Abstract
Objective: Determine the accuracy of a questionnaire on xerostomia as a screening tool for hyposalivation. Methods: A total of 402 adults awaiting dental care at a public healthcare service answered an eight-item questionnaire addressing xerostomia and were submitted to stimulated sialometry, with ≤ 0.7 mL/min considered indicative of hyposalivation. Reproducibility and internal consistency of the questionnaire were evaluated. The correlation between the score and salivary flow was investigated. The total score was also compared between groups with and without hyposalivation and diagnostic precision measures were calculated. Results: Hyposalivation was identified in 162 participants (40.3%) and a total of 229 (57.0%) answered affirmatively to at least one question. The responses to each question revealed variable reproducibility (κ = 0.450–0.785) and satisfactory internal consistency (Cronbach’s α = 0.70). Individuals with a larger number of positive answers had lower salivary flow (Spearman’s ρ = –0.193; P < 0.001). The mean number of positive answers was greater in the group with a clinical diagnosis of hyposalivation compared to those without low salivary flow. The sensitivity of the screening tool was 64.8%, with an area under the ROC curve of 0.60 (95% confidence interval: 0.547–0.645; P < 0.001). Conclusion: The questionnaire proved to be useful for the epidemiological screening of individuals with possible hyposalivation.
Key words: Surveys and questionnaires, xerostomia, screening, salivation
INTRODUCTION
Diverse external factors and natural alterations can lead reduced salivary flow1., 2.. This condition is known as hyposalivation3., 4. and affected individuals may or may not have a sensation of ‘dry mouth’, known as xerostomia2., 5., 6., 7.. Both hyposalivation and xerostomia are conditions that can occur in an isolated manner, but xerostomia is most often the result of a reduction in salivary secretion and is therefore a strong indicator of hyposalivation8., 9., 10..
The causes of hyposalivation and xerostomia range from momentary factors, such as stress and anxiety10., 11., 12., to autoimmune diseases and chronic conditions, such as rheumatoid arthritis13, Parkinson’s disease13., 14., Sjögren’s syndrome10., 15., 16. and diabetes mellitus17., 18., 19., 20., 21.. The two conditions may also be found in patients requiring treatment for head and neck cancer3., 6., 13., 14., 21. and those with diseases specific to the salivary glands1., 19.. Moreover, one of the major causes of reduced salivary flow is the use of particular medications14., 16., 20., 22..
Hyposalivation is diagnosed using sialometry, which consists of collecting saliva to measure the amount of secretion produced per minute12., 18.. The diagnosis of hyposalivation is necessary, because this condition can exert a negative impact on activities of daily living and quality of life3., 13., 17., 23.. Another consequence of hyposalivation is a higher frequency of oral infectious diseases/conditions, such as caries, candidiasis, etc. Hyposalivation in the presence of a lowered immune response favors the occurrence of oral infections2., 9., such as dental caries due to the buildup of plaque and the low buffering capacity of the saliva3. Fungal infections, especially candidiasis, can occur as a result of the imbalance in the oral microbiota3., 9..
One of the major challenges to community dentistry is the identification and screening of patients with hyposalivation, because sialometry requires time, is uncomfortable for patients and is often impractical in large epidemiological studies. Detecting hyposalivation without the use of sialometry is limited to the subjective interpretation of the individual, and there is no other specific measure for the determination of this condition11., 24.. It is therefore fundamental to develop assessment tools, such as questionnaires, that enable satisfactory screening for the identification of patients with potential hyposalivation.
The identification of hyposalivation with the questionnaire used in the present study was proposed by Torres et al.8 in a study in which questions related to xerostomia were administered to individuals in a random manner. However, only those who answered positively to at least one of the items were sent for the determination of salivary flow. In 2015, Nunes et al.25 administered the same questionnaire to a sample of 182 institutionalized older adults in the city of Natal, Brazil. The data were analyzed to validate the uni-dimensionality of the questionnaire using confirmatory factor analysis and the authors suggested the removal of two of the questions. The questionnaire was confirmed to be valid for use as a screening tool for hyposalivation in institutionalized older adults.
Hyposalivation can affect quality of life and negatively impact health and well-being. Given changes in lifestyle and the increasing use of medications that contribute to changes in saliva among adults, it is essential to identify probable cases that require appropriate dental treatment and management. Therefore, the efficacy of the questionnaire needs to be tested in the general population, as its use has only previously been tested on specific groups, such as older adults. Another important aspect of the present study is the fact that all participants were submitted to sialometry, unlike what occurred in the study by Torres et al.,8 in which only those who answered affirmatively to the questionnaire were evaluated by the measurement of salivary flow.
Thus, there is a need to analyze the accuracy of the questionnaire25 compared to the gold standard (sialometry) for application in the general population. Because patients with xerostomia often have hyposalivation, the aim of the present study was to analyze the accuracy of the questionnaire proposed by Torres et al.8 for use as a screening tool for probable cases of hyposalivation in epidemiological surveys.
METHODS
Study design
A diagnostic study was conducted to analyze the accuracy of a questionnaire on xerostomia as a screening tool for cases of hyposalivation. The Standards for Reporting Diagnostic Accuracy (STARD checklist) were used for the design of the study and guidance of the reporting. Although this was not a validation study, the recommendations of the consensus-based standards for the selection of health status measurement instruments (COSMIN checklist) were also considered.
Characterization of sample
A total of 402 individuals awaiting dental care at a primary health service and the clinic of the dental school of the Universidade Estadual da Paraíba (UEPB) participated in this study. The sample size was calculated using the following formula:in which n is the sample size, p is the estimate of the sensitivity measure of the screening tool (70%, based on a preliminary analysis of data in a pilot study) and EF is the error factor or expected error (5%, in this case). Considering a 95% confidence interval (CI), a minimum sample of 322 was determined, which was increased to 402 to compensate for a possible 20% dropout rate.
Eligibility criteria
The participants were selected by convenience. Those who agreed to participate after receiving clarifications regarding the objectives and procedures of the study and met the following eligibility criteria were included in the study: age of ≥9 years, not having smoked or ingested any food in the previous 2 hours24, not having ingested food more than 3 hours earlier, not having received dental treatment on the same day as the collection of saliva and not having performed oral hygiene in the hour prior to the collection of saliva.
Calibration exercise and pilot study
Prior to data collection, we conducted training and calibration procedures and a pilot study. Training and calibration exercises consisted of two stages (theoretical and clinical). The theoretical stage involved a discussion of the criteria for the diagnosis of hyposalivation. A specialist in epidemiological surveys (gold standard) coordinated this stage, instructing the examiner on how to perform the examinations. The clinical stage was performed at a dental school that was not part of the main study. A pilot study was conducted with a convenience sample of 30 adults (age ≥19 years) to test the methods proposed for the main study. The results of the pilot study revealed that no changes to the methods were necessary.
Data collection
Interviews were conducted by two interviewers for the collection of data on age, sex and the administration of the questionnaire on xerostomia proposed by Torres et al.8 using the modified eight-item version proposed by Nunes et al.25 Stimulated salivary flow was always performed between 7:30 and 9:30 am. The collection occurred in a quiet, reserved room to reduce the interference of external factors, avoid the generation of stress and anxiety for the participant and ensure privacy during the exam.
Each individual chewed a tablet (1.5 × 1.5 cm) made from a piece of parafilm measuring 5 × 3 cm. The participant swallowed the saliva produced during the first minute and then spat into a recipient for the subsequent 5 minutes. The saliva produced was deposited in a graduated glass test tube for the determination of salivary flow. A rate <0.7 mL/min was considered indicative of hyposalivation7., 20..
Analysis of the questionnaire as a screening tool for hyposalivation
The reliability of the questionnaire was determined based on reproducibility (test–retest) and internal consistency. Reproducibility was tested in a sample of 100 participants on two occasions with a 1-week interval26. Internal consistency regards the homogeneity of the instrument in relation to the construct, that is, the extent to which the items on the questionnaire are correlated for the evaluation of hyposalivation. The correlation between the score on the questionnaire (number of affirmative answers) and the salivary flow rate was also investigated. Moreover, the total score on the questionnaire was compared between the groups of participants with and without a diagnosis of hyposalivation.
To confirm whether the questionnaire can be considered a screening tool for hyposalivation, its accuracy (A) and practical application were determined based on consistent results after the administration of the questionnaire to a population-based sample. The results questionnaire scores were compared to the salivary flow rates using diagnostic precision measures.
The accuracy of the test was defined as the percentage of times in which the questionnaire correctly identified individuals with and without hyposalivation. For such, the sensitivity and specificity of the instrument were determined and expressed graphically by the receiver operating characteristic (ROC) curve, comparing multiple cutoff points to determine the best cutoff point for the diagnostic test. After the determination of sensitivity and specificity, the positive and negative predictive values (PV+ and PV–, respectively) were determined. Positive and negative likelihood ratios (LR+ and LR–, respectively) were also determined, which regard how often the result of the diagnostic test is more likely (or less likely) in individuals with hyposalivation compared to those without hyposalivation.
Data analysis
Descriptive statistics were performed to characterize the sample, followed by analyses to determine the efficacy of the questionnaire on xerostomia as a screening tool for hyposalivation. The first step consisted of the evaluation of the reliability of the instrument, which was determined based on reproducibility and internal consistency of the answers on the questionnaire. The questionnaire was administered to 100 individuals on two separate occasions (test–retest process). The reproducibility of the answers of the participants to each question (nominal variable with two categories) was analyzed using the Kappa statistic, considering a 5% significance level. The reproducibility of the final score was determined using the intraclass correlation coefficient (ICC) for the determination of the level of agreement, because this coefficient considers systematic differences as part of the measurement error26. The ICCs were interpreted based on the scale traditionally used in the literature27: ≤0.40 = poor to fair, 0.41–0.60 = moderate, 0.61–0.80 = excellent, and 0.81–1.00 = nearly perfect. The homogeneity of the questions in relation to the construct (internal consistency) was determined using Cronbach’s alpha coefficient, with ≥0.70 considered satisfactory26.
The second step consisted of the evaluation of indicators that attest to the predictive value of the questionnaire for screening hyposalivation (measures that lend credibility to the questionnaire as an epidemiological screening tool). To determine the accuracy of the instrument, it is necessary to compare it to the result of a diagnostic test considered the reference standard. Therefore, the clinical consistency of the questionnaire was investigated by comparing its result to the diagnosis of hyposalivation determined by sialometry during the same session in which the questionnaire was administered. Spearman’s correlation coefficients were calculated between the total score on the questionnaire and the salivary flow rates. Moreover, the nonparametric Mann-Whitney was used to compare the total score on the questionnaire between two groups of patients (with and without a diagnosis of hyposalivation).
Based on the questionnaire score and diagnosis of hyposalivation, the area under the ROC curve (AUC) and the sensitivity, specificity, A, LR+, LR–, PV+ and PV– values were analyzed. The best cutoff point was that indicating the most balanced proportion between the percentages of true positives and true negatives, establishing the sensitivity of the instrument, since the intention is to use it as a screening tool for hyposalivation. The following criteria were taken into consideration during the interpretation of the results: (i) percentage values of sensitivity and specificity range from 0 to 100, with higher values denoting greater accuracy; (ii) LR+ values >1 suggest that the test is informative; and (iii) LR– values <1 suggest that the test is informative28. The AUC was interpreted as follows: 0.50–0.59 = poor accuracy, 0.60–069 = satisfactory accuracy, 0.70–0.79 = good accuracy, 0.80–0.89 = very good accuracy, and 0.90–1 = excellent accuracy28., 29.. Moreover, an association analysis between each item in the questionnaire and the diagnosis of hyposalivation was performed using Pearson’s chi-square test.
Ethical considerations
This study received approval from the Human Research Ethics Committee of Universidade Estadual da Paraíba (certificate number: 2.412.702) and was conducted in compliance with Resolution 466/12 of the Brazilian National Board of Health and international norms (Declaration of Helsinki). Participants with hyposalivation received counseling with regard to the stimulation of salivary flow and were sent for a medical examination to investigate possible systemic conditions associated with hyposalivation.
RESULTS
Characterization of sample
A total of 402 individuals participated in the present study. The majority of the participants were female (n = 274; 68.2%) and did not smoke (n = 369; 91.8%) or use alcohol (n = 292; 72.6%). The largest age group was those 29 years or younger (n = 102; 25.4%). Hyposalivation was identified in 162 participants (40.3%; Table 1). Table 2 shows the answers to each item on the questionnaire. The items with the highest frequencies (>15%) were ‘feel dry mouth at night or upon waking’ (n = 166; 41.3%), ‘feel dry mouth during the day’ (n = 91; 22.6%), ‘frequently wake up thirsty at night’ (n = 85; 21.1%) and ‘perceive little saliva in the mouth most of the time’ (n = 71; 17.7%).
Table 1.
Sociodemographic and clinical characterization of sample
| Variables | n | % |
|---|---|---|
| Sex | ||
| Male | 128 | 31.8 |
| Female | 274 | 68.2 |
| Age group | ||
| ≤29 years | 102 | 25.4 |
| 30–39 years | 76 | 18.9 |
| 40–49 years | 72 | 17.9 |
| 50–59 years | 69 | 17.2 |
| ≥60 years | 83 | 20.6 |
| Tobacco use | ||
| Yes | 33 | 8.2 |
| No | 369 | 91.8 |
| Ex-tobacco user | ||
| Yes | 87 | 21.6 |
| No | 315 | 78.4 |
| Alcohol intake | ||
| Yes | 110 | 27.4 |
| No | 292 | 72.6 |
| Clinical diagnosis of hyposalivation | ||
| Yes | 162 | 40.3 |
| No | 240 | 59.7 |
| Sialometry (mL/min) | ||
| Mean (SD) = 0.89 (0.53) | ||
| Median (IQR) = 0.80 (0.50–1.12) | ||
| Total score of questionnaire on xerostomia | ||
| Mean (SD) = 1.33 (1.60) | ||
| Median (IQR) = 1.00 (0.00–2.00) | ||
IQR, interquartile range (25th percentile–75th percentile); SD, standard deviation.
Table 2.
Distribution of participants according to answers to each item of questionnaire on xerostomia
| Variables | n | % |
|---|---|---|
| 1. Feel dry mouth during meals | ||
| Yes | 46 | 11.4 |
| No | 356 | 88.6 |
| 2. Have difficulty swallowing food | ||
| Yes | 33 | 8.2 |
| No | 369 | 91.8 |
| 3. Perceive small amount of saliva in your mouth most of the time | ||
| Yes | 71 | 17.7 |
| No | 331 | 82.3 |
| 4. Feel dry mouth at night or upon waking | ||
| Yes | 166 | 41.3 |
| No | 236 | 58.7 |
| 5. Feel dry mouth during the day | ||
| Yes | 91 | 22.6 |
| No | 311 | 77.4 |
| 6. Chew gum or mints to relieve the sensation of dry mouth | ||
| Yes | 16 | 4.0 |
| No | 386 | 96.0 |
| 7. Frequently wake up thirsty at night | ||
| Yes | 85 | 21.1 |
| No | 317 | 78.9 |
| 8. Have a burning sensation on your tongue | ||
| Yes | 26 | 6.5 |
| No | 376 | 93.5 |
Reliability
Table 3 displays the results of the test–retest analysis of the instrument. The answers to each question individually revealed variable reproducibility (κ = 0.450–0.785). The ICC was 0.917 (95% CI: 0.877–0.944), which demonstrates excellent reliability of the final score produced by the questionnaire. Regarding the homogeneity of the instrument, Cronbach’s alpha was 0.70.
Table 3.
Test–retest analysis for answers to each item of questionnaire on xerostomia
| Variables | Kappa |
|---|---|
| 1. Feel dry mouth during meals | 0.656 |
| 2. Have difficulty swallowing food | 0.593 |
| 3. Perceive small amount of saliva in your mouth most of the time | 0.635 |
| 4. Feel dry mouth at night or on waking | 0.736 |
| 5. Feel dry mouth during the day | 0.570 |
| 6. Chew gum or mints to relieve the sensation of dry mouth | 0.556 |
| 7. Frequently wake up thirsty at night | 0.785 |
| 8. Have a burning sensation on your tongue | 0.450 |
Correlation between questionnaire score and salivary flow rate
A statistically significant correlation was found between the total score on the questionnaire and the salivary flow rate (Spearman’s ρ = –0.193; P < 0.001), demonstrating that individuals with higher scores on the questionnaire tend to have lower stimulated salivary flow.
Comparison of questionnaire score between groups
The total score on the questionnaire was significantly higher among the patients diagnosed with hyposalivation (mean: 1.69; SD: 1.78) compared to those without a clinical diagnosis of hyposalivation (mean: 1.09; SD: 1.43; P = 0.001, Mann-Whitney test).
Clinical consistency
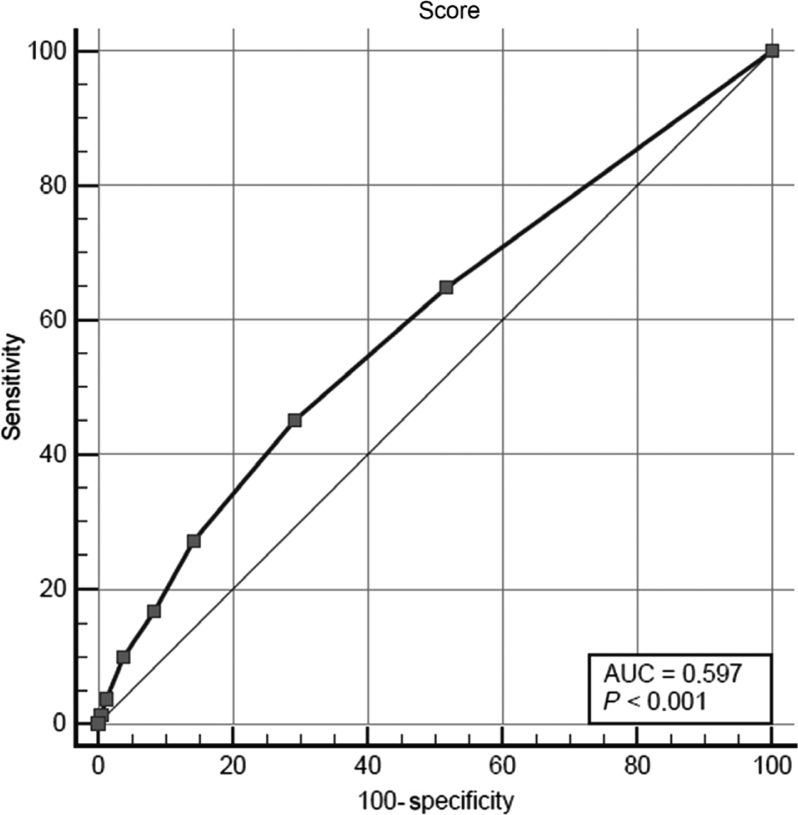
Considering the ROC curve (Figure 1), the AUC was 0.597 (95% CI: 0.547–0.645; P < 0.001). Table 4 displays the indicators of clinical consistency of the questionnaire for the diagnosis of hyposalivation considering different cutoff points.
Figure 1.
ROC curve showing performance of questionnaire on xerostomia for screening hyposalivation
Table 4.
Indicators of clinical consistency of questionnaire for epidemiological screening of hyposalivation using different cutoff points
| Cutoff point | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | LR+ | LR– | PV+ | PV– |
|---|---|---|---|---|---|---|---|
| ≥1 | 64.8 (56.9–72.1) | 48.3 (41.9–54.9) | 55.0 (50.0–59.9) | 1.25 | 0.73 | 45.9 | 67.1 |
| ≥2 | 45.1 (37.2–53.1) | 70.8 (64.6–76.5) | 60.5 (55.5–65.3) | 1.54 | 0.78 | 51.0 | 65.6 |
| ≥3 | 27.2 (20.5–34.7) | 85.8 (80.8–90.0) | 62.2 (57.3–67.0) | 1.92 | 0.85 | 56.4 | 63.6 |
| ≥4 | 16.7 (11.3–23.3) | 91.7 (87.4–94.8) | 61.4 (56.5–66.2) | 2.00 | 0.91 | 57.4 | 62.0 |
| ≥5 | 9.9 (5.8–15.5) | 96.3 (93.0–98.3) | 61.4 (56.5–66.2) | 2.63 | 0.94 | 64.0 | 61.3 |
| ≥6 | 3.7 (1.4–7.9) | 98.8 (96.4–99.7) | 60.5 (55.5–65.3) | 2.96 | 0.98 | 66.7 | 60.3 |
| ≥7 | 1.2 (0.1–4.4) | 99.6 (97.7–100.0) | 60.0 (55.0–64.8) | 2.96 | 0.99 | 66.7 | 59.9 |
CI, confidence interval; LR, likelihood ratio; PV, predictive value.
Best cutoff point (that with highest sensitivity) in bold type.
Analysis of association between items on questionnaire and hyposalivation
Table 5 displays the associations between each item on the questionnaire and altered salivary flow. Associations with five items were statistically significant (P < 0.05).
Table 5.
Associations between clinical diagnosis of hyposalivation and each item of questionnaire on xerostomia
| Variables | Hyposalivation | Total n (%) | P-value† | |
|---|---|---|---|---|
| Yes | No | |||
| n (%) | n (%) | |||
| 1. Feel dry mouth during meals | ||||
| Yes | 28 (60.9) | 18 (39.1) | 46 (100.0) | 0.003* |
| No | 134 (37.6) | 222 (62.4) | 356 (100.0) | |
| 2. Have difficulty swallowing food | ||||
| Yes | 15 (45.5) | 18 (54.5) | 33 (100.0) | 0.529 |
| No | 147 (39.8) | 222 (60.2) | 369 (100.0) | |
| 3. Perceive small amount of saliva in your mouth most of the time | ||||
| Yes | 42 (59.2) | 29 (40.8) | 71 (100.0) | <0.001* |
| No | 120 (36.3) | 211 (63.7) | 331 (100.0) | |
| 4. Feel dry mouth at night or upon waking | ||||
| Yes | 79 (47.6) | 87 (52.4) | 166 (100.0) | 0.012* |
| No | 83 (35.2) | 153 (64.8) | 236 (100.0) | |
| 5. Feel dry mouth during the day | ||||
| Yes | 48 (52.7) | 43 (47.3) | 91 (100.0) | 0.006* |
| No | 114 (36.7) | 197 (63.3) | 311 (100.0) | |
| 6. Chew gum or mints to relieve the sensation of dry mouth | ||||
| Yes | 11 (68.8) | 5 (31.2) | 16 (100.0) | 0.018* |
| No | 151 (39.1) | 235 (60.9) | 386 (100.0) | |
| 7. Frequently wake up thirsty at night | ||||
| Yes | 38 (44.7) | 47 (55.3) | 85 (100.0) | 0.351 |
| No | 124 (39.1) | 193 (60.9) | 317 (100.0) | |
| 8. Have a burning sensation on your tongue | ||||
| Yes | 12 (46.2) | 14 (53.8) | 26 (100.0) | 0.529 |
| No | 150 (39.9) | 226 (60.1) | 376 (100.0) | |
P < 0.05.
Pearson’s chi-squared test.
DISCUSSION
Screening tools must be able to identify the maximum number of true positives with a minimum number of false positives. Therefore, high sensitivity and PV– values suggest that a test is informative30., 31.. In the present study, the probability of the questionnaire correctly indicating the presence of hyposalivation among the participants reached nearly 65%. A similar value (67.1%) was found for the probability of the absence of hyposalivation among individuals that the questionnaire classified as not having hyposalivation (PV–). These results reveal acceptable indicators to suspect low salivary flow in the adult population, enabling affected individuals to be sent for a confirmatory diagnosis and treatment, if necessary.
Regarding the likelihood ratios, the LR+ demonstrated a 1.25-fold greater likelihood that the questionnaire would indicate the presence of hyposalivation in individuals with this condition compared to those without the condition. The LR– demonstrated a 0.73-fold greater probability that the questionnaire would indicate the absence of hyposalivation in individuals with hyposalivation compared to those without the condition. These values fit the parameters for a test to be considered informative (LR+ >1 and LR– <1)28.
When analyzed individually, the answers to each question revealed variable reproducibility, with agreement between the test and retest of the questionnaire higher than 0.63. According to Landis and Koch27, Kappa coefficients between 0.61 and 0.80 can be considered indicative of good agreement. Analyzing the homogeneity of the questions in relation to the construct, the questionnaire exhibited satisfactory internal consistency. Very low Cronbach’s alpha coefficients suggest no correlations among the items on the questionnaire, whereas very high coefficients indicate redundancy among the items. A measure with good internal consistency has values between 0.70 and 0.9026. In the present study, Cronbach’s alpha was 0.70, indicating satisfactory internal consistency of the instrument. In addition, a statistically significant correlation was found between the total score on the questionnaire and the salivary flow rate, demonstrating that individuals with higher scores on the questionnaire tend to have lower stimulated salivary flow. Although the correlation coefficient was not high, these findings were expected. The weak correlation can be explained by the fact that not every individual perceives symptoms of hyposalivation. Generally, people are more aware of problems arising from caries and periodontal disease. In contrast, many patients are unaware of a decline in saliva production and the problems they may suffer because of this condition.
In the bivariate analysis of each item on the questionnaire and the diagnosis of hyposalivation, the following questions proved not to be associated with the outcome: ‘Do you have difficulty swallowing food?’, ‘Do you frequently wake up thirsty at night?’ and ‘Do you have a burning sensation on your tongue?’. In contrast, the question ‘Do you perceive a small amount of saliva in your mouth most of the time?’ was strongly associated with hyposalivation. A nearly constant feeling of dry mouth is an indicator of a low amount of saliva, meaning that xerostomia emerges as a result of a reduction in salivary flow rate in most cases10., 32., 33.. In the study by Torres et al.,8 no statistically significant association was found with this question, which the authors attributed to a possible sensation of excess saliva due to greater salivary viscosity. The question ‘Do you feel dry mouth at night or upon waking?’ received the largest number of positive answers, which is similar to the finding described by Torres et al.8 This may be explained by the physiological reduction in the production of saliva at night and other factors that can exert an influence on the sensation of dry mouth, such as the use of medications14., 16., 20., 22. or the presence of systemic conditions23., 24.. The association between this question and hyposalivation was statistically significant, but less so compared to the other items on the questionnaire.
It is possible that the measures of diagnostic precision of the questionnaire did not achieve higher values due to the choice of the standard test. The method used to measure salivary flow (stimulated sialometry) may be a source of measurement bias. The determination of total salivary flow can be performed in a stimulated manner (chewing on an object) or through the drainage of saliva in the absence of stimulus24., 34.. However, there is no universally accepted standard test35. Mulligan et al.36 and Navazesh and Christensen37 state that the non-stimulated collection method is more indicated and cite some of the limitations to the analysis of stimulated flow, such as the influence of the consistency and size of the product being chewed, an uncontrolled chewing pace and the triggering of the gag reflex. In contrast, Jornet and Fenol38 report that the non-stimulated method is imprecise, as factors such as age, sex and psychological issues can affect the results. According to Thomson34, mechanically stimulated sialometry is the more indicated method for large-scale collections, as occurs in epidemiological studies, due to its ease of use, which is a fundamental characteristic in studies with a large number of participants. Therefore, the stimulated method was selected in the present study due to its reliability, practicality, ease of use and less discomfort on the part of the participants.
This study has some limitations that should be considered. The answers to the questions may have been subject to information bias. Moreover, only stimulated salivary flow was tested. On the other hand, this study contributes to the advancement of scientific knowledge considering two main points. First, it is one of the few studies in the literature to investigate the use of a questionnaire to identify probable cases of hyposalivation. Second, the authors evaluated a considerable number of patients. Future studies should be conducted using a different reference standard of salivary flow, such as non-stimulated sialometry, to compare the results and confirm the use of this questionnaire as a hyposalivation screening tool.
CONCLUSION
The results suggest that the accuracy of the questionnaire on xerostomia as a screening tool for cases of hyposalivation was satisfactory. The questionnaire analyzed can serve as an epidemiological screening tool for the selection of probable cases of hyposalivation. If the results of the questionnaire are positive, the individual should be referred for tests to confirm the diagnosis.
LIMITATIONS
The method used to measure salivary flow (stimulated sialometry) may be a source of measurement bias due to variables related to the consistency and size of the product chewed and uncontrolled chewing pace.
CLINICAL RELEVANCE
The information provided by the test enables a change in the clinical conduct of health professionals, who can employ a fast, low-cost, easy-to-administer assessment tool in cases for which the standard examination is unviable, such as in epidemiological studies.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors declare that they have not received funding.
Ethical approval
All procedures in this study involving human subjects were performed in compliance with the ethical standards of the institutional and/or national review board as well the Declaration of Helsinki from 1964 and subsequent amendments or comparable ethical standards.
Informed consent
Informed consent was obtained individually from all participants in the study.
REFERENCES
- 1.Saleh J, Figueiredo MAZ, Cherubini K, et al. Salivary hypofunction: an update on a etiology, diagnosis and therapeutics. Arch Oral Biol. 2015;60:242–255. doi: 10.1016/j.archoralbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Silva IJO, Almeida ARP, Falcão NC, et al. Hyposalivation: etiology, diagnosis and treatment. Rev Bahiana Odonto. 2016;7:140–146. [Google Scholar]
- 3.Bolstad AI, Skarstein K. Epidemiology of Sjögren’s syndrome – from an oral perspective. Curr Oral Health Rep. 2016;3:328–336. doi: 10.1007/s40496-016-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasidi MQZBM, Jain AR. Knowledge, attitude and practice on hyposalivation in complete denture patients among dental interns in Chennai, India. J Pharm Sci Res. 2017;9:225–229. [Google Scholar]
- 5.Ohga N, Yamazaki Y, Sato J, et al. Elimination of oral candidiasis may increase stimulated whole salivary flow rate. Arch Oral Biol. 2016;71:129–133. doi: 10.1016/j.archoralbio.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Charalambous A, Lambrinou E, Katodritis N, et al. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: A feasibility randomized control trial. Eur J Oncol Nurs. 2017;27:1–8. doi: 10.1016/j.ejon.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Islas-Granillo H, Borges-Yáñez A, Fernández-Barrera MÁ, et al. Relationship of hyposalivation and xerostomia in Mexican elderly with socioeconomic, sociodemographic and dental factors. Sci Rep. 2017;7:40686. doi: 10.1038/srep40686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres SR, Lotti RS, Peixoto CB, et al. Determination of the efficacy of a questionnaire for the detection of hyposalivation. Rev Assoc Paul Cir Dent. 2002;56:227–231. [Google Scholar]
- 9.Paranhos HFO, Salles AES, Macedo LD, et al. Complete denture biofilm after brushing with specific denture paste, neutral soap and artificial saliva. Braz Dent J. 2013;24:47–52. doi: 10.1590/0103-6440201301946. [DOI] [PubMed] [Google Scholar]
- 10.Niklander S, Veas L, Barrera C, et al. Risk factors, hyposalivation and impact of xerostomia on oral health-related quality of life. Braz Oral Res. 2017;31:1–9. doi: 10.1590/1807-3107BOR-2017.vol31.0014. [DOI] [PubMed] [Google Scholar]
- 11.Monasterios FL, Llabrés XR. Etiopathogenesis and diagnosis of dry mouth. Av Odontoestomatol. 2014;30:121–128. [Google Scholar]
- 12.Varoni EM, Federighi V, Decani S, et al. The effect of clinical setting on the unstimulated salivary flow rate. Arch Oral Biol. 2016;69:7–12. doi: 10.1016/j.archoralbio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Barbe AG, Schmidt-Park Y, Hamacher S, et al. Efficacy of GUM® Hydral versus Biotène® Oral balance mouthwashes plus gels on symptoms of medication-induced xerostomia: a randomized, double-blind, crossover study. Clin Oral Invest. 2018;28:169–180. doi: 10.1007/s00784-017-2096-0. [DOI] [PubMed] [Google Scholar]
- 14.Burci LM, Barbosa PBD, Silva CB, et al. Patentability potential of natural products for xerostomia treatment. Braz Dent Sci. 2016;19:4–12. [Google Scholar]
- 15.Ladino RM, Gasitulli OA, Campos MX. Sjögren’s syndrome: clinical case. Rev Chil Pediatr. 2015;8:47–51. doi: 10.1016/j.rchipe.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Kuraji M, Matsuno T, Satoh T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J Clin Biochem Nutr. 2016;59:79–85. doi: 10.3164/jcbn.15-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oncul B, Karakis D, Dogruman F. The effect of two artificial salivas on the adhesion of Candida albicans to heat polymerized acrylic resin. J Adv Prosthodont. 2015;7:93–97. doi: 10.4047/jap.2015.7.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lone MA, Shaikh S, Lone MM, et al. Association of salivary gland hypofunction with diabetes mellitus and drugs among the elderly in Karachi, Pakistan. J Investig Clin Dent. 2016;8:1–6. doi: 10.1111/jicd.12209. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita JM, Moura-Grec PG, Capelari MM, et al. Oral manifestations in patients with diabetes mellitus: a systematic review. Rev Odontol UNESP. 2013;42:211–220. [Google Scholar]
- 20.Morais EF, Macedo RAPM, Lira JAS, et al. Factors related to dry mouth and low salivary flow rates in diabetic elderly: a systematic literature review. Rev Bras Geriatr Gerontol. 2014;17:417–423. [Google Scholar]
- 21.Munemasa T, MukaiboT Kondo Y, et al. Salivary gland hypofunction in KK-Ay Type 2 diabetic mice. J Diabetes. 2018;16:18–27. doi: 10.1111/1753-0407.12548. [DOI] [PubMed] [Google Scholar]
- 22.Cockburn N, Pateman K, Taing MW, et al. Managing the oral side effects of medications used to treat multiple sclerosis. Aust Dent J. 2017;62:331–336. doi: 10.1111/adj.12510. [DOI] [PubMed] [Google Scholar]
- 23.Abrão ALP, Santana CM, Bezerra ACB, et al. What rheumatologists should know about orofacial manifestations of autoimmune rheumatic diseases. Rev Bras Reumatol. 2016;56:441–450. doi: 10.1016/j.rbre.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Falcão DP, Mota LMH, Pires AL, et al. Sialometry: aspects of clinical interest. Rev Bras Reumatol. 2013;53:525–531. doi: 10.1016/j.rbr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Nunes RS, Pinheiro NC, Maia ML, et al. Factorial validation of a questionnaire for the detection of hyposalivation in institutionalized older adults. Rev Ciência Plural. 2015;1:67. [Google Scholar]
- 26.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–211. [PMC free article] [PubMed] [Google Scholar]
- 29.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 30.Pernambuco L, Espelt A, Lima KC. Screening for voice disorders in older adults (RAVI)—Part III: cutoff score and clinical consistency. J Voice. 2016;31:117.e17–117.e22. doi: 10.1016/j.jvoice.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Hatanaka VMA, Benseñor IM. In: Epidemiology: Practical Approach. 2nd ed. Benseñor IM, Lotufo PA, editors. Sarvier; São Paulo: 2011. Evaluation of diagnostic tests; pp. 272–302. [Google Scholar]
- 32.Nonzee V, Manopatanakul S, Khovidhunkit SOP. Xerostomia, hyposalivation and oral microbiota in patients using antihypertensive medications. J Med Assoc Thai. 2012;95:96–104. [PubMed] [Google Scholar]
- 33.Artico G, Freitas RS, Santos Filho AM, et al. Prevalence of Candida spp., xerostomia, and hyposalivation in oral lichen planus – a controlled study. Oral Dis. 2014;20:36–41. doi: 10.1111/odi.12120. [DOI] [PubMed] [Google Scholar]
- 34.Thomson WM. Issues in the epidemiological investigation of dry mouth. Gerodontology. 2005;22:65–76. doi: 10.1111/j.1741-2358.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 35.Pupo DB, Bussoloti Filho I, Liquidato BM, et al. Proposal for a practical method of sialometry. Rev Bras Otorrinolaringol. 2002;68:219–222. [Google Scholar]
- 36.Mulligan R, Navazesh M, Wood GJ. A pilot study comparing three salivary collection methods in an adult population with salivary gland hypofunction. Spec Care Dentist. 1995;15:154–158. doi: 10.1111/j.1754-4505.1995.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 37.Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61:1158–1162. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- 38.Jornet PL, Fenoll AB. Sialométrie sur 159 sujets sains. Facteurs physiologiques qui influencent la sécrétion salivaire non stimulée. Rev Stomatol Chir Maxillofac. 1995;96:342–346. [PubMed] [Google Scholar]