Abstract
Background: To examine the relationships of rare genetic diseases affecting skeletal development, socio-demographic characteristics, and oral health-related behaviours with dental clinical measures in children and adolescents. Methods: A cross-sectional study paired by age, gender and social class included 61 children and adolescents with osteogenesis imperfecta (n = 40) or mucopolysaccharidoses (n = 21) and those without genetic rare diseases (n = 60). Participants were selected at two referral hospitals for rare genetic diseases in the city of Belo Horizonte, Brazil. Caregivers completed a questionnaire to obtain age, gender, caregiver’s schooling, social class, patterns of dental attendance and duration of breastfeeding. Oral hygiene, dental caries, dental anomalies and malocclusion were assessed through dental examinations. The relationships between variables were estimated through Pathway analysis using the maximum likelihood method. Results: Rare genetic diseases affecting skeletal development were directly associated with dental caries (β = 0.22), dental anomalies (β = 0.36) and malocclusion (β = 0.29). They were also inversely linked to a preventive pattern of dental attendance (β = –0.25). Rare genetic diseases affecting skeletal development were associated with poor oral hygiene (β = 0.28) and shorter breastfeeding duration (β = –0.21). Rare genetic diseases affecting skeletal development were linked indirectly with dental caries, a reduced pattern of dental attendance and poor oral hygiene (β = 0.43). Patterns of dental attendance mediated the link between rare genetic diseases affecting skeletal development and malocclusion (β = –0.05). Conclusion: Rare genetic diseases affecting skeletal development were associated with poor oral health. Patterns of dental attendance and poor oral hygiene mediated the link between rare genetic diseases affecting skeletal development and dental clinical measures.
Key words: Dental care for disabled, disabled children, rare diseases, oral health, osteogenesis imperfecta, mucopolysaccharidosis
INTRODUCTION
The prevalence of rare diseases is approximately 65 cases per 100,000 individuals1. This estimate reflects the existence of 7,000 different types of rare diseases, the majority of which are genetic in nature. Rare diseases of genetic origin are often associated with other co-morbid conditions, which are often characterised by debilitating and chronically degenerative conditions related to phenotypes2. Patients with rare diseases can sometimes exhibit challenging behaviour and frequently experience physical, mental and sensory impairments, which can have an impact on functioning and participation in everyday life2., 3., 4., 5..
Mucopolysaccharidoses (MPS) and osteogenesis imperfecta (OI) are two rare genetic diseases. MPS is a group of inherited metabolic diseases caused by the absence or malfunctioning of single specific lysosomal enzymes in the body, which are needed to break down glycosaminoglycans6. The absence or malfunctioning of these enzymes means that over time, glycosaminoglycans (GAG) build up in the cells, blood, brain and spinal tissue. The build-up of GAG can result in severe morbidity and reduced life expectancy7., 8.. Although symptoms of MPS can be similar, they can also vary from individual to individual, making early diagnosis difficult9. Seven types of MPS have been identified, namely MPS I, MPS II, MPS III (A, B, C and D), MPS IV (A and B), MPS VI, MPS VII and MPS IX. Classification occurs according to the type of enzyme involved in the GAG degradation pathway. The overall incidence of MPS is estimated from 1:20,000 to 1:25,00010. MPS can affect appearance, skeletal development, physical abilities, organ and system functioning and, in most cases, cognitive development11.
Osteogenesis imperfecta is a genetic condition present from birth. It is characterised by a reduction in connective tissue, which can be of low quality, or missing altogether. The reduction or lack of connective tissue can create joint hypermobility, brittle bones, which are liable to fracture without a cause and the growth of the jaws and teeth may be affected12. Dentinogenesis imperfecta (DI) is frequently associated with OI and results in dentine dysplasia13., 14.. Recent estimates suggest that OI affects one in 10,000 to 20,000 live births15. OI can be classified into four types, according to phenotype and genotype characteristics: type I (mild), type II (lethal), type III (severe) and type IV (moderate)16.
Individuals with MPS or OI usually have poor oral health17., 18., 19., 20., 21., 22., 23., 24., 25.. The genetic alterations related to MPS and OI also exert an impact on the stomatognathic system. People with these particular genetic disorders usually experience a high prevalence of malocclusion, microdontia, tooth rotation and tooth agenesis. Enamel developmental defects are common in individuals with MPS, whereas DI is frequently observed in individuals with OI21., 22., 23., 25., 26.. Motor co-ordination limitations frequently accompany these clinical conditions, making performing oral hygiene practices such as toothbrushing challenging, while limited access to oral health education, dental services and knowledge barriers for healthcare professionals may contribute to a greater susceptibility of dental caries for patients with MPS and OI27., 28., 29., 30..
Current evidence suggests that children with MPS and OI experience poor oral health. The majority of the oral health research has used case series study designs, which limits valid conclusions concerning the role of genetic rare diseases on oral health because of a lack of group comparison17., 18., 19., 20., 22., 24., 25.. Within the evidence base, some studies using a control group did not adjust the association between rare genetic diseases and oral conditions for possible confounders, limiting the conclusions21., 23., 25..
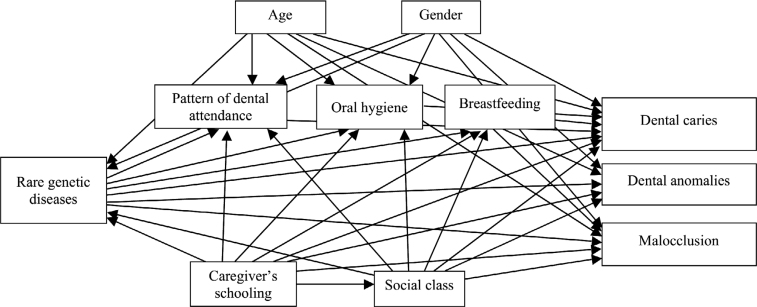
The aim of this study was to examine the possible relationships of rare genetic diseases affecting skeletal development, socio-demographic characteristics, patterns of dental attendance, oral hygiene status and breastfeeding duration with dental clinical measures in children and adolescents. A theoretical model was developed to test the above-mentioned relationships (Figure 1). The theoretical model hypothesised that rare genetic diseases affecting skeletal development would be directly associated with patterns of dental attendance, poor oral hygiene status, lower duration of breastfeeding, dental caries, dental anomalies and malocclusion. Age and gender were hypothesised to be associated with rare genetic diseases affecting skeletal development. Indirect effects of rare genetic diseases affecting skeletal development on dental clinical measures via dental attendance, poor oral hygiene status and duration of breastfeeding were also hypothesised.
Figure 1.
Full theoretical model on the relationships of rare genetic diseases affecting skeletal development, demographics, socioeconomic status, oral health-behaviours, pattern of dental attendance with dental clinical measures in children and adolescents.
METHODS
Study design and sample
A cross-sectional study was conducted in two public hospitals from the Brazilian national health system that are referral centres for rare genetic diseases in the city of Belo Horizonte, Brazil. A convenience sample of 122 children and adolescents and their caregivers was selected according to the presence or absence of rare genetic diseases affecting skeletal development. The rare genetic diseases affecting skeletal development group included 61 children and adolescents with OI (n = 40) or MPS (n = 21). A control group of 61 patients without rare genetic diseases were paired according to age, gender and socioeconomic status and recruited from the outpatient clinics of the same two hospitals. The studied sample included participants aged between 3 and 18 years, with and without OI and MPS. Children and adolescents with mental or physical impairments, other rare and systemic diseases and developmental disorders were excluded.
A sample size of 122 people would lend a power 90% detected in a structural equation model to ascertain a minimum effect size of 0.13, with a 5% level of significance (α = 0.05) directed toward hypothesis testing for complex models with 11 observed variables31.
Data collection
Initially, information regarding the presence and type of rare genetic diseases affecting skeletal development was obtained from patient’s medical records. Clinical oral examinations were carried by two calibrated examiners to obtain dental clinical measures using a plain dental mirror (Duflex 5) and a CPI probe (Golgran, São Paulo, SP, Brazil). Personal protective equipment was used in all exams. Children and adolescents were examined in a chair under artificial light (Petzl Zoom head lamp, Petzl America, Clearfield, UT) at the medical offices at the referral hospitals for rare genetic diseases to assess dental caries, dental anomalies, malocclusion and oral hygiene status. Caregivers completed a structured questionnaire in their native language of Portuguese to assess their age and gender, level of caregiver’s schooling, family social class, child/adolescent pattern of dental attendance and duration of breastfeeding.
Measures
Demographics and socioeconomic characteristics included age and gender of the participants, level of caregiver’s schooling and social class. Age was recorded according to date of birth. Level of caregiver’s schooling was measured according to the number of consecutive years at school and was registered as 0 = <8 years and 1 = 8 or more years. Social class was measured using economic classification criteria based on market power comprised of a group of specific indicators and level of education of the head of household. Data were categorised into three groups: high (A, B1 and B2), middle (C1 and C2) and low (D and E)32.
Patterns of dental attendance were assessed based on the reason for the last dental visit using the following response options: 0 = never been to the dentist, 1 = dental treatment or pain and 2 = prevention or tooth eruption. Duration of breastfeeding was categorised as 0 = <6 months or 1 = 6 months or more.
Oral hygiene status was measured using the Simplified Oral Hygiene Index (OHI-S)33. Initially, each dental surface was categorised according to the presence of dental plaque and dental calculus as follows: 0 = absence of dental plaque/dental calculus; 1 = dental plaque/dental calculus covering <1/3 of the dental surface; 2 = dental plaque/dental calculus covering >1/3 and <2/3 of the dental surface; and 3 = dental plaque/dental calculus covering >2/3 of the dental surface. The codes were added and divided by the number of teeth to obtain the OHI-S final score: 0 to 1 = satisfactory, 1.1 to 2 = fair, 2.1 to 3 = deficient, 3.1 or higher = poor. The classification was dichotomised as adequate (satisfactory and fair) or inadequate (deficient and poor).
Data for dental caries was collected using the component “decayed” according to the WHO guidelines for oral health surveys34. Each sound, missing or filled tooth was coded as “0,” and each primary and permanent teeth with a cavitated lesion was coded as “1.” The codes were totalled to obtain a final dental caries score for each participant34. Dental anomalies were registered based on the presence of the following dental conditions: conical tooth, tooth agenesis, tooth rotation, developmental defects of enamel and DI. Tooth agenesis was considered as a possible diagnosis, because the oral examination was only performed clinically. Participants were considered as having dental anomalies if one or more of the above-mentioned conditions were present35. Malocclusion was measured using the following occlusal characteristics: overjet (increased/protrusion, anterior crossbite, absent), overbite (increased/deep bite, anterior open bite, absent, top) and posterior crossbite. Malocclusion occurred when one or more occlusal characteristics were clinically detected35.
Reliability study and pilot study
Inter-examiner reliability for dental clinical measures was tested in 20 individuals without rare congenital diseases not included in the main study. Individuals were selected from one of the hospitals of the main study where they were examined with 8-day intervals between examinations. Measurements obtained from a dental epidemiologist were used as the gold standard to assess the reliability of the two examiners. Kappa coefficients varied from 0.76–0.98 for the investigated dental conditions.
A pilot study involving 10 individuals with rare genetic diseases affecting skeletal development and their caregivers was conducted at the two public hospitals to assess the methodology of the fieldwork data collection. The data collection protocol was considered adequate and no changes were needed. The participants of the pilot study were included in the main study.
Data analysis
Descriptive analysis reported the distribution of the variables through means and standard deviations (continuous variables) and proportions (categorical variables) between participants with and without rare genetic diseases affecting skeletal development. The differences between the groups were assessed using Mann-Whitney (continuous variables) and Chi-squared (categorical variables) statistical tests.
Pathway analysis examined the direct and indirect relationships between variables according to the theoretical framework (Figure 1) using STATA 22.0 software. The standardised direct effects represented a direct path from one variable to another. Substantial indirect effects between variables were used to assess mediation when one variable was the pathway between two other variables given our assumptions. The standardised effects and 95% confidence intervals (95% CIs) were estimated to evaluate compatibility intervals consistent with our data using the maximum likelihood method to assess whether mediation was present by assessing the importance of indirect effects. Non-substantial direct paths (P value >0.20) were removed from the full model to estimate a parsimonious model compatible with our data.
The adequacy of the full and parsimonious models was evaluated according to the following fit indexes and threshold values: root-mean-square error of approximation (RMSEA) ≤0.06, standardised root-mean-square residual (SRMR) ≤0.08, comparative fit index (CFI) ≥0.90 and Tucker-Lewis index (TLI) ≥0.9036.
RESULTS
Initially, 131 children and adolescents were invited to participate. Five were excluded based on the eligibility criteria. Four of the 126 eligible participants did not agree to participate, resulting in a final sample of 61 children and adolescents with rare disease and 61 without rare disease (response rate was 97%). The mean age of the 122 participants was 8.7 (SD = 4.9) years old and 61.5% of them were male children and adolescents. Most of the sample was from middle class families (60.7%). Sixty-eight per cent of the caregivers had eight years of more of schooling. There was a substantial difference compatible with our data in patterns of dental attendance, oral hygiene status and duration of breastfeeding between groups. Levels of dental caries, prevalence of dental anomalies and malocclusion were statistically higher among children and adolescents with rare genetic diseases affecting skeletal development when compared with the group without rare genetic diseases (Table 1).
Table 1.
Sociodemographic characteristics, pattern of dental attendance, oral hygiene, breastfeeding period and dental clinical measures between participants with and without rare genetic diseases affecting skeletal development
| Variables | Total | Rare genetic diseases (n = 61) | No rare genetic diseases (n = 61) | P |
|---|---|---|---|---|
| Age, mean (SD) | 8.7 (4.9) | 8.7 (4.9) | 8.7 (4.9) | 0.998† |
| Gender, n (%) | ||||
| Male | 75 (61.5) | 37 (60.7) | 38 (62.3) | 0.852‡ |
| Female | 47 (38.5) | 24 (39.3) | 23 (37.7) | |
| Caregiver’s schooling, n (%) | ||||
| <8 | 39 (32.0) | 23 (37.7) | 16 (26.2) | 0.174 ‡ |
| 8+ | 83 (68.0) | 38 (62.3) | 45 (73.8) | |
| Social class, n (%) | ||||
| A/B | 39 (32.0) | 21 (34.4) | 18 (29.5) | 0.539‡ |
| C | 74 (60.7) | 37 (60.7) | 37 (60.7) | |
| D/E | 9 (7.4) | 3 (4.9) | 6 (9.8) | |
| Pattern of dental attendance, n (%) | ||||
| Never been to the dentist | 59 (48.4) | 39 (63.9) | 20 (32.8) | 0.002‡ |
| Dental treatment or pain | 23 (18.9) | 6 (9.8) | 17 (27.9) | |
| Prevention or tooth eruption | 40 (32.8) | 16 (26.2) | 24 (39.3) | |
| Oral hygiene, n (%) | ||||
| Regular/satisfactory | 75 (61.5) | 29 (47.5) | 46 (75.4) | 0.002‡ |
| Poor/very poor | 47 (38.5) | 32 (52.2) | 15 (24.6) | |
| Breastfeeding period, n (%) | ||||
| <6 months | 52 (42.6) | 32 (52.5) | 20 (32.8) | 0.028‡ |
| 6 months or more | 70 (57.4) | 29 (47.5) | 41 (67.2) | |
| Dental caries, mean (SD) | 2.6 (3.5) | 3.6 (4.1) | 1.6 (2.4) | 0.002† |
| Dental anomalies, n (%) | ||||
| Yes | 38 (31.1) | 29 (47.5) | 9 (14.8) | <0.001‡ |
| No | 84 (68.9) | 32 (52.5) | 52 (85.2) | |
| Malocclusion, n (%) | ||||
| No malocclusion | 36 (29.5) | 11 (18.0) | 25 (41.0) | 0.005‡ |
| Malocclusion | 86 (70.5) | 50 (82.0) | 36 (59.0) |
P value refers to Mann-Whitney test for comparison between rare genetic diseases groups.
P Value refers to Chi-square test for comparison between rare genetic disease groups.
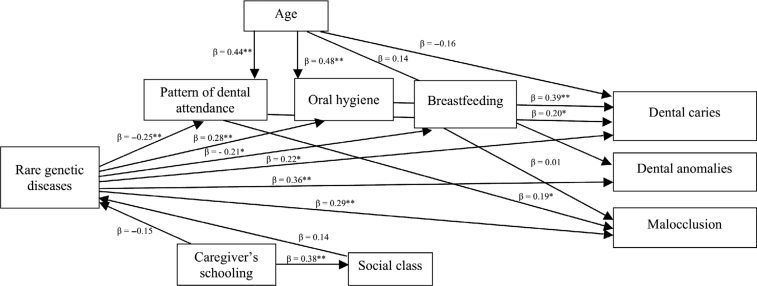
The hypothesised model (full model) was initially tested through Pathway analysis. The following fit indices values supported the full model: RMSEA = 0.029, SRMR = 0.034, CFI = 0.990, TLI = 0.961. Thereafter, the parsimonious model compatible with our data was tested after the removal of direct links with non-substantial association using a P value below 0.20 as a threshold. The variable gender was also removed because gender was not associated with other variables. The parsimonious model also met all the criteria used to evaluate the model fit: RMSEA = 0.001, SRMR = 0.040, CFI = 1.000, TLI = 1.008.
The direct relationships of the parsimonious model are presented in Figure 2. Dental caries (β = 0.22), dental anomalies (β = 0.36) and malocclusion (β = 0.29) were directly associated with rare genetic diseases affecting skeletal development. Rare genetic diseases affecting skeletal development were also associated with poor pattern of dental attendance (β=–0.25). The former was directly linked to poor oral hygiene (β = 0.28) and shorter duration of breastfeeding (β=–0.21). Greater age was directly associated with better patterns of dental attendance (β = 0.44) and poor oral hygiene (β = 0.48). Patterns of dental attendance were linked to dental caries (β = 0.20) and malocclusion (β = 0.19). Poor oral hygiene was associated with more dental caries (β = 0.39). Caregiver’s schooling was directly linked to higher social class (β = 0.38).
Figure 2.
Parsimonious model of associations between rare genetic diseases affecting skeletal development, demographics, socioeconomic status, oral health-behaviours, pattern of dental attendance and dental clinical measures in children and adolescents. The variable “gender” was removed from this model as it was not statistically correlated with any variables. *P < 0.05; **P < 0.01.
Significant indirect pathways between variables were observed. Rare genetic diseases affecting skeletal development were indirectly linked to more dental caries via poor patterns of dental attendance and oral hygiene (β = 0.43). Rare genetic diseases affecting skeletal development were indirectly linked to malocclusion via poor patterns of dental attendance (β = –0.05). Age was indirectly linked to dental caries (β = 0.20) and malocclusion (β = 0.01) via poor patterns of dental attendance. In addition, age was indirectly associated with dental caries via poor oral hygiene (β = 0.20).
DISCUSSION
The present research investigated the possible relationships of rare genetic diseases affecting skeletal development, socio-demographic characteristics, patterns of dental attendance, oral hygiene status and breastfeeding duration with dental clinical measures in children and adolescents. There were increased levels of dental caries, dental anomalies and malocclusion in children and adolescents with rare genetic diseases affecting skeletal development compared to those without rare genetic diseases. Pathway analysis revealed that rare genetic diseases affecting skeletal development were directly linked to dental caries, dental anomalies and malocclusion. This finding highlights oral health disparities related to rare genetic diseases affecting skeletal development amongst children and adolescents. Children and adolescents with rare genetic diseases affecting skeletal development also experienced poor oral hygiene, poor patterns of dental attendance and lower duration of breastfeeding than those without rare genetic diseases. Therefore, rare genetic diseases affecting skeletal development were also related to inequalities in oral health-related behaviours. The mediation effect of patterns of dental attendance and oral hygiene on the link between rare genetic diseases affecting skeletal development and dental caries revealed possible pathways through which rare genetic diseases affecting skeletal development may influence dental caries. In addition, it was observed that there was a mediation effect of patterns of dental attendance on the link between rare genetic diseases affecting skeletal development and malocclusion.
The lack of direct association between socio-demographic factors and rare genetic diseases affecting skeletal development in the studied sample was expected, because the former variables were paired between groups with and without rare genetic diseases. Nonetheless, demographics and socioeconomic characteristics were included in the theoretic model to reduce the effect of residual confounding of the abovementioned variables on the association between rare genetic diseases and dental clinical measures. In addition, it was also possible to explore whether socio-demographic factors were directly associated with dental clinical outcomes and if these links were mediated through patterns of dental attendance, oral hygiene and duration of breastfeeding.
The present research suggests that rare genetic diseases affecting skeletal development can influence the oral health status of children and adolescents. Previous findings corroborate this result showing a higher propensity of individuals with rare diseases to dental caries, dental anomalies and malocclusion21., 25., 30.. In contrast, another study did not report statistical differences on the prevalence of dental caries between individuals with and without rare diseases3. The possible reasons for such divergence might be related to differences on the protective factors (e.g. exposure to fluorides) and risk factors (e.g. high sugar diet) for dental caries between Swedish and Brazilian children. It can further be suggested that there are structural and systemic differences between the two countries and these may exert an effect on oral health status. The relationship of inadequate patterns of dental attendance and poor oral hygiene with rare diseases has already been reported, but the reasons for this have remained unexplored3., 37..
There is a greater likelihood of people with rare genetic diseases affecting skeletal development developing malocclusion and dental anomalies22., 25., 26.. Developmental defects of enamel and DI in patients with OI results from genetic mutations involved in the signalling pathway of the formation of dental tissues21., 38.. In addition, developmental defects of enamel and DI are associated with an increase in dental caries39. Greater prevalence of malocclusion in patients with MPS occurs as a result of upper airway involvement, macroglossia and ogival palate. These characteristics are predisposed to anterior open bite and crossbite that are the most common malocclusion characteristics20., 22.. Patients with OI experience growth deficiency of the skull base, which restricts growth and displacement of the maxilla in the anteroinferior direction. Thus, the prevalence of posterior crossbite and Class II malocclusion is high among individuals with OI21., 26..
Previous research suggests that parents and caregivers face challenges in caring for oral health and there are additional barriers to accessing adequate oral health care for children with disabilities. This means that care givers tend to seek dental treatment for their children in emergency situations and not for preventive reasons37., 40.. Other barriers to access are the shortage of dentists with appropriate training to treat children with disabilities, then there are competing priorities for co-morbid medical conditions and hospital admissions that have an impact on oral health and care11., 12..
The present study has some limitations. First, the use of a convenience sample limits the generalisation of results. All children and adolescents were selected from hospitals for rare genetic diseases and therefore the participants possibly do not represent the characteristics of the general population. Nonetheless, specialised hospitals are ideal settings for studies involving participants with rare diseases. Children and adolescents from the control group were selected from the same hospitals as those with rare genetic diseases, suggesting the groups are comparable because all participants are from the same source population. Second, only children and adolescents with OI and MPS were included in the group for rare genetic diseases affecting skeletal development. Thus, our findings should not be generalised to patients with other rare diseases. Third, tooth agenesis was considered as one of the dental anomalies but only a clinical examination was used for the diagnosis and this method may have introduced classification bias due to lack of radiographs. Fourth, relevant risk factors for dental caries, such as cariogenic diet and use of fluorides, were not investigated. Finally, even though malocclusion was assessed using different measures related to occlusal deviations, it is a dichotomous variable and does not discriminate between the severity and extent of malocclusion.
Future studies should investigate the possible influence of other genetic rare diseases on children’s and adolescent’s oral health. The use of longitudinal designs in future research on this topic addressing other important risk factors for dental conditions may contribute towards increasing the understanding of the role of genetic rare diseases on oral health.
CONCLUSIONS
Within the limits of this study, our findings suggest the need for public health policies to promote the oral health of children and adolescents with rare genetic diseases affecting skeletal development. The main thrust should be that just because someone has a rare genetic disease that affects skeletal development, it does not automatically mean they have poor oral health. Parents need education and support with their child’s oral health to ensure that a non-communicable disease such as dental caries does not become the norm for these groups. The Brazilian National Health System should advocate for the development of strategies to screen children at birth and at regular intervals, referring to appropriate services for education, support and where necessary treatment. There is also the need to improve access to preventive dental care as well as dental treatment for patients with rare genetic diseases affecting skeletal development.
Acknowledgements
The authors wish to thank Research England/HEFCE, HEIF (United Kingdom) QR GCRF - University of UFMG PUMP PRIMING Awards for providing funding that supported this research.
Conflicts of interest
The authors declare no conflict of interest.
Ethics statement
The present study was conducted in accordance with the Declaration of Helsinki and the research protocol was approved by the Ethics Committee of the Federal University of Minas Gerais, according to the following certificate numbers: 01480212.4.0000.5149 (mucopolysaccharidoses) and 03027612.7.000.5149 (Osteogenesis Imperfecta).
REFERENCES
- 1.World Health Organization (WHO). Priority Medicines for Europe and the World 2013; 2013a. Available at http://www.who.int/medicines/areas/priority_medicines/Ch6_19Rare.pdf [updated January 6, 2020].
- 2.Boycott KM, Rath A, Chong JX, et al. International cooperation to enable the diagnosis of all rare genetic diseases. Am J Hum Genet. 2017;100:695–705. doi: 10.1016/j.ajhg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjögreen L, Andersson-norinder J, Bratel J. Oral health and oromotor function in rare diseases - a database study. Swed Dent J. 2017;39:23–27. [PubMed] [Google Scholar]
- 4.Slade A, Isa F, Kyte D, et al. Patient reported outcome measures in rare diseases: a narrative review. Orphanet J Rare Dis. 2018;13:61. doi: 10.1186/s13023-018-0810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanz AP, van de Sande Lee J, Pinheiro B, et al. Health-related quality of life of children and adolescents with osteogenesis imperfecta: a cross-sectional study using PedsQL™. BMC Pediatr. 2018;18:95. doi: 10.1186/s12887-018-1077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neufeld EU, Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, editor. McGraw-Hill; New York, NY: 2001. The mucopolysaccharidoses; pp. 3421–3452. [Google Scholar]
- 7.Archer LD, Langford-Smith KJ, Bigger BW, et al. Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J Inherit Metab Dis. 2014;37:1–12. doi: 10.1007/s10545-013-9613-3. [DOI] [PubMed] [Google Scholar]
- 8.Cimaz R, La Torre F. Mucopolysaccharidoses. Curr Rheumatol Rep. 2014;16:389. doi: 10.1007/s11926-013-0389-0. [DOI] [PubMed] [Google Scholar]
- 9.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology. 2011;50:v4–12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 10.Beck M, Arn P, Giugliani R, et al. The natural history of MPS I: global perspectives from the MPS I Registry. Genet Med. 2014;16:759–765. doi: 10.1038/gim.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez-Guerrero JL, Gómez Higuera PJ, Arias Flórez JS, et al. Mucopolysaccharidosis: clinical features, diagnosis and management. Rev Chil Pediatr. 2016;87:295–304. doi: 10.1016/j.rchipe.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Van Dijk FS, Sillence DO. Osteogenesis Imperfecta: Clinical Diagnosis, Nomenclature and Severity Assessment. Am J Med Genet A. 2014;164A:1470–1481. doi: 10.1002/ajmg.a.36545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge H, Finn S, Robinson BG. Hereditary opalescent dentin, III: histological, chemical and physical studies. J Dent Res. 1940;19:521–536. [Google Scholar]
- 14.Huber MA. Osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:314–320. doi: 10.1016/j.tripleo.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.OIFE. Osteogenesis Imperfecta Federation Europe. http://www.oife.org/index.php/EN. Accessed 10 January 2020.
- 16.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz S, Tsipouras P. Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol. 1984;57:161–167. doi: 10.1016/0030-4220(84)90206-8. [DOI] [PubMed] [Google Scholar]
- 18.O’Connell AC, Marini JC. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:189–196. doi: 10.1016/s1079-2104(99)70272-6. [DOI] [PubMed] [Google Scholar]
- 19.James A, Hendriksz CJ, Addison O. The oral health needs of children, adolescents and young adults affected by a Mucopolysaccharide disorder. JIMD Reports. 2012;2:51–58. doi: 10.1007/8904_2011_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antunes LA, Nogueira AP, Castro GF, et al. Dental findings and oral health status in patients with mucopolysaccharidosis: a case series. Acta Odontol Scand. 2013;71:157–167. doi: 10.3109/00016357.2011.654255. [DOI] [PubMed] [Google Scholar]
- 21.Rizkallah J, Schwartz S, Rauch F, et al. Evaluation of the severity of malocclusions in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofac Orthop. 2013;143:336–341. doi: 10.1016/j.ajodo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro EM, Fonteles CS, Freitas AB, et al. A clinical multicenter study of orofacial features in 26 brazilian patients with different types of mucopolysaccharidosis. Cleft Palate Craniofac J. 2015;52:352–358. doi: 10.1597/13-204. [DOI] [PubMed] [Google Scholar]
- 23.Carneiro NCR, Deps TD, França EC, et al. Oral health of children and adolescentes with mucopolysaccharidosis and mother’s sense of coherence. Spec Care Dent. 2017;37:223–229. doi: 10.1111/scd.12238. [DOI] [PubMed] [Google Scholar]
- 24.Ballikaya E, Eymirli PS, Yıldız Y, et al. Oral health status in patients with mucopolysaccharidoses. Turkish J Pediatr. 2018;60:400–406. doi: 10.24953/turkjped.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Marçal FF, Ribeiro EM, Costa FWG, et al. Dental alterations on panoramic radiographs of patients with osteogenesis imperfecta in relation to clinical diagnosis, severity, and bisphosphonate regimen aspects: a STROBE-compliant case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:621–630. doi: 10.1016/j.oooo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Jabbour Z, Al-Khateeb A, Eimar H, et al. Orthod Craniofac Res. 2018;21:71–77. doi: 10.1111/ocr.12218. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Yu D, Luo W, et al. Impact of oral health behaviors on dental caries in children with intellectual disabilities in Guangzhou, China. Int J Environ Res Public Health. 2014;11:11015–11027. doi: 10.3390/ijerph111011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira SA, Santos PCM, Batista AR, et al. Assessment of oral hygiene in mentally disabled children. Rev Odonto Cienc. 2015;30:65–70. [Google Scholar]
- 29.Akyol MU, Alden TD, Amartino H, et al. Recommendations for the management of MPS IVA: systematic evidence- and consensus-based guidance. Orphanet J Rare Dis. 2019;14:137. doi: 10.1186/s13023-019-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prado HV, Carneiro NCR, Perazzo MF, et al. Assessing a possible vulnerability to dental caries in individuals with rare genetic diseases that affect the skeletal development. Orphanet J Rare Dis. 2019;14:145. doi: 10.1186/s13023-019-1114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1998. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 32.Associação Brasileira de Empresas de Pesquisa (ABEP) Associação Brasileira de Empresas de Pesquisa (ABEP); São Paulo: 2018. Novo Critério de Classificação Econômica Brasil. [Google Scholar]
- 33.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . World Health Organization; Geneva: 2013. Oral Health Surveys. Basic Methods. [Google Scholar]
- 35.Oliveira AC, Paiva SM, Campos MR, et al. Factors associated with malocclusions in children and adolescents with Down syndrome. Am J Orthod Dentofacial Orthop. 2008;133(489):e1–489.e8. doi: 10.1016/j.ajodo.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 37.Leroy R, Declerck D. Oral health care utilization in children with disabilities. Clin Oral Invest. 2013;17:1855–1861. doi: 10.1007/s00784-012-0874-2. [DOI] [PubMed] [Google Scholar]
- 38.Andersson K, Dahllöf G, Lindahl K, et al. Mutations in COL1A1 and COL1A2 and dental aberrations in children and adolescents with osteogenesis imperfecta - a retrospective cohort study. PLoS ONE. 2017;12:e0176466. doi: 10.1371/journal.pone.0176466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma MS, Najirad M, Taqi D, et al. Caries prevalence and experience in individuals with osteogenesis imperfecta: A cross-sectional multicenter study. Spec Care Dentist. 2019;39:214–219. doi: 10.1111/scd.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Agili DE, Roseman J, Pass MA, et al. Access to dental care in Alabama for children with special needs. JADA. 2004;135:490–495. doi: 10.14219/jada.archive.2004.0216. [DOI] [PubMed] [Google Scholar]