Abstract
Campylobacter jejuni colonizes the intestines of domestic and wild animals and is a common cause of human diarrheal disease. We identified a two-component regulatory system, designated the RacR-RacS (reduced ability to colonize) system, that is involved in a temperature-dependent signalling pathway. A mutation of the response regulator gene racR reduced the organism’s ability to colonize the chicken intestinal tract and resulted in temperature-dependent changes in its protein profile and growth characteristics.
Campylobacter jejuni is the most common bacterial cause of food-borne gastroenteritis (18). The bacterium asymptomatically colonizes the intestinal tracts of many animals used for food (1, 11). Contaminated meat, especially poultry, is a major source of C. jejuni infection. Motility is the major factor that has been directly implicated (38) in intestinal colonization. Understanding the processes involved in the colonization of the avian gut is a priority for the development of intervention strategies to control transmission.
Colonization is a multifactorial process involving adaptation by the bacterium to different microenvironments in the intestine (31). Two-component regulatory (TCR) systems are commonly used by bacteria to respond to specific environmental signals. These systems depend on two families of proteins, the sensory histidine kinases and response regulators (RR), which cooperate to transmit environmental signals to the bacterial response machinery (16). TCR systems are of particular importance for the regulation of gene expression in some bacteria. Helicobacter pylori, for example, utilizes a reduced number of global regulatory proteins compared to the number Escherichia coli uses, and TCR systems seem to be a fundamental constituent of the regulatory organization (33). C. jejuni is closely related to H. pylori, and its genome is currently being sequenced (28a). Preliminary analysis indicates that although the C. jejuni genome contains few environment-dependent regulators in total, several putative TCR systems are evident. Therefore, TCR systems may be significant in the transcriptional regulation of the gene expression of C. jejuni responses to environmental pressures. In this report, we describe a novel TCR system important for the growth and possibly survival of C. jejuni in its natural intestinal habitat.
Bacterial strains, plasmids, growth characteristics, and general methods.
E. coli XL1-Blue (6) was cultured aerobically in Luria-Bertani medium (28). C. jejuni 81116 (NCTC 11828) (23) was cultured in Mueller-Hinton (MH) broth and agar (Oxoid) or campylobacter blood-free selective agar (Oxoid) at 37 or 42°C under microaerobic conditions (6% hydrogen, 5% carbon dioxide, 5% oxygen, and 84% nitrogen). Where appropriate, growth medium was supplemented with kanamycin (50 μg/ml) or ampicillin (200 μg/ml). DNA manipulation was carried out as described by Sambrook et al. (28). A nested deletion kit was used to sequence pALB3 according to the manufacturer’s instructions (Pharmacia Biotech). The sequencing reaction mixtures were prepared with a Taq DyeDeoxy Terminator Cycle sequencing kit and were analyzed on an automated DNA sequencer. Sequence data was processed with the Wisconsin Molecular Biology software package (version 8, September 1994) from the Genetics Computer Group. The chicken colonization assay was performed as described previously (38). Two-dimensional (2-D) electrophoresis was carried out by using 20 μg of protein with Immobiline DryStrip (Pharmacia) isoelectric focusing in the first dimension (precast Immobiline DryStrip polyacrylamide gel; 180 mm; pH 3 to 10) and ExcelGel (Pharmacia) in the second dimension (precast ExcelGel sodium dodecyl sulfate-polyacrylamide gels; 245 by 180 mm; 12 to 14%). Protein bands were visualized by silver staining according to the manufacturer’s instructions. N-terminal sequencing was carried out at the Protein and Nucleic Acid Chemistry Laboratory, University of Leicester, with standard Edman degradation.
Cloning and sequencing a response regulator gene.
A C. jejuni F132 genomic library in λZAP II (19) was probed with a DNA fragment isolated by PCR with degenerate primers (41) designed to amplify RR gene family members. A 1.5-kb XbaI/HindIII fragment containing the target sequence was subcloned into pUC19 to generate pALB3 (data not shown). The subcloned fragment was sequenced in both directions, showing the presence of one complete open reading frame (orf1) and the 189 bp of a second open reading frame corresponding to the N terminus (orf2). The 671-bp-long orf1 encodes a predicted protein with an Mr of 24,500, and it includes a DNA sequence identical to that of the 320-bp RR probe. Comparisons of deduced amino acid sequences by FASTA (25) and BLASTP (3) revealed an extensive degree of identity (33 to 38%) between Orf1 and known RR proteins (GenEMBL and SwissProt) and closest similarity between them (approximately 60% with orf1) and members of the OmpR subfamily (24, 32).
The start codon of the partial orf2 overlaps the orf1 stop codon, indicating that the two genes are part of one operon. The analysis of the predicted amino acid sequence with Tmpred (17) showed that Orf2 contains two transmembrane domains spanning from amino acids 11 to 32 and 132 to 174; thus, Orf2 is probably a transmembrane protein. Comparative sequence alignments (15) between Orf2 and protein sequences available in the database (GenEMBL and SwissProt) revealed that Orf2 has approximately 25% identity to other histidine protein kinases. A subsequent comparison of the orf1 and partial orf2 library clone C. jejuni genome DNA sequences (28a) showed 100% nucleotide identity, and therefore, genome sequence data was used for the remaining unknown orf2 3′ DNA sequence.
Given that orf1 and orf2 are part of one operon, it is probable that Orf2 is the cognate sensory kinase of the Orf1 RR. The RR (Orf1) has been named RacR, and the histidine kinase (Orf2) has been named RacS (see below). The RR protein CheY (42) plays a role in the posttranslational regulation of chemotaxis, but RacR-RacS is the first full transcriptional TCR system described for C. jejuni.
Construction of a mutant by insertional inactivation.
An insertional mutation was constructed in orf1 by inverse PCR mutagenesis (40) (primers 5′ GAA GAT CTA AAT CAG ACA ATC ATA GG 3′ and 5′ GAA GAT CTT TAC CTG GAA TTG ATG 3′) and the subsequent insertion of a kanamycin resistance gene (34). The new construct, pALB5, was transferred into C. jejuni 81116 by electroporation (39). Two kanamycin-resistant mutants, designated AB1 and AB2, were isolated in separate electrotransformations. PCR and Southern hybridization confirmed that the mutants resulted from the allelic replacement of the wild-type RR gene by the insertionally mutated copy (data not shown). 2-D protein profiles (see Table 1) showed the absence of an approximately 25-kDa protein in both AB1 and AB2 compared to parent’s protein profile. N-terminal sequencing established this 25-kDa protein to be RacR, confirming that both mutants no longer express RacR.
TABLE 1.
Protein profiles of the parental strain and racR mutants
| Group | Protein | Approximate mass (kDa) | Approximate pI | Expression by:
|
|||
|---|---|---|---|---|---|---|---|
| Parental strain
|
racR mutants
|
||||||
| 37°C | 42°C | 37°C | 42°C | ||||
| I | 1 | 25 | 6.0–6.5 | + | + | − | − |
| 2 | 27 | 6.5 | + | + | − | − | |
| 3 | 21 | 7.0–7.5 | + | ±a | − | − | |
| 4 | 25 | 9.0–9.5 | + | + | − | − | |
| 5 | 28 | 7.0 | + | + | − | − | |
| II | 6 | 29 | 7.0–7.5 | − | − | + | + |
| 7 | 26 | 7.0–7.5 | − | − | + | + | |
| III | 8 | 41 | 5.0–5.5 | + | − | + | + |
| 9 | 41 | 6.0 | + | − | + | + | |
| 10 | 41 | 6.5–7.0 | + | − | + | + | |
| 11 | 6.5 | 7.0–7.5 | + | − | + | + | |
±, protein expressed at a lower level.
Comparison between the parent’s and mutants’ growth profiles.
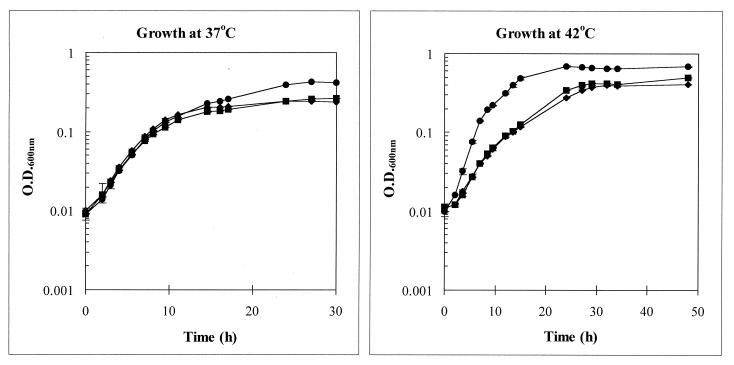
The mutant strains form smaller colonies than the wild-type strain on either MH agar or campylobacter blood-free selective agar at both 37 and 42°C (data not shown). The growth characteristics of AB1 and AB2 were compared with those of 81116 in MH broth. When grown at 37°C, the mutants showed a growth rate similar to that of the parent strain, but they entered stationary phase earlier (Fig. 1). However, at 42°C, the optimum growth temperature of C. jejuni, the mutants showed a growth rate that was lower than that of 81116 (Fig. 1). At both temperatures, the mutants did not achieve the parental level of cell density. Therefore, RacR affects growth in vitro in a temperature-dependent manner.
FIG. 1.
Growth patterns of the parent and mutant strains. The growth profiles of 81116, AB1, and AB2 were analyzed when the strains were incubated in MH broth at 37 and 42°C. Each point represents the mean (± standard deviation) of two cultures. ●, 81116; ⧫, AB1; ■, AB2. O.D., optical density.
Colonization of chickens.
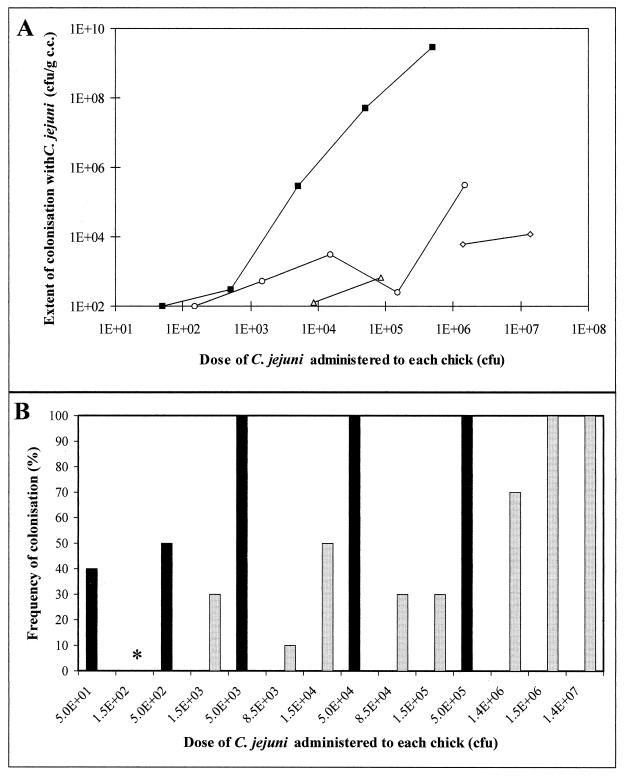
The mutant AB1 was tested in a chicken colonization model (38). Doses between 102 and 107 CFU were administered orally to groups of 10 1-day-old chicks housed in isolators. Colonization was evaluated by the level of colonization (number of viable bacteria recovered per gram of cecal contents [Fig. 2A]) and by the frequency of colonization (percentage of chicks colonized per dose group [Fig. 2B]). Recovered bacteria were checked by restriction fragment length polymorphism of flaAB and proven to be typical 81116-like bacteria (20) (data not shown). The mutant did not colonize the chicken intestine as well as the parent strain. The maximum level of colonization (Fig. 2A) observed with AB1 was approximately 104-fold lower than that of 81116, and only doses of AB1 above 106 CFU resulted in 100% colonization (Fig. 2B), whereas doses of wild-type 81116 above 103 CFU colonized all inoculated chicks.
FIG. 2.
Colonization of 1-day-old chicks with C. jejuni. Shown is a comparison of the abilities of 81116 and AB1 to colonize the intestines of 1-day-old chicks. The threshold of detection in this model is 100 CFU/g of cecal contents (c.c.). (A) Number of viable bacteria recovered from the cecal contents of chicks. Each point on the graph is the mean for 10 chicks. The data for the mutant strains was derived from three separate experiments. ■, 81116; ○, AB1 (experiment 1); ▵, AB1 (experiment 2); ◊, AB1 (experiment 3). (B) Frequency of chick colonization. Black bars, 81116; gray bars, AB1 (experiments 1 to 3). The asterisk signifies no colonization with AB1.
The inactivation of racR reduced the ability of the organism to colonize the alimentary tract in chickens. The mutant phenotype did not arise from changes in motility, as determined by both dark-field analysis and on swarm plates (data not shown). In Salmonella spp., a reduction in the ability to colonize chickens correlated with changes in the lipopolysaccharide profile (8, 35); however, no differences were observed between the lipopolysaccharide profile of 81116 and that of the racR mutant (data not shown). In the case of the racR mutant, it is likely that the reduced growth rate at 42°C (Fig. 1), as well as its inability to achieve the parental level of cell density, contributes to poor colonization. It is possible, however, that other racR-dependent gene products are important for colonization.
Protein profile of the mutants.
Protein profiles at 37 and 42°C of the wild type and mutants (AB1 and AB2) were compared by 2-D gel electrophoresis (summarized in Table 1). At least 11 differentially expressed proteins were identified as members of the RacR regulon, and these proteins could be grouped (Table 1) according to the effect of the RacR mutation on expression. Three resolved protein spots were sequenced. Protein 1 (Table 1) was identified as RacR, and proteins 9 and 10 corresponded to two isoforms of a cytochrome c peroxidase homolog.
As observed with other RRs (2, 4, 5, 14, 26), RacR acts both as a transcriptional activator (group I) and as a repressor (group II). Group III proteins appear to be subject to thermoregulation, being absent from the parent strain only at 42°C. The disruption of racR negated thermoregulation of group III proteins, and thus RacR, like some other RR proteins (27, 32, 36), responds to temperature changes. Since the physiological temperature of chickens is 42°C, the disruption of the expression of a group III protein(s) may be responsible for reduced intestinal colonization by the racR mutant. In addition to thermoregulation, protein 8 and Ccp (proteins 9 and 10) are under iron regulation (37), and therefore, the racR phenotype may be influenced by interaction with different regulatory pathways. However, as determined by 2-D gel electrophoresis (data not shown), RacR does not respond to changes in iron levels. The expression pattern of group I and II proteins indicates that some RacR regulon members are not thermoregulated. Therefore, in addition to temperature, RacR-RacS signal transduction may be influenced by other environmental conditions.
Thermoregulation in bacteria is often controlled by complex, overlapping systems that exert pleiotropic effects on the bacterial cell. Temperature-dependent regulation is important in in vivo bacterial responses (10, 13), and the AraC-like family of regulators and the histone-like protein H-NS are associated with temperature-dependent signal transduction (7, 9, 12, 22, 30). Given that H. pylori (33) lacks hns and araC-like family members, thermoregulation through a RacR-RacS-dependent pathway may play an important role in C. jejuni.
The determinants of colonization by C. jejuni are presumably expressed in response to conditions encountered in intestinal microenvironments. RacR and RacR-dependent genes are important for growth and survival in the avian intestine, and RacR-RacS is a signal transduction system responsive to temperature. Given the likely exposure of C. jejuni to temperature stress, RacR-RacS may also be required during the transmission of the bacterium from the intestine to the environmental reservoirs and vice versa. A C. jejuni dnaJ gene (21) is adjacent to racR, and we have evidence that the gene is under the transcriptional control of RacR (data not shown). C. jejuni dnaJ is also required for efficient avian colonization (21), and as in E. coli (29), C. jejuni dnaJ is involved in the heat shock response (21). Taken together, the evidence suggests that C. jejuni intestinal colonization involves temperature-associated adaptive responses mediated through the RacR-RacS signal transduction system.
Nucleotide sequence accession numbers.
The complete nucleotide sequence of racR and the nucleotide sequence of racS corresponding to the N terminus have been deposited in the GenBank database under accession no. AF053960 and AF053961, respectively.
Acknowledgments
This study was supported by JNICT (Portugal; A. M. Brás), the BBSRC, and a Royal Society University Research Fellowship to J. M. Ketley.
We thank K. Wooldridge for valuable advice, H. Goossens (Department of Microbiology, University Hospital Antwerp, UIA, Antwerp, Belgium) for F132, M. Kiernan for the C. jejuni genomic library, and S. Cawthraw for performing the chicken colonization.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Food. Interim report on Campylobacter. London, United Kingdom: Her Majesty’s Stationery Office; 1993. [Google Scholar]
- 2.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearson B L, Wilson L, Foster J W. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–2417. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullock W O, Fernandez J M, Short J M. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques. 1987;5:376–379. [Google Scholar]
- 7.Cornelis G R, Seniters C, Delor I, Geib D, Kaniga K, Lambert de Rouvroit C, Sony M P, Vanooteghem J C, Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol Microbiol. 1991;5:1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 8.Craven S E, Cox N A, Bailey J S, Stern N J, Meinersmann R J, Blankenship L C. Characterization of Salmonella california and Salmonella typhimurium strains with reduced ability to colonize the intestinal tract of broiler chicks. Avian Dis. 1993;37:339–348. [PubMed] [Google Scholar]
- 9.Dorman C J, Porter M E. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol Microbiol. 1998;29:677–684. doi: 10.1046/j.1365-2958.1998.00902.x. [DOI] [PubMed] [Google Scholar]
- 10.Dorman C J. Genetics of bacterial virulence. Oxford, England: Blackwell Scientific Publications; 1994. pp. 204–259. [Google Scholar]
- 11.Doyle M P, Jones D M. Food-borne transmission and antibiotic resistance of Campylobacter jejuni. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 45–48. [Google Scholar]
- 12.Göransson M, Sonden B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin B E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990;344:682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- 13.Gross R. Signal transduction and virulence regulation in human and animal pathogens. FEMS Microbiol Rev. 1993;104:301–326. doi: 10.1111/j.1574-6968.1993.tb05873.x. [DOI] [PubMed] [Google Scholar]
- 14.Heath J D, Charles T C, Nester E W. Ti plasmid and chromosomally encoded two-component systems important in plant cell transformation by Agrobacterium species. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 367–385. [Google Scholar]
- 15.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignments on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 17.Hofmann K, Stoffel W. TMbase: a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 18.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan M. Molecular genetic approaches to the investigation of Campylobacter jejuni. Ph.D. dissertation. Leicester, United Kingdom: University of Leicester; 1997. [Google Scholar]
- 20.Koenraad P M F J, Ayling R, Hazeleger W C, Rombouts F M, Newell D G. The speciation and subtyping of Campylobacter isolates from sewage plants and waste-water from a connected poultry abattoir using molecular techniques. Epidemiol Infect. 1995;115:485–494. doi: 10.1017/s0950268800058647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konkel M E, Kim B J, Klena J D, Young C R, Ziprin R. Characterization of the thermal stress response of Campylobacter jejuni. Infect Immun. 1998;66:3666–3672. doi: 10.1128/iai.66.8.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert de Rouvroit C, Seniters C, Cornelis G R. Role of the transcriptional activator, Vir F, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol. 1992;6:395–409. [PubMed] [Google Scholar]
- 23.Palmer S R, Gully P R, White J M, Pearson A D, Suckling W G, Jones D M, Rawes J C L, Penner J L. Water-borne outbreak of Campylobacter gastroenteritis. Lancet. 1983;i:287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- 24.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 25.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:160–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 27.Prugnola A, Arico B, Manetti R, Rappuoli R, Scarlato V. Response of the bvg regulon of Bordetella pertussis to different temperatures and short-term temperature shifts. Microbiology UK. 1995;141:2529–2534. doi: 10.1099/13500872-141-10-2529. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28a.The Sanger Centre. Campylobacter jejuni. 26 January 1999, revision date. [Online.] http://www.sanger.ac.uk/Projects/C_jejuni/. [8 April 1999, last date accessed.]
- 29.Sell S M, Eisen C, Ang D, Zylicz M, Georgopoulos C. Isolation and characterization of dnaJ null mutants of Escherichia coli. J Bacteriol. 1990;172:4827–4835. doi: 10.1128/jb.172.9.4827-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 31.Stern N J, Bailey J S, Blankenship L C, Cox N A, McHan F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988;32:330–334. [PubMed] [Google Scholar]
- 32.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 34.Trieu-Cuot P, Gerbaud G, Lambert T, Courvalin P. In vivo transfer of genetic information between Gram-positive and Gram-negative bacteria. EMBO J. 1985;4:3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner A K, Lovell M A, Hulme S D, Zhang-Barber L, Barrow P A. Identification of Salmonella typhimurium genes required for colonization of the chicken alimentary tract and for virulence in newly hatched chicks. Infect Immun. 1998;66:2099–2106. doi: 10.1128/iai.66.5.2099-2106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ullrich M, Peñaloza-Vázquez A, Bailey A-M, Bender C L. A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol. 1995;177:6160–6169. doi: 10.1128/jb.177.21.6160-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vliet A H M, Wooldridge K G, Ketley J M. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassenaar T M, van der Zeijst B A M, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 39.Wassenaar T M, Bleuminkpluym N M C, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wren B W, Henderson J, Ketley J M. A PCR-based strategy for the rapid construction of defined bacterial mutants. BioTechniques. 1994;16:7–8. [PubMed] [Google Scholar]
- 41.Wren B W, Colby S M, Cubberley R R, Pallen M J. Degenerate PCR primers for the amplification of fragments from genes encoding response regulators from a range of pathogenic bacteria. FEMS Microbiol Lett. 1992;99:287–291. doi: 10.1016/0378-1097(92)90042-m. [DOI] [PubMed] [Google Scholar]
- 42.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]