Abstract
An adverse drug reaction (ADR) is an undesirable effect of a drug. ADRs are possible with any medication that is prescribed or administered in the dental office. While most pharmacological agents in use today have favourable drug profiles and are relatively safe, the prudent clinician must be aware of the potential ADRs that can occur and be prepared to manage any complications. Here we review the most commonly used agents in dentistry, namely local anaesthetics, sedatives, analgesics and antibiotics, and their ADRs and management.
Key words: Adverse reactions, drug interactions, dental pharmacology, analgesics, antibiotics
INTRODUCTION
An adverse drug reaction (ADR) can be defined as any undesirable effect of a drug1, 2. It is important to distinguish between an ADR and an allergy: an allergy refers to an immune-mediated response to a medication, for example anaphylaxis, whereas an ADR can be any effect that is not therapeutically desired, for example sedation. In essence, an allergy can be an ADR but not all ADRs are allergies3.
True drug allergies can be identified by the presentation of any definite immunological mechanism (T-cell or drug-specific antibody). Such reactions occur immediately or after a delay, and may be life-threatening to the patient. Immediate drug allergies commonly present as urticaria, rhinitis, angioedema, bronchospasm, conjunctivitis, gastrointestinal (GI) upset or anaphylaxis (which has the potential to cause cardiovascular collapse)4, 5. Delayed drug allergies commonly present on the skin with possible symptoms, including delayed urticaria, maculopapular eruptions, vasculitis and blistering diseases6. If a practitioner is concerned about a patient having an allergic drug reaction, they should immediately stop administration of the suspected drug, treat the reaction, document the drugs taken (dose, type, duration), and record any signs and symptoms7, 8. In severe cases, emergency medical services should be summoned and the patient accompanied to the nearest emergency room. A referral should be made to the patient’s family physician to investigate the allergy further.
In modern dental practice only a few categories of pharmacological agents are ubiquitously used; these include local anaesthetics (LA), central nervous system depressants (e.g. nitrous oxide, benzodiazepines and general anaesthetics), analgesics [e.g. non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen and opioids] and antibiotics (e.g. penicillin, clindamycin and metronidazole). It is critical that the dental healthcare team is well equipped to manage any foreseeable and unforeseeable ADRs that occur in the dental office setting. In addition, it is the responsibility of the healthcare professional to thoroughly understand the health status of their patients whenever prescribing new drugs or altering dosages to avoid provoking avoidable ADRs in their patients.
LOCAL ANAESTHETICS AND THEIR VASOCONSTRICTORS
Local anaesthetics are the most commonly used drugs in dental practice9. It is estimated that each Canadian dentist will administer close to 1,800 cartridges per year; in the USA alone, over 300 million cartridges are administered per year10. LA are generally considered to be safe10; however, the healthcare practitioner must take responsibility to ensure that the dosage and concentration is tailored towards each patient to avoid potential ADRs, both local and systemic.
Local complications associated with LA administration include tissue necrosis and direct neurotoxicity. Tissue necrosis occurs due to disruption of the vasculature-supplying tissue, and is related to the irritating nature of the solution, large volumes being administered, or constriction of the vasculature by vasoconstrictors in the LA cartridge (e.g. epinephrine or levonordefrin). Management of these conditions is largely symptomatic in nature and avoidance is most prudent. Careful administration of LA, particularly in areas of tightly-bound tissue such as in the palate, will reduce the likelihood of ischaemia.
Direct neurotoxicity to nerve trunks resulting in paresthesia has been reported in the literature to occur at higher frequency with anaesthetic solutions of higher concentration such as 4% articaine and prilocaine when administered as blocks (see Haas and Lennon10 and Garisto et al.11). Others suggested that this may not be the case when using articaine12, 13, 14. The clinician must carefully consider whether the benefits of a 4% solution outweigh the potential for complications. Management of paresthesia entails monitoring and potential referral to an oral surgeon with experience and background in the management of these conditions. Fortunately most cases of paresthesia are transient and resolve within 8 weeks; however, in some cases neurological deficits may be permanent10. Other complications associated with LA are a combination of ADR and technique, such as intramuscular injection resulting in trismus.
As LA is removed in the cardiovascular system (CVS) and may be administered directly into the CVS due to poor technique, systemic complications can occur. All LAs are central nervous system depressants, and can result in drowsiness, seizures and even coma at high enough blood levels in cerebral circulation10. While unlikely to occur in an adult patient save for extreme administration of LA, this is much more of a concern in paediatric patients who have much lower body weight10. Clinicians should be aware of calculations of maximum dosages for LA (Table 1). Another possible systemic ADR is methemoglobinemia, an uncommon reaction most often associated with prilocaine, and topical benzocaine. Methemoglobinemia results in cyanosis that does not respond to 100% oxygen, and can result in nausea, sedation, seizures, and coma at high levels. For patients with congenital methemoglobinemia, those mentioned anaesthetics should be avoided10.
Table 1.
Canadian maximum recommended dosages (MRD)* of LA and sample calculation for determining maximum amount of LA that can be safely administered. Modified from Haas and Lennon10
| Anaesthetic | Maximum | Sample calculation |
|---|---|---|
| Lidocaine (e.g. Xylocaine) | 7 mg/kg up to 500 mg | Patient weighs 20 kg (44 lb), dentist administers lidocaine 2% |
| Articaine (e.g. Astracaine) | 7 mg/kg up to 500 mg | Maximum = 7 mg/kg * 20 kg = 140 mg |
| Bupivacaine (e.g. Vivacaine) | 2 mg/kg up to 200 mg | Lidocaine 2% = 20 mg/mL of drug |
| Mepivacaine (e.g. Scandonest) | 6.6 mg/kg up to 400 mg | Max amount = 140 mg/ 20 mg/mL = 7 mL |
| Prilocaine (e.g. Citanest) | 8 mg/kg up to 500 mg | Max cartridge = 7 mL/ 1.8 mL/ cartridge = 3.9 cartridges |
| Therefore, the dentist can administer up to 3.9 cartridges of lidocaine 2%. |
Values in Table 1 represent Canadian MRDs and therefore may vary significantly from country to country. It is recommended that each clinician investigate the manufacturer’s recommendations.
Today, the most common vasoconstrictor in LA formulations is epinephrine, and is available in 1:50,000, 1:100,000 and 1:200,000 formulations. Epinephrine is a potent cardiovascular agent, and at high doses can result in increased heart rate, blood pressure, and potential cardiovascular emergency. The clinician must be cautious with regards to how much is administered.
SEDATIVES AND GENERAL ANAESTHETICS
All sedatives and general anaesthetic agents are capable of causing respiratory depression in a dose-dependent manner, with the intensity of depression increased more readily when multiple sedatives are administered. It is important to distinguish a physiological depression of respiration from an anatomical one – i.e. one that is caused due to loss of muscle tone. Loss of muscle tone in the glossopharyngeal musculature reduces pharyngeal patency, as does malpositioning of the tongue. Such anatomical obstruction can occur at any level of sedation, and can be managed with airway techniques such as a head tilt, chin lift or an oropharyngeal airway.
Physiological depression relates to alterations to the mechanisms related to respiration. Respiration is dependent upon both a central hypercapnic drive and a peripheral hypoxemic drive with the sedative classes impacting these drives differently. As dosages increase, the differences become increasingly minimal, and both central and peripheral drives are affected. With regards to tidal volume and respiratory rate, in general, the inhalation anaesthetics (e.g. sevoflurane) depress tidal volume while increasing the respiratory rate, while intravenous agents and opioids depress both. Benzodiazepines tend to depress respiration the least. Outside of respiratory effects, sedatives and general anaesthetics also have impacts on the CVS and may result in changes to heart rate and arterial blood pressure. Finally, the benzodiazepines and propofol result in anterograde amnesia, which may be of concern where instructions need to be given to the patient or where changes in treatment plan may occur during the procedure (Table 2).
Table 2.
Impacts of common sedatives and general anaesthetics on heart rate, arterial pressure and ventilation. Modified from Butterworth et al.29
| Agent | Heart rate | Arterial pressure | Ventilation |
|---|---|---|---|
| Diazepam | = + * | − | − − |
| Midazolam | + * | − − | − − |
| Propofol | = | − − | − − − |
| Ketamine | + + | + + | = − |
| Fentanyl | − − | = − | − − − |
| Nitrous oxide | = | = | = |
| Desflurane | = + | − − | − − |
| Sevoflurane | = | − | − |
‘*’ Increase due to reflex response to decreased arterial pressure. ‘+’ indicates an increase. ‘−’ indicates a decrease. ‘=’ indicates no change.
It is also worth reviewing the sedation continuum. At minimal sedation (‘anxiolysis’), patients respond normally to verbal stimulation. At moderate sedation (‘conscious sedation’), purposeful response is made to verbal or tactile stimulation. Deep sedation involves purposeful response to repeated or painful stimulus. At general anaesthesia, a patient does not respond to pain. A purposeful response is not to be considered withdrawal from a painful stimulus. See Table 3 for a more detailed examination and summary of the continuum.
Table 3.
The sedation continuum
| Minimal sedation | Moderate sedation | Deep sedation | General anaesthesia | |
|---|---|---|---|---|
| Responsiveness | Either verbal or tactile | Verbal and/or tactile in combination | To painful repeated stimulus | No response even to pain |
| Airway | Maintained | Usually maintained | Intervention may be needed | Intervention usually needed |
| Ventilation | Unaffected | Adequate | May require supplementation | Frequently requires supplementation |
| Cardiovascular function | Unaffected | Usually maintained | Usually maintained | May be impaired |
| Protective reflexes | Intact | Intact | Partial loss | Absent |
Modified from Ref. 30.
ANALGESICS
While numerous analgesics are available, here we focus on the most commonly employed analgesics in practice: acetaminophen, the NSAIDs, and opioids.
Acetaminophen
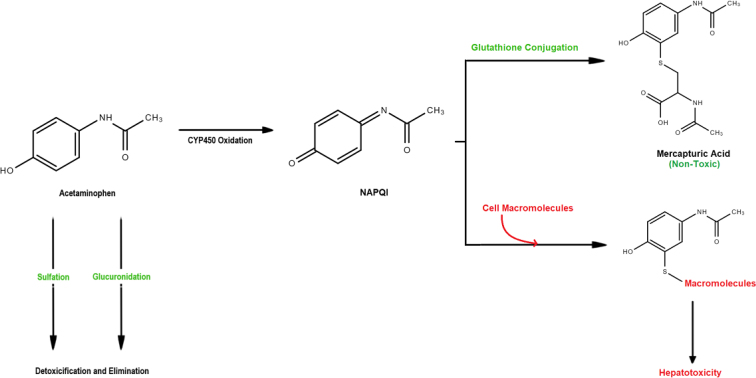
Acetaminophen is one of the safest analgesics available with almost no adverse effects when administered to healthy individuals at conventional doses.10 The most significant ADR associated with acetaminophen is hepatotoxicity. Hepatotoxicity occurs due to the accumulation of N-acetyl-p-benzoquinone imine (NAPQI), a potentially toxic metabolite of acetaminophen15, 16. This metabolite is formed from approximately 5%−15% of total acetaminophen intake, with the remaining 85%−95% undergoing immediate renal elimination after sulphation or glucuronidation (Figure 1). For healthy individuals NAPQI is conjugated by glutathione and is also eliminated. However, in the presence of excessive acetaminophen dosages, or in individuals with compromised hepatofunction, hepatotoxicity becomes a grave concern.
Figure 1.
Main pathway of acetaminophen metabolism.
The commonly accepted maximum dosage of acetaminophen in a healthy individual is 4 g per day; however, recent suggestions have been made to reduce this dosage to 3 g per day17. Hepatotoxicity may occur if a large bolus is taken at 7.5–10 g for adults (150 mg/kg in children), with fatalities potentially occurring at 20−25 g. Because the dosage that induces hepatotoxicity is approximately double that of the daily maximum, the likelihood of adverse reactions is low assuming proper drug instructions are given. In an emergency overdose situation, individuals should be treated with high-dose acetylcysteine to restore glutathione enzyme reserves.
Non-steroidal anti-inflammatory drugs
While generally well tolerated, the most common ADR associated with the NSAIDs relates to GI complications, with the most widely feared relating to GI bleeding and toxicity. GI toxicity is not considered uncommon, with over 16,500 deaths in the USA attributed to NSAIDs18. GI bleeding arises due to the mechanism of action of NSAIDs, which inhibit prostaglandin synthesis, thereby diminishing the protective effect prostaglandins have on mucosa. The prudent clinician should therefore avoid prescribing NSAIDs to any patient with a history of gastric ulcers or bleeding, and should consider acetaminophen as the drug of choice.
More rare is the possibility of triggering an anaphylactoid reaction with NSAIDs. Bronchospasm and other signs and symptoms of allergy can occur in susceptible patients due to the leukotriene pathway of action19. For patients suffering from severe asthma, the clinician should avoid administration of NSAIDs, as well as acetylsalicylic acid. Other potential ADRs include impairment of renal function; however, these typically require long-term, chronic administration of NSAIDs.
Opioids
All opioids produce dose-dependent respiratory depression, sedation and GI upset, including constipation, nausea and vomiting17. Mood alterations are also possible, and may appear as euphoria or dysphoria (if unpleasant). Unfortunately, opioid misuse and addiction is rising at a devastating rate throughout Canada and the USA, to the point where it is now considered an ‘opioid crisis’20. Patients suffering from opioid use disorder often require long-term sustained treatment, most commonly methadone, in order to maintain recovery. As prescribers of approximately 12% of all opioids in North America, dentists must make efforts to avoid opioid prescriptions unless absolutely necessary21. See Table 4 for recommended post-surgical pain management protocol. Opioid prescriptions should be written judiciously after an extensive review of the patient’s health history to minimise the patient’s risk for developing physical dependence, a devastating opioid adverse drug effect.
Table 4.
Post-operative pain management protocol in dentistry
| Anticipated post-operative pain | Initial 24 hours | After 24 hours |
|---|---|---|
| Mild to moderate | 400–600 mg ibuprofen q6h | 400 mg ibuprofen q4–6h |
| Moderate to severe | 400–600 mg ibuprofen +500 mg acetaminophen q6h |
400 mg ibuprofen +500 mg acetaminophen q6h |
| Severe | 400–600 mg ibuprofen +650 mg acetaminophen +10 mg hydrocodone q6h for 24–48 hours |
400–600 mg ibuprofen +500 mg acetaminophen q6h |
‘q’ = every, ‘h’ = hours.
From Ref.31.
ANTIBIOTICS
Excluding anaphylactic reactions, the most commonly used antibiotics in dentistry today are tolerated well. The most common ADR for all antibiotics involves GI upset (e.g. diarrhoea, nausea, vomiting). Approximately 2%–10% of all antibiotics used will result in diarrhoea, reaching upwards of 25% in the case of Augmentin® (amoxicillin and clavulanic acid)22. Of greater concern is the potential for development of more serious opportunistic infections like Clostridium difficile or yeast infections following administration of antibiotics. The former is most commonly associated with the usage of clindamycin, though any antibiotic may potentially result in C. difficile infection. The protocol for the prevention and management of C. difficile infection is outlined in Table 5. If a patient suspects they may have a C. difficile infection, they should immediately contact their family physician to be evaluated. In cases where a patient does not have a family physician or is displaying severe symptoms, such as bloody diarrhoea or urine, they should go to their nearest hospital emergency room. The prevention protocol is outlined in Table 6. Fungal infections are generally managed through prescription of a topical antifungal such as nystatin.
Table 5.
Management protocol for the management of Clostridium difficile infection
| Stop all antibiotics |
|---|
| Keep the patient hydrated |
| Refer to a physician |
| Prescribe: |
| Vancomycin 500 mg po qid for 2 days (if severe) |
| Vancomycin 125 mg po qid for 10–14 days |
| Metronidazole 500 mg po tid for 7–14 days |
Table 6.
Preventive protocol for Clostridium difficile infection
| Only prescribe antibiotics when absolutely necessary |
| Wash hands with soap and warm water between each patient |
| Use disposable gloves and gowns when in the same room as infected patients |
| Thorough disinfection of room/chair with chlorine bleach between patients |
It is also extremely important to educate patients on their restrictions when taking specific antibiotics to avoid the potential for ADRs. Users prescribed metronidazole, an antibiotic effective at treating anaerobic bacterial infections, should be advised to avoid alcohol intake due to metronidazole’s ability to inhibit enzymes in the ethanol degradation pathway24, 25. Users may experience flushing, headaches, nausea and cardiac palpitations26. Metronidazole users should avoid alcohol intake for at least 3 days after completing the full antibiotic course.
Another, more life-threatening, adverse drug interaction is that of clarithromycin, erythromycin and azithromycin with digoxin25. These antibiotics inhibit the elimination of digoxin from the bloodstream and thus have the potential to severely elevate digoxin blood levels, causing digitalis toxicity27, 28. Signs of digitalis toxicity commonly include nausea, vomiting and irregular heartbeat. Less common symptoms may include experiences of confusion, blurry vision, blind spots and bodily oedema.
CONCLUSION
By and large, the pharmacological armamentarium for the practicing dentist today is relatively safe. However, the prudent clinician must be aware of potential ADRs that can arise from drug administration and be comfortable with the management of such complications.
Acknowledgements
None.
Conflict of interest
None.
Funding information
No fundings was received for this review.
REFERENCES
- 1.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 2.Pirmohamed M, Breckenridge AM, Kitteringham NR, et al. Adverse drug reactions. BMJ. 1998;316(7140):1295–1298. doi: 10.1136/bmj.316.7140.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourpak Z, Fazlollahi MR, Fattahi F. Understanding adverse drug reactions and drug allergies: principles, diagnosis and treatment aspects. Recent Pat Inflamm Allergy Drug Discov. 2008;2(1):24–46. doi: 10.2174/187221308783399289. [DOI] [PubMed] [Google Scholar]
- 4.Limsuwan T, Demoly P. Acute symptoms of drug hypersensitivity (urticaria, angioedema, anaphylaxis, anaphylactic shock) Med Clin North Am. 2010;94:691–710. doi: 10.1016/j.mcna.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Pipa-Vallejo A, García-Pola-Vallejo MJ. Local anesthetics in dentistry. Med Oral Patol Oral Cir Bucal. 2004;9(5):440–443. 438–440. [PubMed] [Google Scholar]
- 6.Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges. 2009;7:142–160. doi: 10.1111/j.1610-0387.2008.06878.x. [DOI] [PubMed] [Google Scholar]
- 7.Demoly P, Adkinson NF, Brockow K, et al. International Consensus on drug allergy. Allergy. 2014;69(4):420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 8.Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43–61. doi: 10.1111/j.1365-2222.2008.03155.x. [DOI] [PubMed] [Google Scholar]
- 9.Haas DA. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002;68(9):6. [PubMed] [Google Scholar]
- 10.Haas DA, Lennon D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61(4):319–320. 323–326, 329–330. [PubMed] [Google Scholar]
- 11.Garisto GA, Gaffen AS, Lawrence HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141(7):836–844. doi: 10.14219/jada.archive.2010.0281. [DOI] [PubMed] [Google Scholar]
- 12.Albalawi F, Lim JC, Direnzo KV, et al. Effects of lidocaine and articaine on neuronal survival and recovery. Anesth Prog. 2018;65:82–88. doi: 10.2344/anpr-65-02-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malet A, Faure MO, Delegate N, et al. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015;120:589–596. doi: 10.1213/ANE.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 14.DiRenzo KV, Lim JL, Albalawi F, et al. Differential effects of sodium channel blockers on neural survival and responsiveness: 2% lidocaine kills more SHSY-5Y cells and reduces cellular activity as compared to 4% articaine. J Den Res. 2016;95:0790. [Google Scholar]
- 15.Turkoski BB. Acetaminophen: old friend—new rules. Orthop Nurs. 2010;29(1):41–43. doi: 10.1097/NOR.0b013e3181c8cd75. [DOI] [PubMed] [Google Scholar]
- 16.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 17.Haas DA. An update on analgesics for the management of acute postoperative dental pain. J Can Dent Asoc. 2002;68(8):476–482. [PubMed] [Google Scholar]
- 18.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340(24):1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 19.Leuppi JD, Schnyder P, Hartmann K, et al. Drug-induced bronchospasm: analysis of 187 spontaneously reported cases. Respiration. 2001;68(4):345–351. doi: 10.1159/000050525. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Eng J Med. 2017;377(4):391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 21.Denisco RC, Kenna GA, O’Neil MG, et al. Prevention of prescription opioid abuse: The role of the dentist. J Am Dent Assoc. 2011;142(7):800–810. doi: 10.14219/jada.archive.2011.0268. [DOI] [PubMed] [Google Scholar]
- 22.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 23.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell DA. Metronidazole: its use in clinical dentistry. J Clin Periodontol. 1984;11(3):145–158. doi: 10.1111/j.1600-051x.1984.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 25.Hersh EV, Moore PA. Adverse drug interactions in dentistry. Periodontol 2000. 2008;46(1):109–142. doi: 10.1111/j.1600-0757.2008.00224.x. [DOI] [PubMed] [Google Scholar]
- 26.Sellers EM, Lang M, Koch-Weser J, et al. Interaction of chloral hydrate and ethanol in man: I. Metabolism. Clin Pharmacol Ther. 1972;13(1):37–49. doi: 10.1002/cpt197213137. [DOI] [PubMed] [Google Scholar]
- 27.Rengelshausen J, Göggelmann C, Burhenne J, et al. Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin–clarithromycin interaction. Br J Clin Pharmacol. 2003;56(1):32–38. doi: 10.1046/j.1365-2125.2003.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakasugi H, Yano I, Ito T, et al. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin Pharmacol Ther. 1998;64:123–128. doi: 10.1016/S0009-9236(98)90030-3. [DOI] [PubMed] [Google Scholar]
- 29.Butterworth J, Mackey D, Wasnick J. 5th edn. McGraw Hill; New York, NY: 2013. Morgan & Mikhail’s Clinical Anesthesiology. [Google Scholar]
- 30.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96(4):1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Dione R. Breaking down barriers to care with effective pain management. Compendium. 2016;37(5):346–348. [Google Scholar]