Abstract
Background: Maternal serum IgG antibody against Porphyromonas gingivalis is an indicator of both periodontitis and adverse pregnancy outcomes. This study aims to evaluate the anti-P. gingivalis IgG and IgG subclasses1–4 in threatened preterm labour (TPL) patients and their association with small for gestational age (SGA). Methods: Serum, saliva and subgingival plaque samples were collected from 47 TPL patients compared with 48 healthy pregnant women. The amount of P. gingivalis was measured in saliva and plaque using real-time polymerase chain reaction. The serum anti-P. gingivalis IgG titre and anti-P. gingivalis subclasses IgG 1–4 concentration were measured using enzyme-linked immunosorbent assay. Results: The amount of anti-P. gingivalis IgG-1 was significantly lower in the TPL group than in the healthy group. Fourteen subjects delivered SGA infants in the TPL group. The pocket probing depth (PPD), clinical attachment loss, PPD ≥ 5 mm%, amount of P. gingivalis in plaque, anti-P. gingivalis IgG and anti-P. gingivalis IgG-4 were significantly higher in the TPL-SGA group than in the TPL-normal weight group. Moreover, logistic regression analysis revealed the detection frequency of P. gingivalis in plaque and placenta weight were significantly correlated with SGA in TPL. In the receiver operating characteristic curve analysis, an amount of P. gingivalis in plaque ≥ 86.45 copies showed a sensitivity of 0.786 and a specificity of 0.727 (AUC 0.792) for predicting SGA in TPL. Conclusion: Lower anti-P. gingivalis IgG-1 amounts are related to TPL, while higher anti-P. gingivalis IgG and IgG-4 are related with SGA in TPL. Further, greater colonisation of P. gingivalis in plaque might increase the risk of SGA and can be useful in prediction of SGA in TPL.
Key words: Porphyromonas gingivalis, immunoglobulin G subclasses antibody, threatened preterm labour, small for gestational age
INTRODUCTION
Periodontal disease is a chronic inflammatory oral disease induced by bacteria. Accumulating epidemiological evidence suggests a potential association between periodontal disease and adverse pregnancy outcomes (APOs), including preterm birth (PB), low birth weight (LBW), and small for gestational age (SGA)1, 2, 3, 4. SGA, which is defined as a weight below the 10th percentile for the gestational age, is an important prognostic trait because it predicts susceptibility to hypoglycaemia, hypothermia, erythrocytosis, and future growth suppression5, 6. Poor fetal growth is the leading cause of prenatal and infant mortality, and is a major public health problem worldwide.
Porphyromonas gingivalis is known to be a keystone anaerobe in the progression to periodontal disease. It is also associated with progression of APOs7, 8. As a product of the host response against microbial colonisation, serum anti-P. gingivalis immunoglobulin (Ig) G reflects the destructive periodontal disease status. However, the underlying mechanism of how the IgG subclasses for P. gingivalis affect pregnancy outcomes remains unknown.
In our previous study, we reported that P. gingivalis periodontal infection and its antibody response to homologous peptides of beta 2 glycoprotein I might be associated with both threatened preterm labour (TPL) and PB4. TPL is the progression of uterine neck dilatation and shortening caused by regular uterine contractions before 37 weeks of pregnancy, which is a risk factor for PB, LBW and SGA9, 10. Therefore, anti-P. gingivalis IgG is considered to be useful not only as a diagnostic parameter of periodontal disease12, 13, but also as a potential biomarker for APOs4, 14.
Human IgG antibody has four subclasses (IgG-1, -2, -3 and -4), and the immune response to most antigens includes a mixture of all four subclasses15. However, the underlying mechanism of how these IgG subclasses for P. gingivalis affect pregnancy outcomes remains unknown.
In this longitudinal study, we evaluated the periodontal parameters; amount of P. gingivalis in subgingival plaque and saliva samples, serum anti-P. gingivalis IgG, and IgG subclasses in TPL patients; and subsequent SGA status after childbirth to determine the association of the amount of anti-P. gingivalis IgG and IgG subclasses with SGA infant delivery.
MATERIALS AND METHODS
Ethics statement
This study was conducted in full accordance with the World Medical Association Declaration of Helsinki principles. The study and consent procedures were approved by the Institution Ethics Committee of Tokyo Medical and Dental University (No. 498). All human tissues were collected from the Obstetrics and Gynecology Clinic at Tokyo Medical and Dental University following the guidelines issued by Tokyo Medical and Dental University; written consent was obtained from all subjects who participated in the research.
Subjects
A total of 95 subjects, between the ages of 23 and 44 years, were recruited in our previous study4. Forty-seven TPL patients who were hospitalised in the Obstetrics and Gynecology Clinic at Tokyo Medical and Dental University met the inclusion criteria. These criteria included the following: regular uterine contractions occurring at least once every 10 minutes during 30 minutes of monitoring without dilatation and effacement of the cervix, cervical length of < 2 cm, and/or vaginal bleeding between 22 and 28 weeks of gestation. Forty-eight healthy pregnant women visited the Obstetrics and Gynecology Clinic at Tokyo Medical and Dental University for regular prenatal check-ups (H group). The subjects had a minimum of 15 teeth remaining, with no systemic diseases.
After delivery, the gestational age at birth, birth weight and placental weight were recorded. The subjects in the TPL group were divided into the TPL-SGA and TPL-normal weight (NW) groups. SGA was defined as a birth weight of less than the 10th percentile for the gestational age in the Japanese standard. NW was defined as a birth weight of more than the 10th percentile for the gestational age in the Japanese standard17. PB was defined as birth at a gestational age of less than 37 weeks and LBW as a birth weight of < 2,500 g.
Periodontal examination
All 95 pregnant women received a periodontal examination between 26–28 weeks of gestation. The periodontal parameters included the pocket probing depth (PPD), clinical attachment loss (CAL), and bleeding on probing (BOP), which were recorded by one periodontal specialist (S.K.) at all six sites for each tooth using a manual probe (PCP-UNC15, Hu-Friedy, Chicago, IL, USA).
Oral and serum sample collection
Subgingival plaque samples were collected via 30-second insertion of a sterile #40 paper point from the deepest pocket of the Ramfjord teeth18, which include a right upper molar, an upper incisor, a left upper molar, a right lower molar, a lower incisor and a left lower molar. When the representative tooth was missing, the next tooth was used instead. One millilitre of unstimulated saliva was obtained simultaneously. The samples (plaque and saliva) were collected in capped sterile glass tubes and then stored at −80 °C. At 28 weeks of gestation, peripheral blood samples were collected and centrifuged at 1,500 g for 10 minutes at 4 °C. Thereafter, the serum samples were collected and immediately stored at −20 °C.
Bacterial detection
The prepGEM bacteria kit (ZyGEM, Hamilton, New Zealand) was used to extract DNA from the subgingival plaque and saliva samples according to the manufacturer’s instructions. The extracted DNA was then subjected to real-time polymerase chain reaction (PCR) via the TaqMan probe detection method to determine the presence of P. gingivalis. The primers and probes were prepared as follows: forward, TAGCTTGCTAAGGTCGATGG; reverse, CAAGTGTATGCGGTTTTAGT; and probe, TGCGTAACGCGTATGCAACTTGCC. Porphyromonas gingivalis strain ATCC 33277, used as a positive control, was cultured under appropriate culture conditions19. The bacterial cells were counted using a Petroff-Hausser counting chamber, and their DNA was extracted from 102 to 108 cells as the standard. For the amplification reaction, duplicate samples were routinely used. The assays that were performed in a total volume of 25 μL contained the following: 12.5 μL Premix Ex Taq, 0.5 μL each forward and reverse primer (final concentration, 10 nm), 0.5 μL TaqMan probe (final concentration, 10 nm), 1 μL template DNA solution, and an appropriate volume of sterilised DNase- and RNase-free water. The PCR TaqMan Master Mix without DNA was used as a negative control. Amplification and detection were performed using a thermal cycler device (Real-Time System II; Takara-bio, Tokyo, Japan) at 95 °C for 30 seconds, 95 °C for 5 seconds (40 cycles), and 60 °C for 30 seconds.
Serum anti-Porphyromonas gingivalis IgG titre determination
The anti-P. gingivalis IgG antibody titre was determined using a previously described enzyme-linked immunosorbent assay (ELISA) method20. A sonic extracted antigen of P. gingivalis strain ATCC 33277 was prepared using the method of Yano-Higuchi et al.19. Briefly, 96-well assay plates (SUMILON multi-well plate for ELISA; Sumitomo Bakelite, Tokyo, Japan) were coated with the sonicated antigen (10 μg/mL) in carbonate buffer and incubated for 2 hours at 37 °C. After blocking with 2% bovine serum albumin (BSA) in carbonate buffer, the plates were washed three times with phosphate-buffered saline (PBS)-T (1 × PBS, 0.05% Tween 20, pH 7.2). Serially diluted healthy human serum (25–214, 100 μL per well) was added as the standard. The testing serum samples were added to the duplicate wells. The plates were incubated for 2 hours at 37 °C and then washed three times. Thereafter, 100 μL per well of alkaline phosphatase-conjugated goat anti-human IgG (Sigma Chemical, St Louis, MO, USA) was added. The plates were then incubated for 1 hour at 37 °C, washed three times, and developed with phosphate substrate (Sigma-Aldrich, St Louis, MO, USA). The optical density (OD) at 405 nm for each well was measured using the Vmax Microplate Reader (Molecular Devices, Downingtown, PA, USA).
The antibody titre calculation was performed using the method of Ishikawa et al.20. Absorbance was plotted against the dilution ratio and the logarithm of the dilution ratio. The regression curve (obtained with a cubic polynomial expression) intersected at the line expressed by an OD of 0.8 was adopted as the antibody titre for a given sample. The antibody titre was expressed as log2 of the dilution.
Serum anti-Porphyromonas gingivalis IgG subclass concentration determination
Serum anti-P. gingivalis IgG-1, -2, -3 and -4 concentrations were determined using ELISA. Briefly, in the 96-well assay plates, the first two lines that acted as the standards were coated with human IgG-1, -2, -3 or -4 Kappa purified myeloma protein (Sigma-Aldrich, St Louis, MO, USA) with concentrations serially diluted from 50 to 0.195 μg/mL; the rest of the wells were coated with the sonicated antigen (10 μg/mL) in carbonate buffer. The plates were then incubated for 2 hours at 37 °C. After blocking with 2% BSA in carbonate buffer, the plates were washed three times with PBS-T (1 × PBS, 0.05% Tween 20, pH 7.2). Serially diluted serum (25 to 214, 100 μL per well) was added into the duplicate wells, and the plates were incubated for 2 hours at 37 °C. Following incubation, the plates were washed three times. Subsequently, 100 μL per well of dilution buffer and serum samples (1:64 diluted) was added into the standard line and the rest of the plates. Following another 1 hour of incubation at 37 °C, the plates were washed three times and developed with 100 mL secondary antibody (1:10,000 diluted): monoclonal anti-human IgG-1 biotin antibody produced in mice (8c/6), IgG-2 (HP6012), IgG-3 (HP6050) and IgG-4 (HP6025; Sigma-Aldrich); thereafter, they were subsequently incubated for 2 hours at 37 °C. Biotin-conjugated horseradish peroxidase-streptavidin (1:1,000 diluted; Vector Laboratories, Burlingame, CA, USA) was added, and the plates were incubated for 2 hours at 37 °C and washed three times. TMB substrate was added, and the reaction was stopped with 1 m H2SO4 (Sigma-Aldrich, St Louis, MO, USA) after 30 minutes; the plates were then read at 450 nm using the Vmax Microplate Reader.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 21 (IBM, NY, USA). Initially, the data distribution was assessed using the Shapiro–Wilk test. Thereafter, unpaired t-tests were applied to analyse the statistical differences in age, birth week, birth weight and placental weight between the TPL and H groups. Mann–Whitney U-tests were applied to analyse the mean PPD, mean CAL, percentage of sites with a PPD of ≥ 5 mm, percentage of BOP-positive sites, and amount of P. gingivalis in the saliva and plaque samples, anti-P. gingivalis IgG, and IgG subclasses between the TPL and H groups. The amount of P. gingivalis in the saliva and plaque samples that did not demonstrate a normal distribution was categorised into a dichotomous variable. The chi-square test was performed to compare the smoking history; incidence of PB, LBW and SGA; and detection frequency of P. gingivalis in the saliva and plaque samples between the TPL and H groups. The above-mentioned tests were also applied between the TPL-SGA and TPL-NW groups. Potential risk factors for TPL-SGA, including the mean PPD, mean CAL, percentage of sites with a PPD of ≥ 5 mm, detection frequency of P. gingivalis in the plaque samples, serum titre of IgG against P. gingivalis, serum concentration of IgG-4, and placental weight, were selected and used in the logistic regression analysis. The variables were screened using the stepwise approach and then evaluated using a regression model. Odds ratios and 95% confidence intervals (CIs) were also calculated. Receiver operating characteristic (ROC) curve analysis was used for the prediction of cut-off values. The diagnostic value of P. gingivalis in the plaque samples was evaluated by calculating the sensitivity and specificity. P-values of < 0.05 were considered statistically significant.
RESULTS
TPL and H groups before delivery
Table 1 shows the demographic characteristics, periodontal parameters, laboratory values, and final birth outcomes of the subjects in the TPL and H groups. Although no significant difference was found in the demographic characteristics and periodontal parameters between the TPL and H groups, the serum anti-P. gingivalis IgG-1 concentration was significantly lower in the former than in the latter. After labour, 17 (17.9%) patients delivered SGA infants, and 78 (82.1%) delivered NW infants. Among the 17 subjects who delivered SGA infants, 14 and 3 were from the TPL and H groups, respectively. Among the 78 subjects who delivered NW infants, 33 and 45 were from the TPL and H groups, respectively. The birth week and birth weight in the TPL group were significantly lower than those in the H group. The incidence of PB, LBW and SGA was significantly higher in the TPL group than in the H group.
Table 1.
Subject characteristics in the TPL group and H group
| TPL group (n = 47) | H group (n = 48) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 33.5 ± 4.8 | 32.8 ± 3.9 | NS |
| Smoking history (%) | 29.8 (14/47) | 20.8 (10/48) | NS |
| Periodontal parameters | |||
| Mean PPD (mm) | 2.34 | 2.3 | NS |
| CAL (mm) | 2.4 | 2.4 | NS |
| Sites with a PPD of ≥ 5 mm (%) | 0 | 0 | NS |
| BOP (%) | 20.7 | 22.2 | NS |
| Laboratory values | |||
| Porphyromonas gingivalis. in the plaque (16s rDNA copies) | 1.29 | 0 | NS |
| Porphyromonas gingivalis. (+)% in the plaque | 51.0 (24/47) | 43.7 (21/48) | NS |
| Porphyromonas gingivalis. in the saliva (16s rDNA copies) | 43.6 | 204 | NS |
| Porphyromonas gingivalis. (+)% in the saliva | 53.2 (25/47) | 60.4 (29/48) | NS |
| Titre of anti-Porphyromonas gingivalis. IgG | 7.1 | 6.5 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG1 | 17.2* | 23.5 | 0.04 |
| Concentration of anti-Porphyromonas gingivalis. IgG2 | 2.7 | 2.8 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG3 | 3.2 | 3.3 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG4 | 0.4 | 0.5 | NS |
| Serum hs-CRP (ng/mL) | 1048 | 1326 | NS |
| Pregnancy outcomes | |||
| PB rate | 27.7 (13/47)* | 2.1 (1/48) | 0.0004 |
| LBW rate | 29.8 (14/47)* | 6.25 (3/48) | 0.003 |
| SGA rate (%) | 29.8 (14/47)* | 6.25 (3/48) | 0.003 |
| Birth week | 37.9 ± 2.2* | 39.4 ± 2.8 | 0.0003 |
| Birth weight (g) | 2914.7 ± 490.1* | 3107.6 ± 419.1 | 0.0438 |
| Placenta weight (g) | 617.0 ± 136.8 | 596.7 ± 113.4 | NS |
Parametric continuous variables were tested using an unpaired t-test and are given as means ± standard deviations; non-parametric continuous variables were tested using the Mann–Whitney U-test and are given as medians.
BOP, bleeding on probing; CAL, clinical attachment loss; H, healthy; hs-CRP, high-sensitivity C-reactive protein; Ig, immunoglobulin; LBW, low birth weight; NS, not significant; PB, preterm birth; PPD, pocket probing depth; SGA, small for gestational age; TPL, threatened preterm labour.
Significantly different from the H group (P < 0.05).
TPL-SGA and TPL-NW groups after delivery
In the TPL group, 14 patients delivered SGA infants (TPL-SGA group), and 33 delivered NW infants (TPL-NW group). Table 2 shows the demographic characteristics, periodontal parameters, laboratory values, and pregnancy outcomes of the subjects in the TPL-SGA and TPL-NW groups. Regarding the periodontal parameters, the mean PPD and CAL and the percentage of sites with a PPD of ≥ 5 mm were significantly higher in the TPL-SGA group than in the TPL-NW group. Regarding the laboratory values, the anti-P. gingivalis antibody titre, P. gingivalis IgG-4 concentration, and amount and detection frequency of P. gingivalis in the subgingival plaque samples were significantly higher in the TPL-SGA group than in the TPL-NW group. The birth week, birth weight and placental weight in the TPL-SGA group were significantly lower than those in the TPL-NW group.
Table 2.
Subject characteristics in TPL-SGA and TPL-NW groups
| TPL (47) |
|||
|---|---|---|---|
| TPL-SGA group (n = 14) | TPL-NW group (n = 33) | P-value | |
| Demographic characteristics | |||
| Age (years) | 35.1 ± 5.0 | 32.8 ± 4.6 | NS |
| Smoking history (%) | 42.8 (6/14) | 24.2 (8/33) | NS |
| Periodontal parameters | |||
| Mean PPD (mm) | 2.6* | 2.2 | 0.004 |
| Mean CAL (mm) | 2.6* | 2.3 | 0.008 |
| Sites with a PPD of ≥ 5 mm (%) | 2.1* | 0 | 0.019 |
| BOP (%) | 23.8 | 19 | NS |
| Laboratory values | |||
| Porphyromonas gingivalis. in the plaque (16s rDNA copies) | 728* | 0 | 0.002 |
| Porphyromonas gingivalis. (+)% in the plaque | 78.6 (11/14)* | 39.3 (13/33) | 0.014 |
| Porphyromonas gingivalis. in the saliva (16s rDNA copies) | 146 | 0 | NS |
| Porphyromonas gingivalis. (+)% in the saliva | 71.4 (10/14) | 45.5 (15/33) | NS |
| Titre of anti-Porphyromonas gingivalis. IgG | 8.6* | 6.8 | 0.048 |
| Concentration of anti-Porphyromonas gingivalis. IgG1 | 18.4 | 17.1 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG2 | 3.1 | 2.3 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG3 | 3.2 | 3.2 | NS |
| Concentration of anti-Porphyromonas gingivalis. IgG4 | 0.9* | 0.3 | 0.023 |
| Serum hs-CRP (ng/mL) | 1444 | 1408 | NS |
| Pregnancy outcomes | |||
| Birth week | 36.7 ± 1.2* | 38.4 ± 2.3 | 0.019 |
| Birth weight (g) | 2327.8 ± 114.4* | 3153.1 ± 364.3 | < 0.001 |
| Placenta weight (g) | 525.4 ± 92.9* | 651.3 ± 135.8 | 0.005 |
Parametric continuous variables were tested using an unpaired t-test and are given as means ± standard deviations; non-parametric continuous variables were tested using the Mann–Whitney U-test and are given as medians.
BOP, bleeding on probing; CAL, clinical attachment loss; hs-CRP, high-sensitivity C-reactive protein; Ig, immunoglobulin; NS, not significant; NW, normal weight; PPD, pocket probing depth; SGA, small for gestational age; TPL, threatened preterm labour.
Significantly different from the TPL-NW group (P < 0.05).
Potential risk factor of SGA in the TPL group
The logistic regression analysis showed that a high detection frequency of P. gingivalis in the plaque samples and the placental weight were significantly correlated with SGA in the TPL group (Table 3). The estimated odds ratios of P. gingivalis in the plaque samples (%) and the placental weight were 3.6 and 0.987, respectively.
Table 3.
Potential risk indicators for SGA in TPL subjects
| Estimated odds ratio | 95% CI | |
|---|---|---|
| Porphyromonas gingivalis. (+)% in the plaque | 3.6* | 1.2–22.8 |
| Placenta weight | 0.987* | 0.977–0.998 |
CI, confidence interval.
Statistically significant (P < 0.05; ordinal logistic regression analysis).
Predictive factor of SGA in the TPL group
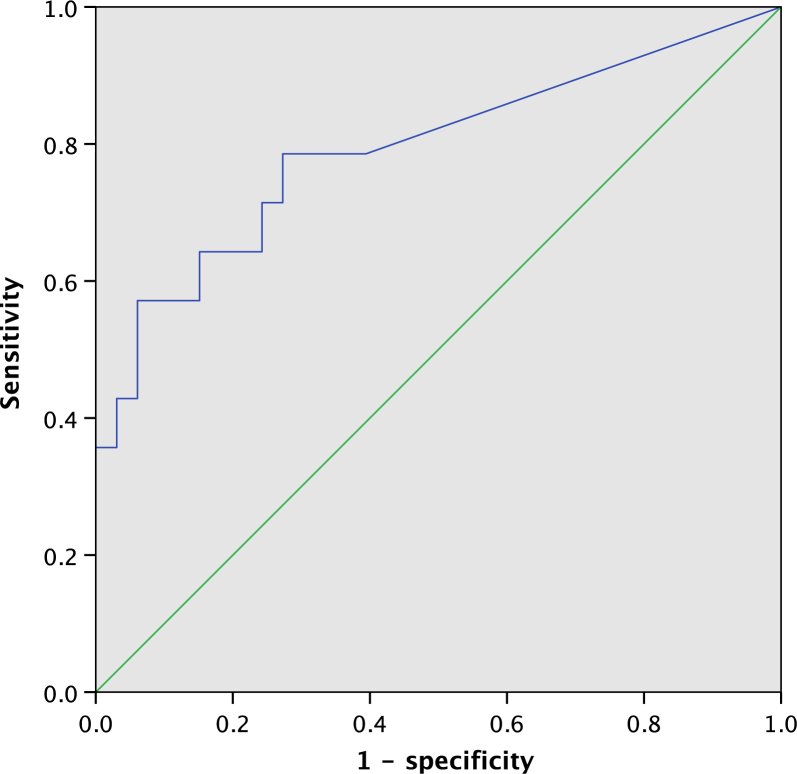
Figure 1 shows the ROC curve for the amount of P. gingivalis in the plaque samples for predicting SGA in the TPL patients. The area under the ROC curve for the amount of P. gingivalis in the plaque samples was 0.792 (95% CI, 0.634–0.951), and the cut-off value was 86.45 copies number, with a sensitivity of 0.786 and a specificity of 0.727.
Figure 1.
Receiver operating characteristic (ROC) curve for the amount of Porphyromonas gingivalis in the plaque samples for predicting small for gestational age (SGA) in threatened preterm labour (TPL). The area under the curve (AUC) for the amount of P. gingivalis in the plaque samples was 0.792 [95% confidence interval (CI), 0.634-0.951].
DISCUSSION
The relationship between periodontal disease and pregnancy outcomes has been examined for the past 30 years. Previous studies have demonstrated that existing maternal periodontal disease might be a risk factor for APO2, 21. In the present study, we found increased severity of periodontal disease, including mean PPD, mean CAL and percentage of sites with a PPD of ≥ 5 mm, in the TPL-SGA group. These results correlate with previous findings. Boggess et al.3 reported an increased SGA rate among women with moderate or severe periodontal disease as compared with that among healthy women. Our data support the finding that severe periodontal conditions in pregnant women are also related to intrauterine growth restriction (IUGR), which is highly associated with neonatal mortality and lifelong co-morbidities.
To the best of our knowledge, no study has yet evaluated the role of anti-P. gingivalis IgG subclasses in APOs. Only Sasahara et al.22 demonstrated that the anti-P. gingivalis IgG-1 concentration was lower in patients who had IUGR and PB than in healthy subjects. This might indicate the protective role of anti-P. gingivalis IgG-1 against APOs. Our study also demonstrated that the serum anti-P. gingivalis IgG-1 concentration in the TPL group was lower than that in the H group at the gestational age of 28 weeks.
Interestingly, the study findings correlate with those of Dasanayake et al.16 where an increment in the total IgG concentration in response to P. gingivalis in the mid-trimester was associated with the risk for LBW infant delivery. Conversely, Ebersole et al.23 and Lin et al.14 have shown that women who delivered preterm infants had significantly lower total serum concentrations of IgG against P. gingivalis at the first trimester than women who delivered NW infants. Moreover, Madianos et al.24 demonstrated that a lack of maternal IgG against P. gingivalis within 48 hours after delivery was associated with an increased rate of premature birth. Different blood collection times and immunological alterations during pregnancy might contribute to these opposite conclusions. Hormones modulate the immunological shift that occurs during pregnancy25. In early pregnancy, the maternal immune responses can control local and low-grade infections, and maternal IgG might protect the fetus from exposure to pathogens. As hormones change with progression of pregnancy, a shift from Th1 to Th2 immunity increases antibody production and pregnancy-related attenuation in immune responses. This will ultimately increase the susceptibility to and risk of developing complications of infections25, 26.
Optimal growth and function of the placenta are essential for fetal development. Lower placental weight not only affects the neonatal size but also results in a lower than expected body size late in childhood27. Another risk factor in this study was the high detection frequency of P. gingivalis in the subgingival plaque samples. Animal studies revealed that after oral administration of P. gingivalis, a higher incidence of PB and LBW was found28, 29. Based on the results of the ROC analysis, the amount of P. gingivalis in subgingival plaques can be applied as a potential predictor to identify patients who are at a high risk of delivering SGA infants with TPL. Hence, periodontal therapy should be performed to reduce P. gingivalis colonisation in high-risk populations.
To the best of our knowledge, this is the first study to evaluate anti-P. gingivalis IgG subclasses in the serum and their possible relationship with APOs. Its limitations include the small number of patients included in the SGA group and its partial cross-sectional design, which makes it difficult to determine the changes in the IgG titres during pregnancy. Further investigation is necessary to reveal the mechanism and examine these findings in a larger population.
CONCLUSION
The present study demonstrated that low concentrations of anti-P. gingivalis IgG-1 were related to TPL, while high concentrations of total anti-P. gingivalis IgG and IgG-4 were related to SGA in TPL. The high prevalence of P. gingivalis in the plaque samples might be associated with an increased risk of SGA and might be useful in the prediction of SGA in TPL.
Acknowledgement
The authors appreciate the help of Dr Yoshihito Momohara, Dr Masaki Sekiguchi, Dr Satoko Takamine from Maternal and Women’s Clinic, Medical Hospital, Tokyo Medical and Dental University in recruiting patients. This work was supported by a grant (81600875) from National Natural Science Foundation of China, a Grant-in-Aid (A) (16K11826) from the Japan Society for the Promotion of Science, Tokyo, Japan and a grant (2015-HM01-00088-SF) from the Chengdu Science and Technology Bureau.
Conflicts of interest
No conflicts of interest are reported for this study.
Author contributions
Hiroaki Kobayashi conceived and designed the research. Changchang Ye performed the experiments and wrote the manuscript with the help of Ryutaro Kuraji, Takeuchi, Yasuo, Hiroaki Kobayashi. Sayaka Katagiri performed the periodontal examinations. Naoyuki Miyasaka recruited subjects. Yuichi Izumi supervised the project. All authors reviewed the manuscript.
REFERENCES
- 1.Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K, Furuichi Y, Shimotsu A, et al. Associations between systemic status, periodontal status, serum cytokine levels, and delivery outcomes in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2003;74:1764–1770. doi: 10.1902/jop.2003.74.12.1764. [DOI] [PubMed] [Google Scholar]
- 3.Boggess KA, Beck JD, Murtha AP, et al. Maternal periodontal disease in early pregnancy and risk for a small-for-gestational-age infant. Am J Obstet Gynecol. 2006;194:1316–1322. doi: 10.1016/j.ajog.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 4.Ye C, Katagiri S, Miyasaka N, et al. The anti-phospholipid antibody-dependent and independent effects of periodontopathic bacteria on threatened preterm labor and preterm birth. Arch Gynecol Obstet. 2013;288:65–72. doi: 10.1007/s00404-013-2741-z. [DOI] [PubMed] [Google Scholar]
- 5.Lunze K, Bloom DE, Jamison DT, et al. The global burden of neonatal hypothermia: systematic review of a major challenge for newborn survival. BMC Med. 2013;11:24. doi: 10.1186/1741-7015-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer MS, Olivier M, McLean FH, et al. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics. 1990;86:707–713. [PubMed] [Google Scholar]
- 7.Swati P, Thomas B, Vahab SA, et al. Simultaneous detection of periodontal pathogens in subgingival plaque and placenta of women with hypertension in pregnancy. Arch Gynecol Obstet. 2012;285:613–619. doi: 10.1007/s00404-011-2012-9. [DOI] [PubMed] [Google Scholar]
- 8.Barak S, Oettinger-Barak O, Machtei EE, et al. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007;78:670–676. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- 9.Lucovnik M, Chambliss LR, Garfield RE. Costs of unnecessary admissions and treatments for "threatened preterm labor". Am J Obstet Gynecol. 2013;209(217):217.e1–217.e3. doi: 10.1016/j.ajog.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinoza J, Kusanovic JP, Kim CJ, et al. An episode of preterm labor is a risk factor for the birth of a small-for-gestational-age neonate. Am J Obstet Gynecol. 2007;196(574):574.e1–574.e6. doi: 10.1016/j.ajog.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig RG, Boylan R, Yip J, et al. Serum IgG antibody response to periodontal pathogens in minority populations: relationship to periodontal disease status and progression. J Periodontal Res. 2002;37:132–146. doi: 10.1034/j.1600-0765.2002.00031.x. [DOI] [PubMed] [Google Scholar]
- 13.Morozumi T, Nakagawa T, Nomura Y, et al. Salivary pathogen and serum antibody to assess the progression of chronic periodontitis: a 24-mo prospective multicenter cohort study. J Periodontal Res. 2016;51:768–778. doi: 10.1111/jre.12353. [DOI] [PubMed] [Google Scholar]
- 14.Lin DM, Moss K, Beck JD, et al. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007;78:833–841. doi: 10.1902/jop.2007.060201. [DOI] [PubMed] [Google Scholar]
- 15.Schur PH. Igg subclasses - a historical-perspective. Monogr Allergy. 1988;23:1–11. [PubMed] [Google Scholar]
- 16.Dasanayake AP, Boyd D, Madianos PN, et al. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J Periodontol. 2001;72:1491–1497. doi: 10.1902/jop.2001.72.11.1491. [DOI] [PubMed] [Google Scholar]
- 17.Uehara R, Miura F, Itabashi K, et al. Distribution of birth weight for gestational age in Japanese infants delivered by Cesarean section. J Epidemiol. 2011;21:217–222. doi: 10.2188/jea.JE20100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodont. 1959;30:51–59. [Google Scholar]
- 19.Yano-Higuchi K, Takamatsu N, He T, et al. Prevalence of Bacteroides forsythus, Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival microflora of Japanese patients with adult and rapidly progressive periodontitis. J Clin Periodontol. 2000;27:597–602. doi: 10.1034/j.1600-051x.2000.027008597.x. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa I, Watanabe H, Horibe M, et al. Diversity of IgG antibody responses in the patients with various types of periodontitis. Adv Dent Res. 1988;2:334–338. doi: 10.1177/08959374880020022301. [DOI] [PubMed] [Google Scholar]
- 21.Xiong X, Buekens P, Fraser WD, et al. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113:135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 22.Sasahara J, Kikuchi A, Takakuwa K, et al. Antibody responses to Porphyromonas gingivalis outer membrane protein in the first trimester. Aust Nz J Obstet Gyn. 2009;49:137–141. doi: 10.1111/j.1479-828x.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 23.Ebersole JL, Novak MJ, Michalowicz BS, et al. Systemic immune responses in pregnancy and periodontitis: relationship to pregnancy outcomes in the obstetrics and periodontal therapy (OPT) study. J Periodontol. 2009;80:953–960. doi: 10.1902/jop.2009.080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madianos PN, Lieff S, Murtha AP, et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001;6:175–182. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- 25.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naeye RL. Do placental weights have clinical significance? Hum Pathol. 1987;18:387–391. doi: 10.1016/s0046-8177(87)80170-3. [DOI] [PubMed] [Google Scholar]
- 28.Fischer LA, Bittner-Eddy PD, Costalonga M. Fetal weight outcomes in C57BL/6J and C57BL/6NCrl mice after oral colonization with Porphyromonas gingivalis. Infect Immun. 2019;87:e00280–19. doi: 10.1128/IAI.00280-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang S, Ren H, Guo H, et al. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol Oral Microbiol. 2018;33:312–321. doi: 10.1111/omi.12227. [DOI] [PubMed] [Google Scholar]