Abstract
Background: The contaminated output water from dental unit waterlines (DUWLs) is a potential risk to both patients and dental personnel who are frequently exposed to this water or aerosols. Aim: The purpose was to evaluate the contamination level and prevalence of bacteria in the output water of DUWLs, and to identify key factors to provide technical support for formulating relevant policies. Methods: We developed a special sampling connector designed for collecting dental handpiece output water and a measurement device to assess retraction of a dental chair unit (DCU). Output water from dental handpieces and air/water syringes were collected as representative of DUWLs. Water samples were tested with reference to China’s national standard. Findings: From 2012 to 2017, 318 DCUs were randomly selected from 64 hospitals in Tianjin, China. Of these DCUs, 78.93% had no disinfection to prevent DUWL contamination. Three-hundred and forty-three (56.14%) samples complied with the guidelines on DUWL output water. The highest concentration of bacteria was 1.8 × 106 colony-forming units (CFUs)/mL. The three key factors of influence were as follows: daily or weekly disinfection of DUWLs; water supply source being hospital self-made purified water or purchased purified bottled water; and DCU with a valid anti-retraction valve. Potential infectious agents, including Bacillus cereus, Burkholderia cepacia and Pseudomonas aeruginosa, were isolated. Conclusion: There was a high rate of contamination in DUWLs. This highlights the need to develop national standards. There is a need to disinfect the DUWLs periodically and use a cleaner source of water; more attention should be paid to the efficacy of DCU anti-retraction valves.
Key words: Dental unit waterlines, retraction, bacteria, water microbiology, dental handpiece, air/water syringe
INTRODUCTION
The dental chair unit (DCU), classified as a medical device according to the EU Medical Devices Directive, is one of the most essential pieces of equipment in the routine practice of dentistry1. The DCU uses water to cool and irrigate dental instruments and tooth surfaces and provides rinse water during dental treatments. A complex network of interconnected narrow-bore plastic dental unit waterlines (DUWLs) supply water to dental instruments. DUWLs are considered as reservoirs for potential pathogens of human or environmental origin2., 3., 4., including Legionella, because the narrow-bore tubing offers an optimal environment for biofilm development5. Many studies have reported that the concentrations of bacteria in DUWLs can reach as high as 104–106 colony-forming units (CFUs)/mL3., 4., 6., which is a potential risk for dental patients and staff, especially for patients with compromised immunity7. There are many factors that contribute to the contamination of DUWLs, including anti-retraction valve failure, the presence of water heaters, a piped water supply or an impure water supply.4., 7., 8., 9., 10., 11., 12., 13.. How to identify the key factors from many influencing factors is an important topic in infection control research. In 2017, Schonning et al.14 reported the case of an elderly immunocompromised man who died from legionellosis at a hospital in Uppsala, Sweden. This report highlighted the risks that are associated with Legionella in the output water of DUWLs. The link was confirmed by pulsed-field gel electrophoresis (PFGE) and whole-genome sequencing (WGS). The previously reported Italian case, to the authors’ knowledge, was the first reported verified case of legionellosis acquired through a DCU15. With the application of molecular biology technology, such as PFGE, WGS and core genome multi-locus sequence typing (MLST), it may be possible to detect more such cases. According to the US Centers for Disease Control and Prevention (CDC), 68.2% of people in the USA made dental visits in 201516. Assuming that this frequency is the same in North America, Europe, Japan, South Korea and Australia – highly developed countries with similar high-quality infectious disease surveillance systems – almost 1 billion people make dental visits annually in these countries17. Together with the rapid economic development in China, the Chinese people are increasingly seeking dental care18. A third National Oral Health Epidemiological Survey found that 63.41% of middle-aged adults and 60.32% of older adults were seeking dental visits19. Tianjin, one of the four municipalities of China, has provincial-level status (i.e. is situated directly below the central government). China has been late in monitoring bacterial contamination of DUWL output water. There were reports by other countries, as early as the 1960s, on the contamination of DUWLs20; in contrast, there were no published papers in Chinese on this subject until 2002. Tianjin CDC, a public institution that performs government functions using government finance, began to monitor contamination of DUWLs from 2012 onwards. To the best of our knowledge, no other Chinese investigators have reported on DUWL output water contamination in non-Chinese journals. The purpose of this study was to evaluate the degree of contamination and prevalence of bacteria in output water of DUWLs and to identify the key factors influencing this contamination in order to provide technical support for formulating relevant policies.
MATERIALS AND METHODS
Dental chair units
A total of 318 DCUs, from 64 hospitals in Tianjin, were selected from the DCU database by simple random sampling. At the time of this writing, there were about 991 DCUs in Tianjin, distributed across 16 districts, of which 10 are agricultural. Of the 318 DCUs, the average age was 5.72 ± 4.82 (range: 0.5–20) years, 14.15% were derived from stomatological hospitals, 66.98% were domestic brands, 33.33% were supplied with bottled water from independent water reservoirs, 16.98% were supplied directly by non-purified municipal water and for 78.93% no disinfection measures were taken to prevent DUWL contamination before our analysis. Of the 67 DCUs with DUWL control measures, 83.58% used chlorine-containing disinfectants, 13.43% applied electrochemically activated solutions and 2.98% used glutaraldehyde.
Collection of water samples
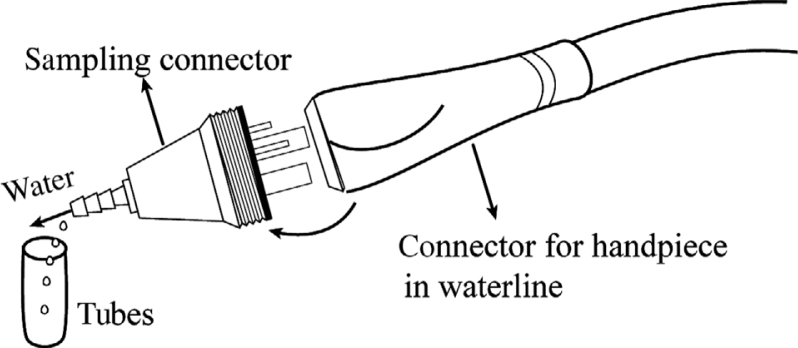
At each sampling, one of the authors randomly selected DCUs according to the sampling plan, and collected two water samples (one from the air/water syringe and the other from the dental handpiece) from each DCU, aseptically, using sterile gloves, single-use masks, and gowns at the beginning of the workday. In 2012, the first year of monitoring, only the air/water syringe output water was monitored because there was no suitable sampling method for collecting output water from the dental handpiece. For this purpose, a special sampling connector (Figure 1), designed for collecting dental handpiece output water, was developed. When this connector is connected to the DUWL, it replaces the dental handpiece; it only allows water to pass through and shields the air flow. Before taking the samples, we removed the dental handpiece and pressed the foot control to flush the waterline for 2 minutes, then installed the sampling connector to collect the water samples. Water samples (20 mL) from air/water syringes were collected, using a conventional approach21, into sterilised test tubes, which were then placed at 4 °C in a sampling box and shipped to the laboratory within 2 hours of sample collection22.
Figure 1.
Sampling connector. The dimensions and thread characteristics of the sampling connector were identical to the hose connectors of air-driven dental handpieces (ISO 9168-2009). Water samples (20 mL) were obtained from a high-speed handpiece with the sterilised sampling connector.
Processing of water samples
Water samples were tested and analysed with reference to China’s national standard ‘the standard examination methods for drinking water - microbiological parameters (GB/T 5750.12-2006)’23. One millilitre of well-mixed water was aseptically pipetted into a sterilised petri dish, then 15 mL of nutrient agar medium that had melted and cooled to about 45 °C was poured into the dish and the dish was immediately shaken to thoroughly mix the water sample with medium. Thereafter, the dishes were incubated at 37 °C for 48 hours. Heterotrophic plate counts (HPCs) were calculated as CFUs/mL. The threshold values established from American Dental Association (ADA) recommendations and US CDC guidelines (i.e. 500 CFUs/mL)24 were used as criteria. The VITEK 2 (Vitek2 compack30; Biomerieux, Marcyl’Etoile, France) analyser was used to identify aerobic bacteria.
Testing the efficacy of DCU anti-retraction valve
In 2014, we developed a measurement device to assess retraction of a DCU21 in accordance with ISO 7494-2:2015(E). If the DCU anti-retraction valve is valid, the volume of water retracted should not exceed 40 μL, according to the American Dental Association/American National Standard (ADA/ANSI) specification #47 or ISO 7494-2:2015(E).
Collection of variables
A questionnaire of more than 10 variables was designed and administered, after each sampling, to a staff member. The variables were recorded and coded as follows: location of hospital (1 = Downtown area, 2 = Rural area); category of hospital (1 = Stomatological hospital, 2 = General hospital); level of hospital (1 = Level 1, 2 = Level 2, 3 = Level 3); type of DCU (1 = Imported, 2 = Domestic); DCU supply water (1 = Municipal water, 2 = Hospital self-made purified water, 3 = Purchased bottled water); DUWLs disinfection (1 = Not disinfected, 2 = Chlorine disinfectant, 3 = Other disinfectant); frequency of DUWLs disinfection (1 = Not disinfected, 2 = Once a day, 3 = Once a week, 4 = Once a month); bottle disinfection (1 = Not disinfected, 2 = Chlorine disinfectant, 3 = Other disinfectant); frequency of bottle disinfection (1 = Not disinfected, 2 = Once a day, 3 = Once a week, 4 = Once a month); and water monitoring (1 = Yes, 2 = No), using hot water (1 = Yes, 2 = No). DCU ID and sampling date were recorded for each water sample.
Statistical analysis
The microbial loads were converted into log10 to normalise the non-normal distributions for comparing the results over years. Chi-square tests were applied for comparing the differences of each value between groups and selecting variables for logistic regression analysis. We conducted a binary logistic regression analysis to correlate the variables with the quality of DUWL output water (0: ≤500 CFU/mL, 1: >500 CFU/mL). A significance level of 0.05 (two-sided) was used. Statistical Package for the Social Sciences Version 24.0 (IBM, Armonk, NY, USA) was used for data analysis.
Ethics approval
This study was independently reviewed and approved by the Institutional Review Board of Tianjin Centers for Disease Control and Prevention.
RESULTS
Microbial culture of water samples
A total of 611 DUWL output samples were collected from 2012 to 2017, comprising 318 samples from air/water syringes and 293 samples from dental handpieces. The microbial contamination values of water samples are shown in Table 1. The highest HPC values of output water samples were 1.8 × 106 CFUs/mL for handpieces and 1.7 × 106 CFUs/mL for air/water syringes. Taken together, 343 (56.14%) of 611 samples complied with the guidelines of the CDC on DUWL output water. Separately, the percent of output water from the dental handpiece and air/water syringes below the threshold were 53.58% and 58.49%, respectively. There were no significant differences between the two groups according to chi-square analysis (χ2 = 1.491, P = 0.222).
Table 1.
Bacterial contamination of water samples from air/water syringes and dental handpieces [colony-forming units (CFUs)/mL]
| Year | Air/water syringes |
Dental handpieces |
Total |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Range | Median (P25–P75) | ≤500 n (%) | ≤100 n (%) | n | Range | Median (P25–P75) | ≤500 n (%) | ≤100 n (%) | n | Range | Median (P25–P75) | ≤500 n (%) | ≤100 n (%) | |
| 2012 | 24 | 2–30,000 | 970 (71.5–2,562.5) | 11 (45.83) | 10 (41.67) | 0 | 24 | 2–30,000 | 970 (71.5–2,562.5) | 11 (45.83) | 10 (41.67) | ||||
| 2013 | 99 | 0–267,120 | 158 (33–1,360) | 53 (53.54) | 52 (52.53) | 99 | 1–718,680 | 406 (100–11,200) | 46 (46.46) | 43 (43.43) | 198 | 0–718,680 | 249.5 (55.25–3,000) | 99 (50) | 95 (47.98) |
| 2014 | 58 | 0–1,700,000 | 125 (8.5–2,775) | 38 (65.52) | 31 (53.45) | 58 | 0–1,800,000 | 390 (35.5–9,375) | 36 (62.07) | 32 (55.17) | 116 | 0–1,800,000 | 235 (14.25–5,975) | 74 (63.79) | 63 (54.31) |
| 2015 | 42 | 0–620,000 | 350 (73.5–1,375) | 28 (66.67) | 27 (64.29) | 41 | 0–550,000 | 430 (140–2,500) | 23 (56.10) | 20 (48.78) | 83 | 0–620,000 | 400 (98–1,600) | 51 (61.45) | 47 (56.63) |
| 2016 | 50 | 0–66,000 | 180 (4–1,975) | 26 (52.00) | 24 (48.00) | 50 | 0–64,000 | 370 (20.75–3,650) | 27 (54.00) | 19 (38.00) | 100 | 0–66,000 | 290 (14.25–2,200) | 53 (53) | 43 (43.00) |
| 2017 | 45 | 0–32,000 | 120 (26–1,600) | 30 (66.67) | 21 (46.67) | 45 | 0–64,000 | 450 (91.5–2,950) | 25 (55.56) | 12 (26.67) | 90 | 0–64,000 | 230 (35–1,925) | 55 (61.11) | 33 (36.67) |
| Total | 318 | 0–1,700,000 | 190 (27.75–1,825) | 186 (58.49) | 165 (51.89) | 293 | 0–1,800,000 | 410 (66.5–4,100) | 157 (53.58) | 126 (43.00) | 611 | 0–1,800,000 | 280 (35–2,450) | 343 (56.14) | 291 (47.63) |
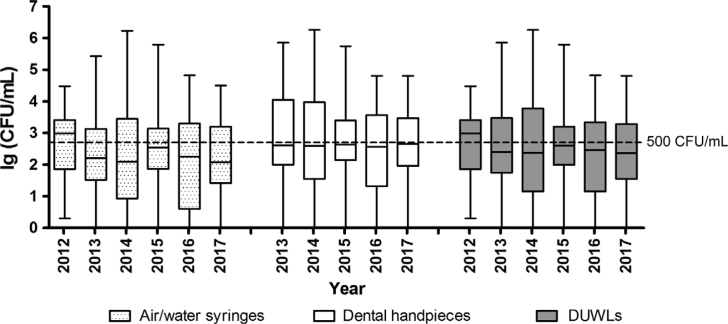
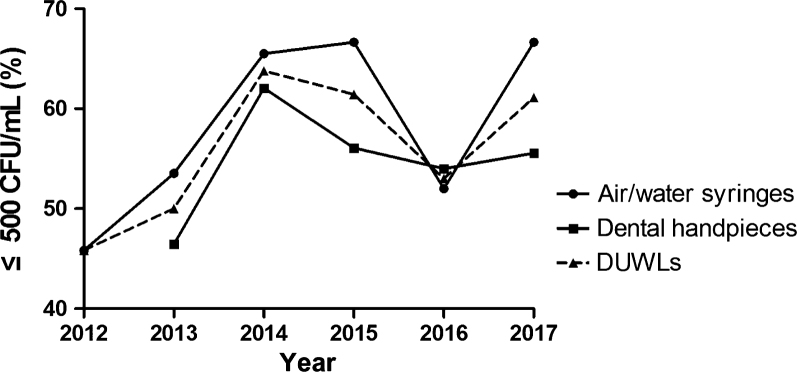
According to the annual statistics, except for 2012, the median of the bacterial concentrations of DUWL output water in all other years was lower than 500 CFUs/mL (Figure 2). From 2012 to 2017, the percent of DUWL and handpiece output water below the threshold was the same; from the low in 2012 the values began to rise yearly and after the peak in 2014 began to decline; in 2017, the percent was higher than in 2016 (Figure 3). By contrast, the percent of the air/water syringe output water below the threshold showed a decline from the peak reached in 2015.
Figure 2.
The values of microbial loads were converted into log10 × to compare the results over the years using box-and-whisker plots. A box-and-whisker plot shows a ‘box’ with a low edge at lower quartile, the high edge at upper quartile, the ‘middle’ of the box at the median and the maximum and minimum as ‘whiskers’. The dotted line in the figure corresponds to lg500 colony-forming units (CFUs)/mL. DUWLs, dental unit waterlines.
Figure 3.
The lines demonstrate the trends of the output water samples that were below the threshold for dental unit waterlines, handpieces and air/water syringes from 2012 to 2017. CFU, colony-forming unit; DUWLs, dental unit waterlines.
Testing the efficacy of DCU anti-retraction
Two-hundred and ninety-three DCUs were tested for anti-retraction, of which 167 (57%) complied with ISO 7494-2:2015(E). Table 2 shows that 334 (57%) of the 586 output water samples from DUWLs were below the threshold for the DCU anti-retraction test. Among these 334 samples, 224 (67.07%) complied with the guidelines of the CDC for DUWL output water (≤500 CFUs/mL). The percent of DUWL output water samples collected from DCUs using a valid anti-retraction valve was higher than that with an invalid anti-retraction valve, and the difference was statistically significant (P < 0.05).
Table 2.
Single-factor analysis for bacterial contamination of dental unit waterlines with the threshold value [500 colony-forming units (CFUs)/mL]
| Factor | Category | ≤500 n (%) | >500 n (%) | χ2 | P |
|---|---|---|---|---|---|
| Distinct category | Downtown area | 140 (58.33) | 100 (41.67) | 0.774 | 0.379 |
| Agricultural area | 203 (54.72) | 168 (45.28) | |||
| Hospital category | Stomatological hospital | 43 (49.43) | 44 (50.57) | 1.856 | 0.173 |
| General hospital | 300 (57.25) | 224 (42.75) | |||
| Anti-retraction handpiece | Y | 282 (55.4) | 227 (44.6) | 0.668 | 0.414 |
| N | 61 (59.8) | 41 (40.2) | |||
| Hot water | Y | 134 (52.14) | 123 (47.86) | 2.879 | 0.090 |
| N | 209 (59.04) | 145 (40.96) | |||
| Imported DCU | Y | 135 (64.29) | 75 (35.71) | 8.628 | 0.003 |
| N | 208 (51.87) | 193 (48.13) | |||
| Sampling season | Spring | 79 (57.66) | 58 (42.34) | 8.886 | 0.012 |
| Summer | 170 (61.37) | 107 (38.63) | |||
| Autumn | 94 (47.72) | 103 (52.28) | |||
| Hospital level | Level 1 hospital | 88 (47.57) | 97 (52.43) | 25.279 | 0.000 |
| Level 2 hospital | 89 (48.37) | 95 (51.63) | |||
| Level 3 hospital | 166 (68.6) | 76 (31.4) | |||
| DCU supply water | Municipal water | 36 (35.29) | 66 (64.71) | 22.958 | 0.000 |
| Hospital self-made purified water | 244 (59.08) | 169 (40.92) | |||
| Purchased bottled water | 63 (65.63) | 33 (34.38) | |||
| DUWL disinfection | Not disinfect | 256 (53.33) | 224 (46.67) | 10.042 | 0.007 |
| Chlorine disinfectant | 76 (69.72) | 33 (30.28) | |||
| Other disinfectant | 11 (50) | 11 (50) | |||
| Frequency of DUWLs disinfection | Not disinfect | 256 (53.33) | 224 (46.67) | 16.945 | 0.001 |
| Once a day | 16 (69.57) | 7 (30.43) | |||
| Once a week | 63 (73.26) | 23 (26.74) | |||
| Once a month | 8 (36.36) | 14 (63.64) | |||
| Bottle disinfection | Not disinfect | 22 (57.89) | 16 (42.11) | 3.435 | 0.180 |
| Chlorine disinfectant | 98 (63.64) | 56 (36.36) | |||
| Other disinfectant | 8 (42.11) | 11 (57.89) | |||
| Frequency of bottle disinfection | Once a day | 30 (88.24) | 4 (11.76) | 16.478 | 0.000 |
| Once a week | 82 (58.99) | 57 (41.01) | |||
| Not disinfect | 16 (42.11) | 22 (57.89) | |||
| Water monitoring | Y | 232 (53.83) | 199 (46.17) | 3.168 | 0.075 |
| N | 111 (61.67) | 69 (38.33) | |||
| DCU anti-retraction valve | Valid | 224 (67.07) | 110 (32.93) | 180.706 | 0.000 |
| Invalid | 29 (11.51) | 223 (88.49) |
DCU, dental chair unit; DUWL, dental unit waterline; N, no; Y, yes.
Factor analysis
Table 2 presents the results of chi-square analysis for bacterial concentration (above or below the threshold of 500 CFU/mL) in DUWL output water, according to various underlying factors.
A logistic regression analysis was conducted to assess whether the model varied significantly. The Hosmer–Lemeshow goodness-of-fit test indicated that the model was well calibrated (P = 0.731). Only three factors were statistically significant after being brought into the model (Table 3). These were DUWL disinfection frequency, DCU supply water and DCU anti-retraction.
Table 3.
Multifactorial logistic analysis of bacterial contamination of dental unit waterlines (DUWLs)
| Factor | β | SE | Wals | P | OR (95% CI) |
|---|---|---|---|---|---|
| Frequency of DUWLs disinfection | |||||
| Not disinfect | 1.000 | ||||
| Once a day | −1.401 | 0.677 | 4.279 | 0.039 | 0.246 (0.065–0.929) |
| Once a week | −0.657 | 0.332 | 3.919 | 0.048 | 0.518 (0.270–0.994) |
| Once a month | 1.871 | 1.165 | 2.577 | 0.108 | 6.494 (0.661–63.760) |
| χ2 (df = 3) | 10.502 | 0.015 | |||
| DCU supply water | |||||
| Municipal water | 1.000 | ||||
| Hospital self-made purified water | −0.875 | 0.276 | 10.027 | 0.002 | 0.417 (0.242–0.716) |
| Purchased purified bottled water | −1.000 | 0.420 | 5.683 | 0.017 | 0.368 (0.162–0.837) |
| χ2 (df = 2) | 10.965 | 0.004 | |||
| DCU anti-retraction (invalid = 0, valid = 1) | −2.473 | 0.404 | 37.523 | 0.000 | 0.084 (0.038–0.186) |
DCU, dental chair unit; df, degrees of freedom; OR, odds ratio.
For DUWL disinfection frequency, daily disinfection [odds ratio (OR) = 0.246; 95% CI: 0.065–0.929] and weekly disinfection (OR = 0.518; 95% CI: 0.270–0.994) were statistically significant compared with non-disinfection regarding whether the DUWL output water was ≤500 CFUs/mL. Daily disinfection was better than weekly disinfection. There was no statistical difference between monthly disinfection and non-disinfection.
Similarly, in the three categories of DCU supply water, hospital self-made purified water (OR = 0.417; 95% CI: 0.242–0.716) and purchased purified bottled water (OR = 0.368; 95% CI: 0.162–0.837) showed a statistically significantly (P < 0.05) lower concentration of bacteria in the DUWL output water compared with municipal water used as the reference.
The difference between the above two factors was that DCU anti-retraction is a binary classification variable. It can be seen from the model results that the valid anti-retraction of the DCU (OR = 0.084; 95% CI: 0.038–0.186) compared with invalid anti-retraction was statistically significant for DUWL output water.
Pathogen detection
A total of 112 strains of bacteria were isolated from the output water samples of DUWLs, including Bacillus cereus (n = 34), Burkholderia cepacia (n = 10), Pseudomonas aeruginosa (n = 7), Alcaligenes faecalis (n = 6), Staphylococcus epidermidis (n = 5) and Pseudomonas fluorescens (n = 5). The other strains isolated were from 14 genera, mainly Micrococcus, Comamonas and Staphylococcus.
DISCUSSION
To our knowledge, this is the longest sampling study of DUWL output water contamination in China. The output water from dental handpieces and air/water syringes used in patient treatment is more likely to affect patients than dental personnel. However, it readily forms microaerosols, putting both patients and dental personnel at risk for inhalation of potentially pathogenic bacteria3., 9..
Contamination of DUWLs with bacteria has been documented by scientific evidence7., 25., 26., 27., 28., 29., 30.. Microbial levels of 104–106 CFUs/mL are frequently reported in water samples from DUWLs25., 26., 27., 28.. The maximum concentration of bacteria in DUWL output water was 1.8 × 106 CFUs/mL in our study, which is higher than the concentrations most frequently obtained in other studies25., 26., 27., 28.. In our samples, 56.14% (343/611) complied with the threshold recommended by ADA or CDC guidelines, and 47.63% (291/611) met the China drinking water standards, that is, the number of bacteria should be ≤100 CFUs/mL. Regarding numerical values the results of this study showed that the percent of DUWL samples below the threshold values were lower than reported by Mardjan Arvand et al.31: 58 (64.44%) of 90 samples complied with the German drinking water standards of a total colony count of ≤100 CFU/mL for all dental units.
The high CFUs/mL and low percent of samples below the threshold were unexpected. There are several possible reasons. The most important, at present, China has not enacted any national standards or industry standards in this field. In China, many studies have evaluated DUWL output water either by the Standards for Drinking Water Quality of China (≤100 CFUs/mL)32 or by the CDC guidelines (≤500 CFUs/mL)24. In addition to the lack of indicator thresholds, there is also a lack of management regulations and control measures for DUWLs, leading to a lack of supervision in hospitals and a lack of knowledge and attention regarding DUWL contamination of dental personnel21.
Besides the lack of national standards, the causes of microbial contamination of DUWL output water are multifactorial7. These include DCU type, sampling season, type of hospital, DCU supply water, DUWL disinfection, DUWL disinfection frequency, bottle disinfection frequency and DCU anti-retraction valve validity1., 8., 24.. These factors play essential roles in the formation of biofilms, which develop in DUWLs and function as a reservoir for continuous contamination of DUWL output water7. In our multifactor logistic regression study, the OR of non-compliance with the standards for the DCU with valid anti-retraction valve versus invalid anti-retraction valve was 0.084. This suggests that the odds of having a DCU with a valid anti-retraction valve were 11.9 (1/OR, 1/0.084) times larger than the odds of a DCU failing anti-retraction testing. Berlutti showed that the overwhelming majority (74%) of anti-retraction devices did not prevent retraction when the turbine stopped running, leading to contamination of the water lines and to consequent possible cross-contamination of patients10. Also, analysis of our preliminary study showed a significant, positive correlation (P < 0.05) between increased concentration of bacteria in the water sample and retracted volume21. In conclusion, DCUs equipped with anti-retraction valves should be periodically monitored and should undergo preventive maintenance to minimise instances of anti-retraction valve failure7.
The exponent of the coefficient of DCU supply water has an OR of 0.368, meaning that the odds of a DCU supplied by purchased bottled water were 2.72 (1/0.368) times larger than the odds of DCU supplied by non-purified municipal water (the reference category). Similarly, the odds of a DCU being supplied by the hospital self-made purified water were 2.39 (1/0.417) times larger than the odds of a DCU being supplied by non-purified municipal water. The result obtained is in agreement with CDC guidelines for infection control in dental health-care settings24.
In our multifactorial logistic regression study, the odds of DUWL disinfection once daily were 4.07 (1/0.246) times larger than the odds of DUWLs without disinfection (the reference category), and the odds of DUWLs disinfection once weekly were 1.93 (1/0.518) times larger than the odds of DUWL without disinfection. The OR of complying with standards for DUWL disinfection monthly versus non-disinfection was 6.494, with no statistical significance. Some studies reported that biofilms could form within the DUWLs of new DCUs within several hours of connection to a water supply33., 34. and reform rapidly following an intermittent treatment. Although continuously applied agents performed better than those used periodically, patients are exposed to residual products7. In the present study, we showed that DUWL disinfection weekly was better than the other frequencies of disinfection. Therefore, when formulating standards in the future, in addition to considering the effect after the implementation of control measures, it is necessary to consider compliance of the clinic staff. Logistic regression and other related categorical-data regression methods have often been used to assess risk factors for various diseases35. However, the authors did not find any reports in which logistic regression models were used to analyse the factors influencing contamination of DUWL output water at levels exceeding the CDC standards. The model developed here for predicting the quality of DUWL output water suggests that DUWLs should be disinfected at least once a week. The water supply for DCUs should be purified water (purchased purified bottled water is better) and the effectiveness of the DCU anti-retraction valve should be maintained.
The bacterial species found in the present study were mostly environmental aerobes, which were also present at low levels in municipal water36. Most of the genera isolated (Micrococcus spp.37, Comamonas spp.38 and Staphylococcus spp.39) are known opportunistic pathogens, as also reported in many other studies40., 41., 42.. Bacillus cereus, occasionally isolated from human dental plaque43, was the strain most frequently detected. It is also indirect proof of the existence of retraction. It is important to note that several strains of B. cereus can enhance biofilm formation. Within established biofilms, B. cereus can form spores, which may lead to contamination of DUWL output water, but this microbe is not common in oral infections43., 44.. Burkholderia cepacia, isolated in various DUWL output water investigations25., 45., 46., is a known opportunistic human pathogen. There is evidence for transmission of B. cepacia to cystic fibrosis patients via pulmonary test equipment, nebulisers and other types of respiratory equipment used both in cystic fibrosis centres and for home-care, but there is little evidence of spread through aerosols, dental equipment, hands, contaminated disinfectants or water supplies41. Further research is needed. Al-Hiyasat et al. reported that P. aeruginosa was detected in 86.7% (26/30) of the DCUs at the beginning of the work day. The high percentage of contamination of the DUWLs tested in that study can be related mainly to the low level of efficiency of the anti-retraction valves and also to the heating system in the DCUs, in addition to the presence of softener that may act as a source of contamination when the water passes through47.
As we did not choose a selective culture medium for the culture of specific bacteria, we did not identify Legionella pneumophila, which has been reported to be associated with DUWL infection14., 15., 17.. Serological studies have shown higher titres of antibodies specific to L. pneumophila in healthy dental personnel than general population9. According to the results of the present study, the presence of bacteria in DUWL is not a matter of grave concern, but their quantity and the presence of potential pathogens and microbial flora in the oral cavity of the patients as a result of retraction are of concern48.
In the future, research should be focused on the health economics of the DUWL problem (cost of testing, disinfection), risks to patients and staff, surveillance of adverse events related to dental treatment and importance of following the advice of dental unit manufacturers.
CONCLUSION
The high rate of contamination in DUWLs highlights the need to develop national standards in China. There is a need to disinfect DUWLs regularly and use a cleaner source of water; more attention should be paid to the efficacy of DCU anti-retraction valves.
Acknowledgements
The authors wish to thank Wei Zhang, Dong-jing Yang, Jie Dong and Ying Zhang for their help and support in this research.
Funding sources
This study was supported by two research funds: (i) Technology Fund of Tianjin Centers for Disease Control and Prevention (CDCKY1302); and (ii) Science and Technology Fund of Tianjin Health and Family Planning Commission (2014KZ043).
Conflict of interest
None declared.
References
- 1.Coleman DC, O’Donnell MJ, Shore AC, et al. The role of manufacturers in reducing biofilms in dental chair waterlines. J Dent. 2007;35:701–711. doi: 10.1016/j.jdent.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Porteous N, Sun Y, Schoolfield J. Evaluation of 3 dental unit waterline contamination testing methods. Gen Dent. 2015;63:41–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Szymańska J, Sitkowska J. Bacterial contamination of dental unit waterlines. Environ Monit Assess. 2013;185:3603–3611. doi: 10.1007/s10661-012-2812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymanska J, Sitkowska J. Opportunistic bacteria in dental unit waterlines: assessment and characteristics. Future Microbiol. 2013;8:681–689. doi: 10.2217/fmb.13.33. [DOI] [PubMed] [Google Scholar]
- 5.Ditommaso S, Giacomuzzi M, Ricciardi E, et al. Efficacy of a low dose of hydrogen peroxide (peroxy Ag+) for continuous treatment of dental unit water lines: challenge test with Legionella pneumophila serogroup 1 in a simulated dental unit waterline. Int J Environ Res Public Health. 2016;13:745. doi: 10.3390/ijerph13070745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacchetti R, De Luca G, Zanetti F. Influence of material and tube size on DUWLs contamination in a pilot plant. New Microbiol. 2007;30:29–34. [PubMed] [Google Scholar]
- 7.O’Donnell MJ, Boyle MA, Russell RJ, et al. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011;6:1209–1226. doi: 10.2217/fmb.11.104. [DOI] [PubMed] [Google Scholar]
- 8.Coleman DC, O’Donnell MJ, Shore AC, et al. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol. 2009;106:1424–1437. doi: 10.1111/j.1365-2672.2008.04100.x. [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst CL, Coulter WA. Do contaminated dental unit waterlines pose a risk of infection? J Dent. 2007;35:712–720. doi: 10.1016/j.jdent.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Berlutti F, Testarelli L, Vaia F, et al. Efficacy of anti-retraction devices in preventing bacterial contamination of dental unit water lines. J Dent. 2003;31:105–110. doi: 10.1016/s0300-5712(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 11.Montebugnoli L, Dolci G, Spratt DA, et al. Failure of anti-retraction valves and the procedure for between patient flushing: a rationale for chemical control of dental unit waterline contamination. Am J Dent. 2005;18:270–274. [PubMed] [Google Scholar]
- 12.Wirthlin MR, Marshall GJ, Rowland RW. Formation and decontamination of biofilms in dental unit waterlines. J Periodontol. 2003;74:1595–1609. doi: 10.1902/jop.2003.74.11.1595. [DOI] [PubMed] [Google Scholar]
- 13.Leoni E, Dallolio L, Stagni F, et al. Impact of a risk management plan on Legionella contamination of dental unit water. Int J Environ Res Public Health. 2015;12:2344–2358. doi: 10.3390/ijerph120302344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonning C, Jernberg C, Klingenberg D, et al. Legionellosis acquired through a dental unit: a case study. J Hosp Infect. 2017;96:89–92. doi: 10.1016/j.jhin.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet. 2012;379:684. doi: 10.1016/S0140-6736(12)60074-9. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Oral and dental health; 2018. Available from: https://www.cdc.gov/nchs/fastats/dental.htm. Accessed 5 January 2018
- 17.Petti S. Healthcare outbreaks associated with dental unit water systems: strong scientific evidence of minimal risk. Clin Infect Dis. 2016;63:1270. doi: 10.1093/cid/ciw534. [DOI] [PubMed] [Google Scholar]
- 18.Hu DY, Hong X, Li X. Oral health in China–trends and challenges. Int J Oral Sci. 2011;3:7–12. doi: 10.4248/IJOS11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fei BI, Chun Y. The research status of the application of oral health services in China. J Kunming Med Univ. 2014;35:162–164. (in Chinese) [Google Scholar]
- 20.Belting CM, Haberfelde GC, Juhl LK. Spread of organisms from dental air rotor. J Am Dent Assoc. 1964;68:648–651. doi: 10.14219/jada.archive.1964.0145. [DOI] [PubMed] [Google Scholar]
- 21.Ji X, Fei C, Zhang Y, et al. Evaluation of bacterial contamination of dental unit waterlines and use of a newly designed measurement device to assess retraction of a dental chair unit. Int Dent J. 2016;66:208–214. doi: 10.1111/idj.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health of the People’s Republic of China . Ministry of Health of the People’s Republic of China; Beijing: 2012. Hygienic Standard for Disinfection in Hospital (GB15982-2012) [Google Scholar]
- 23.Ministry of Health of the People’s Republic of China . Ministry of Health of the People’s Republic of China; Beijing: 2006. Standards Examination Methods for Drinking Water–Microbiological Parameters. [Google Scholar]
- 24.Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep. 2003;52:1–61. [PubMed] [Google Scholar]
- 25.Meiller TF, Depaola LG, Kelley JI, et al. Dental unit waterlines: biofilms, disinfection and recurrence. J Am Dent Assoc. 1999;130:65–72. doi: 10.14219/jada.archive.1999.0030. [DOI] [PubMed] [Google Scholar]
- 26.Walker JT, Bradshaw DJ, Bennett AM, et al. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66:3363–3367. doi: 10.1128/aem.66.8.3363-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo JA, Oertling KM, Andrieu SC. Bacterial biofilm: a source of contamination in dental air-water syringes. Clin Prev Dent. 1990;12:13–20. [PubMed] [Google Scholar]
- 28.Turetgen I, Goksay D, Cotuk A. Comparison of the microbial load of incoming and distal outlet waters from dental unit water systems in Istanbul. Environ Monit Assess. 2009;158:9–14. doi: 10.1007/s10661-008-0560-7. [DOI] [PubMed] [Google Scholar]
- 29.Percival RS, Devine DA, Nattress B, et al. Control of microbial contamination in dental unit water systems using tetra-sodium EDTA. J Appl Microbiol. 2009;107:1081–1088. doi: 10.1111/j.1365-2672.2009.04299.x. [DOI] [PubMed] [Google Scholar]
- 30.Nikaeen M, Hatamzadeh M, Sabzevari Z, et al. Microbial quality of water in dental unit waterlines. J Res Med Sci. 2009;14:297–300. [PMC free article] [PubMed] [Google Scholar]
- 31.Arvand M, Hack A. Microbial contamination of dental unit waterlines in dental practices in Hesse, Germany: a cross-sectional study. Eur J Microbiol Immunol (Bp) 2013;3:49–52. doi: 10.1556/EuJMI.3.2013.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministry of Health of the People’s Republic of China . Ministry of Health of the People’s Republic of China; Beijing: 2006. Standards for Drinking Water Quality (GB 5749-2006) [Google Scholar]
- 33.Barbeau J, Tanguay R, Faucher E, et al. Multiparametric analysis of waterline contamination in dental units. Appl Environ Microbiol. 1996;62:3954–3959. doi: 10.1128/aem.62.11.3954-3959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams HN, Johnson A, Kelley JI, et al. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int. 1995;26:331–337. [PubMed] [Google Scholar]
- 35.Wolkewitz M, von Cube M, Schumacher M. Multistate modeling to analyze nosocomial infection data: an introduction and demonstration. Infect Control Hosp Epidemiol. 2017;38:953–959. doi: 10.1017/ice.2017.107. [DOI] [PubMed] [Google Scholar]
- 36.Shearer BG. Biofilm and the dental office. J Am Dent Assoc. 1996;127:181–189. doi: 10.14219/jada.archive.1996.0166. [DOI] [PubMed] [Google Scholar]
- 37.Adhikari A, Kurella S, Banerjee P, et al. Aerosolized bacteria and microbial activity in dental clinics during cleaning procedures. J Aerosol Sci. 2017;114:209–218. [Google Scholar]
- 38.Stampi S, Zanetti F, Bergamaschi A, et al. Comamonas acidovorans contamination of dental unit waters. Lett Appl Microbiol. 1999;29:52–55. doi: 10.1046/j.1365-2672.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 39.Pye AD, Lockhart DE, Dawson MP, et al. A review of dental implants and infection. J Hosp Infect. 2009;72:104–110. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Pankhurst CL, Harrison VE, Philpott-Howard J. Evaluation of contamination of the dentist and dental surgery environment with Burkholderia (Pseudomonas) cepacia during treatment of children with cystic fibrosis. Int J Paediatr Dent. 1995;5:243–247. doi: 10.1111/j.1365-263x.1995.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 41.Pankhurst CL, Philpott-Howard J. The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients. J Hosp Infect. 1996;32:249–255. doi: 10.1016/s0195-6701(96)90035-3. [DOI] [PubMed] [Google Scholar]
- 42.Hsueh PR, Teng LJ, Pan HJ, et al. Outbreak of Pseudomonas fluorescens bacteremia among oncology patients. J Clin Microbiol. 1998;36:2914–2917. doi: 10.1128/jcm.36.10.2914-2917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189–198. doi: 10.1016/s1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 44.Hayrapetyan H, Muller L, Tempelaars M, et al. Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron. Int J Food Microbiol. 2015;200:72–79. doi: 10.1016/j.ijfoodmicro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Pankhurst CL, Johnson NW, Woods RG. Microbial contamination of dental unit waterlines: the scientific argument. Int Dent J. 1998;48:359–368. doi: 10.1111/j.1875-595x.1998.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 46.Uzel A, Cogulu D, Oncag O. Microbiological evaluation and antibiotic susceptibility of dental unit water systems in general dental practice. Int J Dent Hyg. 2008;6:43–47. doi: 10.1111/j.1601-5037.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 47.Al-Hiyasat AS, Ma’Ayeh SY, Hindiyeh MY, et al. The presence of Pseudomonas aeruginosa in the dental unit waterline systems of teaching clinics. Int J Dent Hyg. 2007;5:36–44. doi: 10.1111/j.1601-5037.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 48.Szymanska J, Sitkowska J, Dutkiewicz J. Microbial contamination of dental unit waterlines. Ann Agric Environ Med. 2008;15:173–179. [PubMed] [Google Scholar]