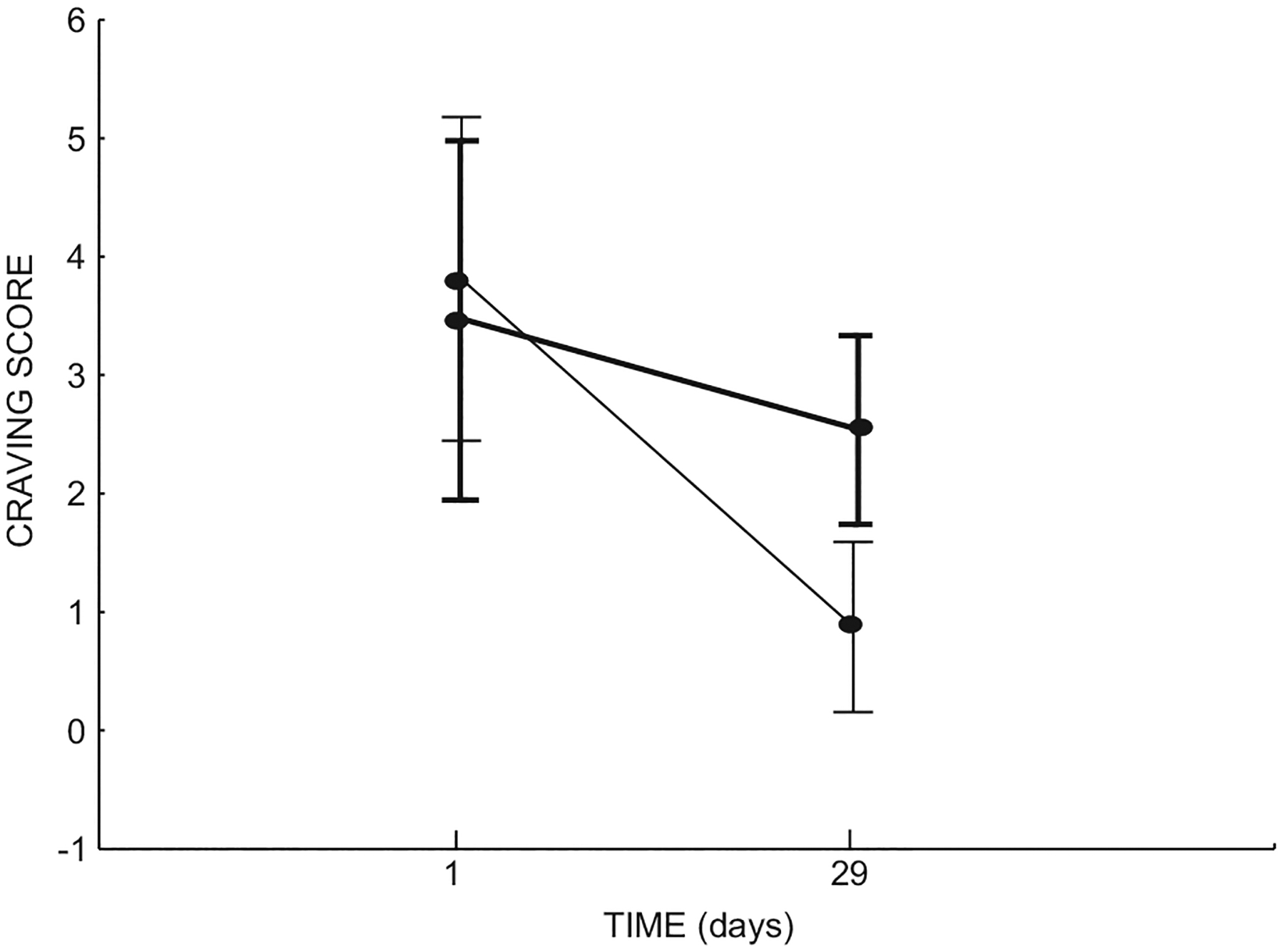
Significant differences in craving scores between the rTMS (thin line) and control (thick line) groups.
5.6. Depressive symptoms
There were no differences on the SCL-90 depression subscale score at baseline (pre-treatment) between the two groups (p>0.05). During the Stage 1 treatment, there was an improvement on depressive symptoms in both groups, but there were no differences between the two groups [F (2,54)=0.70963, p=0.49].
6. Discussion
This study provides strong and novel, albeit preliminary information, on the role of rTMS and the DLPFC in treating cocaine addiction. Study limitations need to be acknowledged, in particular: the small sample, the open-label design, the lack of urine drug screen for benzodiazepines, and the short duration of treatment. Another important limitation was the use of a control group treated pharmacologically (as opposed to a sham group). It is also important to highlight that there are no formal data, in terms of efficacy and safety, on the combined use of these medications, although no serious or unexpected side-effects were reported in our control group.
While disulfiram administration was used to assure compliance to total alcohol abstinence, it has been suggested that disulfiram may have anti-craving properties in cocaine addiction (Suh et al., 2006). As such, disulfiram might also have improved the cocaine use outcomes in our study. However, it is unlikely that disulfiram acted as a confounder to our findings, given that it was used in both groups, and still a significant effect of rTMS on our outcomes was found. Nonetheless, future studies testing the role of rTMS in CUD patients should consider alternative non-pharmacological approaches to assure compliance to alcohol abstinence. This consideration is also important given that the combination of cocaine with the disulfiram–alcohol interaction can be associated with side-effects, including potentially severe cardiovascular problems (Karila et al. 2008).
In spite of these limitations, this study supports both the safety of the use of rTMS in CUD patients, and it represents the first preliminary clinical evidence of rTMS efficacy in treating CUD patients. Notably, this study used a cocaine urine drug screen as the primary outcome, thereby providing an objective marker of response as opposed to self-reported assessments.
From a safety standpoint, while rTMS has been recently established as a safe therapeutic tool, it is important to keep in mind that most of the literature on rTMS does not include CUD patients. These patients may present with long-lasting adaptations and changes in the brain. Given that rTMS treatment results in functional changes in brain activity, establishing the safety of rTMS administration in CUD patients is important. Notably, patients enrolled in this study were all active CUD subjects, and all of them presented with urine drug tests positive for cocaine at the time the first TMS session took place. As such, this study provides much needed safety information on the use of rTMS in this population.
Animal and human studies demonstrate that rTMS alters cortical excitability through the modulation of different neurotransmitters involved in addiction-related processing, including dopamine and GABA (Barr et al., 2011). Combined rTMS/PET studies in healthy subjects demonstrated that rTMS over the DLPFC resulted in increased levels of extracellular dopamine (Cho and Strafella, 2009), and induced dopamine release in the caudate nucleus (Strafella et al., 2001), while dopaminergic activity was reduced during withdrawal (Volkow et al., 2004b). Additionally, rTMS has been shown to enhance GABA neurotransmission (McDonnell et al., 2006) through increased cortical inhibitory activity (Daskalakis et al., 2006).
Previous studies provide additional support for the use of rTMS in cocaine abusers. In fact, two previous clinical studies (Camprodon et al., 2007; Politi et al., 2008) indicated that rTMS over DLPFC resulted in decreased self-reported cocaine craving in cocaine-dependent patients. However, none of these studies (one of which included only six patients) addressed if, beyond reducing a subjective outcome such as cocaine craving, rTMS was useful in reducing cocaine use, which is not only an objective outcome, but also the most relevant outcome from a clinical standpoint. This study also holds important and unprecedented translational significance. The fact that rTMS treatment applied to DLPFC reduced cocaine use and craving in humans is strikingly consistent with recent preclinical findings showing a marked reduction in prelimbic cortex excitability in compulsive cocaine seeking rats (Chen et al., 2013), further highlighting the importance of rodent studies to provide rationale for designing clinical trials.
In this study, there was no significant improvement of depressive symptoms in the rTMS group compared to the control group. This observation is consistent with recent work indicating a lack of antidepressant efficacy for treatment of CUD (Pani et al., 2011), and that changes in depressive symptoms did not predict future cocaine abstinence (Milby et al., 2015). As such, it seems unlikely that our results were due to an improvement in depressive symptoms, given that rTMS was significantly more effective than the control group on the cocaine outcomes, but not on the depressive symptoms. However, it is important to keep in mind the high comorbidity between CUD and depression (Hatzigiakoumis et al., 2011; Roy et al., 2015), as well as the preliminary nature of the present study. As such, future randomized controlled trials and mechanistic studies are needed to shed light on the potential role of rTMS in treatment of CUD patients with depression comorbidity, and on the potential action mechanisms of how rTMS may improve cocaine outcomes, i.e. directly acting on craving for cocaine, and/or indirectly acting via an improvement of depressive symptoms.
Although controlled studies are needed to establish efficacy, the results of this study tentatively suggest that rTMS is an effective treatment for CUD. Not only does our study confirm previous observations on reduced cocaine craving after rTMS (Camprodon et al., 2007; Politi et al., 2008), but it also provides much needed novel information. In fact, this is the first clinical report indicating that rTMS treatment resulted in significant reduction in cocaine use. As such, this study holds critical clinical importance. While reduction in craving is important; cocaine abstinence represents the most critical achievement goal from a clinical standpoint.
In conclusion, while important limitations need to be kept in mind, this study supports the safety and potential efficacy of rTMS in treating cocaine addicts, and paves the way for larger, double-blind, sham-controlled, and randomized clinical trials to build on these promising, yet preliminary findings.
Acknowledgments
The authors would also like to thank Drs. Tommi Raij (Feinberg School of Medicine, Northwestern University), David Epstein, Kenzie Preston, Geoffrey Schoenbaum, and Elliot Stein (NIDA Intramural Research Program) for their very helpful comments and suggestions.
Role of funding sources
The authors would like to acknowledge the following funding sources: IRCCS San Camillo, Venice, Italy (AT, LG), “Novella Fronda” Foundation, Italy (AT, LG), Department of Neuroscience of the University of Padua, Italy (MS, ME), NIDA Intramural Research Program (LL, AB) and NIAAA Division of Intramural Clinical and Biological Research (LL). These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Trial name: Repetitive transcranial magnetic stimulation (rTMS) in cocaine abusers, URL: 〈http://www.isrctn.com/ISRCTN15823943?q=&filters=&sort=&offset=8&totalResults=13530&page=1&pageSize=10&searchType=basic-search〉, Registration number: ISRCTN15823943
Conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Ansseau M, Papart P, Gérard MA, von Frenckell R, Franck G,1990. Controlled comparison of buspirone and oxazepam in generalized anxiety. Neuropsychobiology 24 (2), 74–78. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A, 1998. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37 (4), 407–419. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL, 1985. Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–1107. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ, 2011. Repetitive transcranial magnetic stimulation and drug addiction. Int. Rev. Psychiatry 23 (5), 454–466. [DOI] [PubMed] [Google Scholar]
- Bech P, Bille J, Møller SB, Hellström LC, Østergaard SD, 2014. Psychometric validation of the Hopkins symptom checklist (SCL-90) subscales for depression, anxiety, and interpersonal sensitivity. J. Affect. Disord 160, 98–103. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih M, Pascual-Leone A, 2007. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug. Alcohol Depend 86 (1), 91–94. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau H, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A, 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496 (7445), 359–362. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann E, Hallett M, Cohen L, 1997. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48 (5), 1398–1403. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP, 2009. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLOS One 4, e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, Friauf W, Hallett M, 1990. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr. Clin. Neurophysiol 75 (4), 350–357. [DOI] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM, 2014. Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul. 7 (1), 130–132. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL, 2000. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depression Anxiety 11 (2), 58–65. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R, 2006. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp. Brain Res 174, 403–412. [DOI] [PubMed] [Google Scholar]
- Lazzaro Di, Ziemann V, Lemon RN U, 2008. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 1 (4), 345–362. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2014. Annual Overview of the European Drug Situation on 27 May in a Multilingual, Multimedia Information Package Focusing on Today’s Rapidly Shifting Drug Phenomenon, EMCDDA. [Google Scholar]
- Gass J, Chandler L, 2013. The plasticity of extinction: contribution of the prefrontal cortex in treating addiction through inhibitory learning. Front. Psychiatry 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159 (10), 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12 (11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L, 2011. Anhedonia and substance dependence: clinical correlates and treatment options. Front. Psychiatry 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev 109 (4), 679. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chen BT, Bonci A, Stein EA, 2014. Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies. Addict. Biol 20 (2), 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, et al. , 2008. New treatments for cocaine dependence: a focused review. Int. J. Neuropsychopharmacol 11 (3), 425–438. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H, 2003. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci.: Off. J. Soc. Neurosci 23 (21), 7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, et al. , 2004. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res.: Neuroimaging 130 (3), 283–293. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35 (1), 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, De Ridder D, 2014. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol 125 (11), 2150–2206. [DOI] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF, 2010. Neurochemistry of drug action. Ann. N. Y. Acad. Sci 1187 (1), 148–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RS, 1986. Depression scales derived from the Hopkins symptom checklist. In: Sartorius N, Ban T (Eds.), Assessment of Depression. Springer, Berlin, pp. 232–248. [Google Scholar]
- Mah L, Zarate CA, Nugent AC, Singh JB, Manji HK, Drevets WC, 2011. Neural mechanisms of antidepressant efficacy of the dopamine receptor agonist pramipexole in treatment of bipolar depression. Int. J. Neuropsychopharmacol 14 (4), 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak M, Scharf MB, Woods M, 1985. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep 9 (1 Pt 2), 285–289. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet J, Bolla KI, 2003. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 19 (3), 1095–1102. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U, 2006. The role of GABA (B) receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res 173, 86–93. [DOI] [PubMed] [Google Scholar]
- Milby JB, Conti K, Wallace D, Mennemeyer S, Mrug S, Schumacher JE, 2015. Comorbidity effects on cocaine dependence treatment and examination of reciprocal relationships between abstinence and depression. J. Consult. Clin. Psychol 83, 45–55. [DOI] [PubMed] [Google Scholar]
- Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A, 2012. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug. Alcohol Depend 125 (3), 208–214. [DOI] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Vecchi S, Amato L, 2011. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst. Rev 7 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakes GE, Brogden RN, Heel RC, Speight TM, Avery GS, 1981. Triazolam: a review of its pharmacological properties and therapeutic efficacy in patients with insomnia. Drugs 22 (2), 81–110. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley J, Weinberger D, 2012. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 path-ways. Mol. Psychiatry 17 (1), 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann E, Hallett M, 1994. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117 (Pt 4), 847–858. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E, 2008. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict 17 (4), 345–346. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Cunningham LA, Batey SR, Johnston JA, Ascher JA, 1998. A multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained-release bupropion tablets versus placebo in depressed outpatients. Clin. Ther 20 (3), 505–516. [DOI] [PubMed] [Google Scholar]
- Roy É, Jutras-Aswad D, Bertrand K, Dufour M, Perreault M, Laverdière É, Bene-Tchaleu F, Bruneau J, 2015. Anxiety, mood disorders and injection risk behaviors among cocaine users: results from the COSMO study. Am. J. Addict 24 (7), 654–660. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120 (12), 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A, 2001. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci 21, RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP, 2006. The status of disulfiram: a half of a century later. J. Clin. Psychopharmacol 26 (3), 290–302. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, 2002. Basic mechanisms of TMS. J. Clin. Neurophysiol 19, 322–343. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Swanson JM, 2004a. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9 (6), 557–569. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Swanson JM, 2004b. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9 (6), 557–569. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, 2003. The addicted human brain: Insights from imaging studies. J. Clin. Investig 111 (10), 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, 2000. Basic neurophysiological studies with TMS. In: George M, Belmaker R (Eds.), Transcranial Magnetic Stimulation in Neuropsychiatry., 45 American Psychiatric Press, Washington, DC. [Google Scholar]


