Abstract
Traditional medicinal herbs as Echinacea purpurea and Erigeron canadensis are recommended as a complementary supplementation for the treatment of diseases associated with immunological inflammation (e.g. common cold, coughs, bronchitis, upper respiratory infections, immunodeficiencies). This pathologic conditions are accompanied by the wide range of malfunctions or imbalances of the immune system, thus there is increased necessity for search of novel immunomodulation trends and immunopharmacologically active phytosubstances for effective pharmaco-immunomodulatory therapy. Anti-inflammatory immunobiological activity of polyphenolic polysaccharide-proteins of Echinacea purpurea and Erigeron canadensis are still not studied. Our results demonstrated the immunobiological effectivity of selected herbal polyphenolic polysaccharide-proteins isolated from flowers of medicinal plants Echinacea purpurea and Erigeron canadensis resulting into the significant immunostimulation of inflammatory TNF-α, IL-6, IL-1ß and IL-12 cytokines (p < 0.001). Both herbal polyphenolic polysaccharide-proteins triggered cell release of anti-inflammatory interleukin IL-10 (p < 0.001). Furthermore, the inductive cell release of growth factors M-CSF and GM-CSF has been demonstrated (p < 0.001). E. purpurea and E. canadensis polyphenolic polysaccharide-proteins accelerated the efficacy of cellular phagocytosis and free radical release, more pronounced with Erigeron treatment.
Keywords: Echinacea purpurea, Erigeron canadensis, Polyphenolic polysaccharide-protein, Immunobiological activity, Cytokines, Phagocytosis, Proliferation
Introduction
Currently, according to the increase of immune system disorders as autoimmune diseases, immune deficiencies, allergy (Muñoz-Carrillo et al. 2018) there is an intensive search for new immunobiologically active substances capable to modulate the innate and adaptive immune responses. The immunobiological study of various medicinal plants and the active structural components thereof is promising prerequisite for development new pharmacologically active drugs suitable for effective pharmaco-immunomodulatory therapy. Moreover, the traditional use of medicinal herbs for remedial purposes represented a precondition for successful phytotherapeutic application.
Traditional medicinal herbs as Echinacea purpurea (L.) Moench (Purple coneflower) and Erigeron canadensis L.(synonym Conyza Canadensis, Canadian horseweed), are considered as plants with various biological and pharmacological activities (Manayi et al. 2015; Kozuharova et al. 2019). The main active ingredients of Erigeron canadensis represent flavonoids, essential oils, tannins, choline, gallic acid, resins, waxes and the others. (Strzelecka and Glinkowska 1981; Weaver 2001; Al-Snafi 2017). Plant extracts and essential oils from E. canadensis has been demonstrated to have various pharmacological and biological activities such as anticancer, mutagenic, antioxidant, anti inflammatory, antiproliferative, immunostimulating, anticoagulant and antiplatelet, antiallergic, antibacterial, antifungal (Pawlaczyk et al. 2011; Al-Snafi 2017; Kozuharova et al. 2019). Recently, the radioprotective ability of a polyphenolic polysaccharide-protein (PPP) complex isolated from flowering parts of E. canadensis has been described (Zbikowska et al. 2016).
Echinacea purpurea (L.) Moench (Purple coneflower) is a medical plant traditionally used for wound healing. The treatment of the common cold, cough, bronchitis, upper respiratory infections, bacterial inflammation of the nasal and oral cavities as well as pharynx has been described (Percival 2000; Hudson 2012). The most important biological activities of E. purpurea herb include antioxidant activity, antibacterial and antifungal activities, antiviral and mosquitocidal properties, as well as anti inflammatory, hypoglycaemic, antiproliferative and anticancer effects (reviewed in Manayi et al.2014; Aarland et al. 2016; Sharifi-Rad et al. 2018). Nowadays, several articles pointed out the effective activity of E. purpurea towards Coronavirus disease 2019 (COVID-19) (Aucoin et al. 2020; Nugraha et al.2020; Khalifa et al. 2021; Nagoor Meeran et al. 2019).
The numerous chemical, pharmacological and biological studies have been performed to identify variety of medically important substances in Echinacea species and their possible therapeutic effects. Although many of the active compounds of Echinacea have been isolated and identified to date, their possible biological activities, mechanisms of action or their synergistic effects remain unknown. The studies summarized in the reviews (Liun et al. 2015; Wiesner and Knöss 2017; Dobrange et al. 2019; Percival 2000) identified the main active ingredients related to healthy properties of Echinacea plant. The bioactive compounds of distinct parts of E. purpurea are known for their immunomodulative properties as enhancement of immune responses, immunomodulation of immunocyte (e.g. monocytes, macrophages, NKT cells, T cells and dendritic cells), selected interleukins and growth factors release, enhancement of phagocytosis, modulation of cell proliferation, (reviewed in Manayi et al. 2014; El-Ashmawy et al. 2015). Furthermore, Echinacea is known to induce transcriptional changes activating immunomodulatory pathways (Liun et al. 2015; Dobrange et al. 2019).
The interactive plant-immunocytes sensing and recognition during the course of innate immune responses is processed by conserved species–specific signature structures (arabinogalactan, galactomannan and the others). Immune sensing of these plant structural moieties initiates the cell signaling resulting in activation of transcription factors, interferon regulatory factors, and various cellular responses, including the production of interferons and pro- and anti-inflammatory cytokines. Moreover, these polysaccharides have been shown to increase immunocytes cytotoxic activity against tumor cells and microorganisms (Schepetkin and Quinn 2006; Mahla et al. 2013).Polysaccharides and their conjugates with proteins or phenolics represent a large group of plant macromolecules, which in addition to structural function also exhibit a number of biological functions (Liun et al. 2015).
In our previous studies (Pawlaczyk et al. 2011; Zbikowska et al. 2016) the anticoagulant, antiplatelet and radioprotective activities of PPPs isolated from flowering parts of E. canadensis have been preliminarily studied. In addition, pharmacological effects (antitussive, bronchodilatory, and anti asthmatic) of PPPs isolated from flowering parts of E. purpurea and E. canadensis have been described (Capek et al. 2015; Šutovská et al. 2015, 2022). It should be emphasized that the isolation of these complexes was carried out under hot alkaline conditions, in order to isolate the polymeric material with the highest possible content of phenolics and uronic acids (Pawlaczyk et al. 2011; Zbikowska et al. 2016). Both complexes are interconnected polysaccharides, proteins and phenolic compounds that cannot be separated without destruction of the individual components.
The aim of this study was to compare the chemical composition of two polyphenolic polysaccharide-protein complexes isolated from flowering parts of the medicinal plants E. purpurea and E. canadensis and to verify their possible immunomodulatory effects on RAW 264.7 mouse macrophage cells, as these natural complexes have not yet been tested for the immunobiological effects, characterizing macrophages innate immune responses.
Material and Methods
Plant material and isolation of polyphenolic polysaccharide-proteins
Dry flowering parts of Erigeron canadensis and Echinacea purpurea were obtained from the Central European market (F-DENTAL Hodonin s.r.o, Czech Republic) and a market in Wroclaw, Poland, respectively. The identity of the plants was certified by Prof. Krystyna Kromer and Dr. Magdalena Mularczyk from Wrocław University, Wrocław, Poland, and the voucher specimens, No 019361 for E. canadensis and No 011493 for E. purpurea respectively, have been deposited in the Botanical Garden of the University of Wrocław, Wrocław, Poland. The isolation of the E. purpurea and E. canadensis polyphenolic polysaccharide-proteins was made according to already described procedure (Pawlaczyk et al. 2009).
The isolation of the E. purpurea and E. canadensis polyphenolic polysaccharide-proteins was executed according to previously described method (Pawlaczyk et al. 2009).
Homogeneity and molecular weight distribution analysis of PPPs
The gel permeation chromatography (GPC) of E. canadensis and E. purpurea PPPs was performed on a column (15 × 1000 mm) of Sephacryl S300 HR. Sephacryl was eluted with 0.1 M NaOH solution, with the flow rate of 0.3 mL/min. Fractions were analyzed spectrophotometrically for carbohydrate and phenolic contents (Dubois et al. 1956; Singleton et al. 1999). To evaluate the molecular weight distribution pattern, the GPC column was calibrated using dextran standards with Mp 1, 5, 12, 25, 50, 70 and 500 × 103 g/mol.
General methods
The protein, carbohydrate, uronic acid and phenolic contents were estimated by the phenol– sulfuric acid, Folin–Ciocalteu, Lowry and m-hydroxybiphenyl reagent assays using serum albumin, glucose, galacturonic acid and gallic acid as reference compounds, respectively (Lowry et al. 1951; Dubois et al. 1956; Blumenkrantz and Asboe-Hansen 1973; Singleton et al. 1999). The colorimetric assays were measured using UV- VIS 1800 spectrophotometer (Shimadzu, Japan).
PPPs were hydrolyzed with 2 M TFA for 1 h at 120 °C and the quantitative determination of the neutral sugars was carried out in the form of their alditol acetates by gas chromatography on a TRACE Ultra Gas Chromatograph (Thermo Scientific, USA) equipped with a SP-2330 (Supelco, USA) fused silica capillary column (30 m × 0.25 mm × 0.2 μm) at a temperature program of 80 °C (4 min)—(8 °C/min)-160 °C (4 min)—(4 °C/min)—250 °C (20 min), the flow rate of helium was 0.4 mL/min. The gas chromatograph was coupled with TSQ Quantum XLS mass spectrometer (Thermo Scientific, USA) with EI ionization under standard 70 eV electron energy, emission current 25 μA, ion source temperature 200 °C. A mixture of alditol acetates of Rha, Fuc, Rib, Ara, Xyl, Man, Gal, and Glc was used as a standard.
Cell maintenance and culture, cell exposure
RAW 264.7 cell line murine macrophages (ECACC, Salisbury, UK) were cultured in complete DMEM for 24 h (till confluence of approximately 80%) at 37 °C in a humidified atmosphere with 5% CO2. Cell viability has been assessed with the Trypan blue dye exclusion method using TC20™ automated cell counter (Bio-Rad Laboratories, Inc., USA). The starting inoculum of 1 × 105 cells/mL/well (with 93.1% viability) was plated on 24-well cell culture plate (Sigma-Aldrich, USA), and exposed to 100 μg per well of Erigeron (EC) and Echinacea (EP) PPPs complexes for 24 and 48 h. The sensitized cells were subjected to flow cytometry evaluation of fagocytic activity and phenotyping. The cell surface expression of macrophages antigens F4/80 and CD11b had been used to ascertain phenotypic diversity. Culture media were stored at -20 °C until further use.
Cell proliferation and cytotoxicity
Cell proliferation and cytotoxicity of RAW 264.7 macrophage cells induced by Erigeron (EC) and Echinacea (EP) PPPs complexes exposure was evaluated by bioluminescent measurement of adenosine triphosphate (ATP) using the ViaLight™ plus kit (Lonza, USA) according to the instructions of the manufacturer. The intensity of emitted light was measured with Cytation 5 Cell Imaging Multi-Mode Reader (BioTek Instruments, Inc., USA). Light emission, expressed as relative light units (RLU), was recorded continuously for one second and evaluated on the basis of peak values. Proliferation of unstimulated cells was considered to be the baseline. The proliferation index was determined as ratio of induced proliferation (exposed cells) to baseline (non-exposed cells) proliferation.
Determination of cytokines
The levels of interleukins and growth factors in cell culture supernates following Erigeron (EC) and Echinacea (EP) PPPs exposure, were determined using ELISA method (Quantikine ELISA® Mouse M-CSF (R&D, USA), Platinum ELISA: Mouse MCP-1, Mouse IL-12,Mouse GM-CSF, Mouse IL-17, Mouse IL-2 and Mouse IL-6,; Instant ELISA®: Mouse IL-1β, Mouse TNF-α, Mouse IL-10, (e-Bioscience, USA) according to the instructions of the manufacturer.
Determination of free radicals
Cell culture supernates following Erigeron (EC) and Echinacea (EP) PPPs treatment of RAW 264.7 cells were assayed for total content of free radicals (Free radicals kit; Sedium R&D, Czech Republic). The assay is based on the ability of chlorophyllin to transfer electrons due to its electron-rich double-bonds structure. The quantification of free radicals was assayed using a calibration based on a Fe2+/Fe3+ reactive shift and was expressed as mmol/L Fe2+. Free radical production of the unstimulated cells was used to determine the baseline value.
Immunocytometry
RAW264.7 cells, exposed to Erigeron (EC) and Echinacea (EP) PPP complexes, were subjected to immuno-flow cytometry using a Beckman Coulter FC 500 flow cytometer equipped with CXP software (Beckman Coulter, Fullerton, CA, USA). Gates were set to exclude the debris and damaged cells using FSC vs SSC discrimination. The settings were optimized using proper isotype control (immunophenotyping assay) and C. albicans FITC conjugate (phagocytosis). For each sample fluorescence histograms of 10 000 cells (immunophenotyping) or 5000 cells (phagocytosis) were generated and analyzed (green fluorescence, 525-nm band-pass filter, FL1 channel). All samples were analyzed in duplicates. The data are expressed as percentage (Avg % ± SD) or as a mean of fluorescence intensity (MFI ± SD).
Phagocytosis
RAW264.7 macrophages phagocytosis was evaluated by immunoflow cytometry (CytoFLEX, Beckman Coulter Life Sciences, Inc., Indianapolis, US). For each sample, a fluorescence histogram of 5000 cells was generated and analyzed. Gates were set around the macrophages population to exclude debris. Phagocytosis, i.e. the ingestion of Candida albicans yeast cells took place under controlled conditions using a fluorescein-labeled C. albicans. Aliquots of (30 μL) post-exposed cells were incubated with C. albicans –FITC (3µL) for 15 min at 37 °C. Following treatment, the reaction was ice-stopped. The mean percentage of phagocytic cells represents the percentage of cells ingesting at least one C. albicans –FITC cell.
Statistical analysis
Results of in vitro experiments with RAW 264.7 cells were evaluated as Mean ± SD values from two biological and three technical replicates. Normality of data distribution was evaluated according to Shapiro–Wilk’s test at the 0.05 level of significance. Statistical comparison was performed using one-way ANOVA and post hoc Bonferroni’s tests. The results were significant if the differences equaled or exceeded the 95% confidence level (P < 0.05). Statistics was performed using ORIGIN 2018 software (OriginLab Corporation, Northampton, Massachusetts, USA). Pearson´s correlation coefficient was used to compare the strength of the relationship between immunobiological variables.
Results
Isolation and chemical characterization of Echinacea and Erigeron polyphenolic polysaccharide-protein complexes
Air-dried flowering parts of the medicinal plants of E. purpurea (EP) and E. canadensis (EC) were ground to a powder and subjected to hot alkaline treatments in order to obtain polyphenolic polysaccharide-protein complexes (PPPs) with the highest possible content of phenolics and uronic acids. These polymeric compounds have been found to have interesting anticoagulant, antiplatelet and radioprotective effects (Pawlaczyk et al. 2011; Zbikowska et al. 2016). Dark brown PPPs were isolated in 1.8 wt% (EP) and 0.9 wt% (EC) yields of dried plant materials. In HPLC analyses, they showed molecular weights (Mw) of 10,000 (EP) and 38,000 g/mol (EC) (Pawlaczyk et al. 2009; Šutovská et al. 2015, Šutovská et al. 2022). Compositional analyses of Echinacea and Erigeron PPPs revealed the presence of carbohydrates, phenolics, proteins and uronic acids (Table 1).
Table 1.
Characteristics of E. purpurea (EP) and E. canadensis (EC) polyphenolic polysaccharide-proteins (PPPs)
| Composition of PPP complexes | Ep (w/w) | Ec (w/w) |
|---|---|---|
| Carbohydrates | 26.3 | 13.3 |
| Protein | 14.0 | 16.3 |
| Phenolics | 17.5 | 13.2 |
| Uronic acids | 11.2 | 6.3 |
| Yield | 1.8 | 0.9 |
| Mw (g/mol) | 10,000 | 38,000 |
| Sugars | ||
| Rhamnose | 18.6 | 3.8 |
| Fucose | 1.4 | 1.9 |
| Arabinose | 24.2 | 24.1 |
| Xylose | 12.9 | 12.1 |
| Mannose | 2.9 | 3.8 |
| Galactose | 31.4 | 41.0 |
| Glucose | 8.6 | 13.3 |
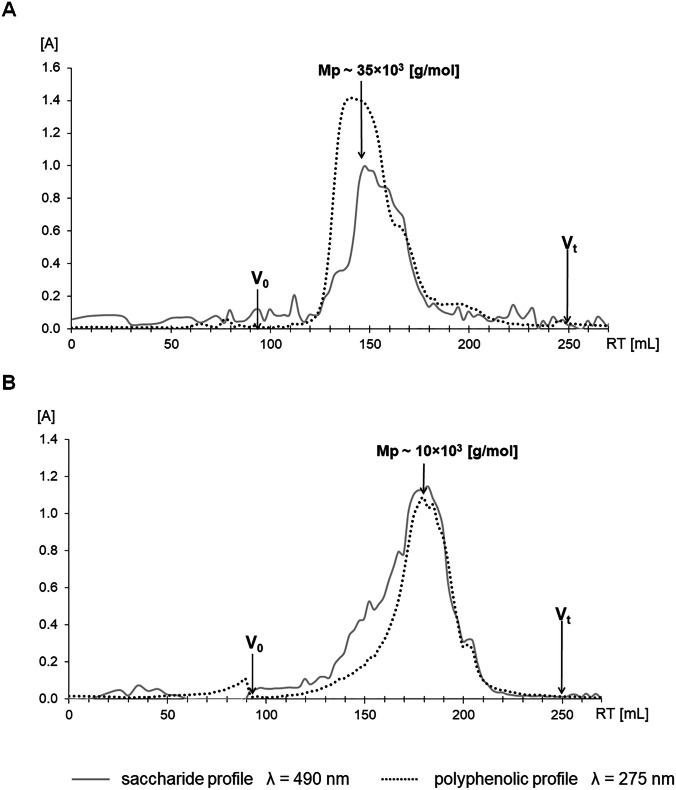
It is evident from Table 1 that both PPP complexes differ in protein, phenolics and uronic acid contents. A relatively significant difference was recorded in the case of yields, e.g. the Echinacea complex showed twice as high a yield as of Erigeron, but in the case of molecular weight it was the opposite, where the Erigeron complex had a significantly higher molecular weight. To verify homogeneity, i.e. whether it is a mixture or a single compound, PPPs were analyzed by gel permeation chromatography. Analysis showed that both PPP complexes have a macromolecular character, the polysaccharide and the polyphenolic components had the same mobility, indicating their interconnection. This is also confirmed by the fact that if the polyphenolic compounds were not bound to the polysaccharide backbone, they would be insoluble (Fig. 1).
Fig. 1.
Gel permeation chromatography (GPC) of the E. canadensis (A) and E. purpurea (B) PPPs, on Sephacryl S300 HR column. Mp—represents peak molecular weight, Vo – void volume and Vt—total volume of the column
Monosaccharide analyses of PPP complexes showed the prevalence of four sugar constituents, i.e. uronic acids, galactose, arabinose, and rhamnose indicating the dominance of arabinogalactan (56%) associated with rhamnogalacturonan (30%) in Echinacea. Other sugars such as fucose, xylose, mannose and glucose, derived from other minor types of polysaccharides, have been found in much smaller amounts (Table 1). The monosaccharide composition of the carbohydrate part of Erigeron PPP differed from Echinacea one mainly in the content of rhamnose, galactose, glucose and uronic acid. Erigeron PPP was shown to have higher levels of galactose and glucose residues, and much lower rhamnose content, while the amounts of other monosaccharides were comparable to Echinacea complex. Based on sugar analysis, the main polysaccharide component in Erigeron PPP appears to be arabinogalactan (~ 65%), while the acidic polysaccharide content is significantly lower (~ 10%) than in the Echinacea complex (Table 1). It is evident that the investigated PPPs differ mainly in the content of dominant polysaccharide components, i.e. arabinogalactan and an acidic heteropolysaccharide, i.e. rhamnogalacturonan and glucuronoxylan.
A characteristic feature of arabinogalactans is the presence of proteins which are bound to saccharide moieties. Similarly, phenolic compounds can be attached to the side chains of the pectin material (arabinogalactan-rhamnogalacturonan, RG-I) by covalent and ester bonds and to form a complex composed of polysaccharides, proteins, and phenolics. The natural products tested are interconnected polysaccharides, proteins and phenolic compounds that cannot be separated without destroying the individual components.
Proliferation of RAW 264.7 cells
The ability of Echinacea and Erigeron PPP complexes to modulate the proliferation of RAW 264.7 macrophage cells was assayed with cell viability indicator adenosine triphosphate using bioluminescence ViaLight™ plus kit (Fig. 2). The cell proliferation of Echinacea and Erigeron PPPs exposed cells has been followed after 24 h and 48 h. Evidently, both formulas reflected after 24 h cell exposure the comparable (p < 0.01) antiproliferative pattern vs. baseline untreated cells. The treatment for the next 24 h exerted the increasing trend vs. values of baseline untreated cells, i.e. for Echinacea complex the ratio between exposed and unexposed cells was 0.9777, and 1.001for Erigeron complex. In contrast, cell mitogen Concanavalin A (ConA) increased the RAW 264.7 macrophage cell proliferation 1.25-fold following 24 h treatment, and 1.4-fold after next 24 h. The impact of time-exposure of Con A on cell proliferation overcame ratios of both Echinacea and Erigeron PPPs: ratio Echinacea vs. ConA 0.53 (24 h) and 0.67(48 h), and ratio Erigeron vs ConA 0.54 (24 h) and 0.71 (48 h).
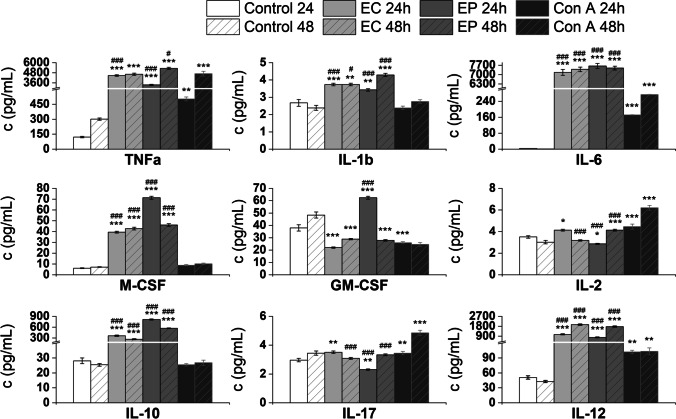
Fig. 2.
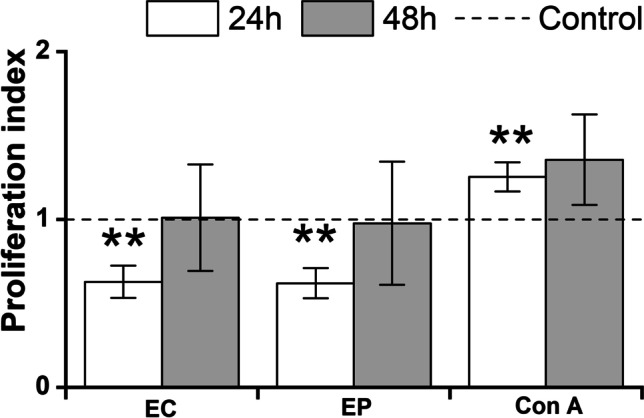
Proliferation of RAW 264.7 macrophages. RAW 264.7 cells were stimulated for 24 h or 48 h with 100 µg/mL of Echinacea (EP) and Erigeron (EC). As a negative control, RAW 264.7 cells cultured without stimulants were used. Concanavalin A (Con A (10 μg/mL)) was used as positive control. Results are expressed as a mean stimulation indexes (average relative light units in the presence of antigens/average relative light units obtained without antigen). All data are presented as a mean stimulation indexes ± SD values from two biological and three technical replicates. The statistical significance of differences between untreated cells and stimulated cells using one-way ANOVA and post hoc Bonferroni’s tests is expressed as: **—0.001 < P < 0.01
Cytokines cell release following exposure of RAW 264.7 macrophages with Erigeron and Echinacea PPPs
The culture media following 24 h and 48 h RAW 264.7 exposure with Echinacea and Erigeron PPPs were subjected to analysis of pro-inflammatory cytokines TNFα, IL-1β, IL-6, IL-2, IL-12, and IL-17, anti-inflammatory interleukin IL-10, and growth factors GM-CSF and M-CSF (Fig. 3). Echinacea and Erigeron PPPs induced after 24 h cell exposure most significant increase in production of IL-6: for Erigeron: 528.168-fold, p = 2.52E−08 and for Echinacea: 563.867-fold, p = 7.60E−09. Next significant media release was noted for TNFα: 36.843-fold, p = 4.67E−10, for Erigeron, and 27.718-fold, p = 4.86E−09 for Echinacea, compared to baseline values. Further effective induction has been demonstrated for IL-12: for Erigeron 20.704-fold, p = 1.58E−07, and for Echinacea 15.295-fold, p = 1.99E−06. The inductive increase of cell release of anti-inflammatory IL-10 has been revealed after Echinacea exposure: 28.897-fold, p = 1.64E−09, to lesser extent following Erigeron exposure: 12.814-fold, p = 1.49E−06.
Fig. 3.
Effect of E. canadensis (EC) and E. purpurea (EP) on RAW 264.7 macrophages cytokines production. Concentration of cytokines (pg/ml) in post-exposure culture media was determined following stimulation of RAW 264.7 macrophages for 24 h or 48 h with 100 µg/mL of Echinacea and Erigeron PPP complexes. Untreated RAW 264.7 cells were used as negative control (Control). Cell mitogen Concanavalin A (Con A, 10 µg/mL) was used as positive control. All data are presented as Mean ± SD values from two biological and three technical replicates. Statistical significance of differences between untreated and stimulated cells using one-way ANOVA and post hoc Bonferroni tests is expressed as: *** P < 0.001, ** 0.001 < P < 0.01, * 0.01 < P < 0.05. Statistical differences between EP and EC exposed cells and ConA treated cells are expressed as: ### – P < 0.001, ## – 0.001 < P < 0.01, # – 0.01 < P < 0.05
Prolonged exposure with both formulas for next 24 h revealed the increasing trends of IL-6, for Erigeron: 621.086-fold, p = 2.62E−10 and for Echinacea: 629.297-fold, p = 2.36E−10. Next 48 h increase has been observed in IL-12 release: for Erigeron 45.419-fold, p = 6.09E−10 and for Echinacea 41.358-fold, p = 1.31E−09, when compared to untreated cell culture. Only slight increase in IL-1α cell release has been determined, for Erigeron 1.566-fold, p = 0.00243 and for Echinacea 1.798-fold, p = 1.99E−04. Comparison between 24 and 48 h cytokine production revealed increasing trends, especially after 48 h treatment with Echinacea for IL-12: 2.274-fold, TNFα: 1.581-fold, IL-2: 1.444-fold, IL-17: 1.442-fold, and IL-1 β: 1.252-fold, compared to 24 h values.
The determination of strength of association among cytokines release induced by Erigeron exposure revealed the tightest correlation between IL-2 and IL-10 (r = 0.99992, p = 8.36E−05), IL-2 and IL-17 (r = 0.99276, p = 0.00724) and for GM-CSF and IL-12(r = 0.99798, p = 0.00202).
For Echinacea, different pattern has been established—the tightest correlation was between pro-inflammatory cytokines TNFα and IL-17 (r = 0.99996, p = 3.97E−05), TNFα and IL-2 (r = 0.99994, p = 5.90E−05), TNFα and IL-12 (r = 0.99932, p = 6.79E−04), and TNFα and IL-1 β (r = 0.99848, p = 0.00152). Next significant correlations were established also for IL-1 β and IL-17 (r = 0.99872, p = 0.00128), IL-1 β and IL-2 (r = 0.99878, p = 0.00122) and IL-1β and IL-12 (r = 0.99593, p = 0.00407). Production of IL-10 is strongly associated with cell release of growth factors M-CSF (r = 0.99998, p = 2.26E−05) and GM-CSF(r = 0.99915, p = 8.54E−04).
The effect of exposure of RAW 264.7 macrophages with Erigeron and Echinacea PPPs on cell phagocytosis
Time-dependent pattern of RAW 264.7 macrophages phagocytic effectivity had revealed the significant acceleration of number of phagocytic cells especially following Erigeron exposure 1.17 fold after 24 h compared to untreated control and 1.12-fold after 48 h (p < 0.01) (Fig. 4). The similar results of cell phagocytosis were observed with Echinacea exposure, after 24 h exposure the ratio between control and exposed cells was significantly raised 1.088-fold and after prolonged treatment up to 48 h 1.12-fold increase (p < 0.01) has been detected. Overall correlation analysis of interactions concerning exposure time period and extent of phagocytosis supported the high correlation r = 0.99 between Echinacea and Erigeron PPPs.
Fig. 4.
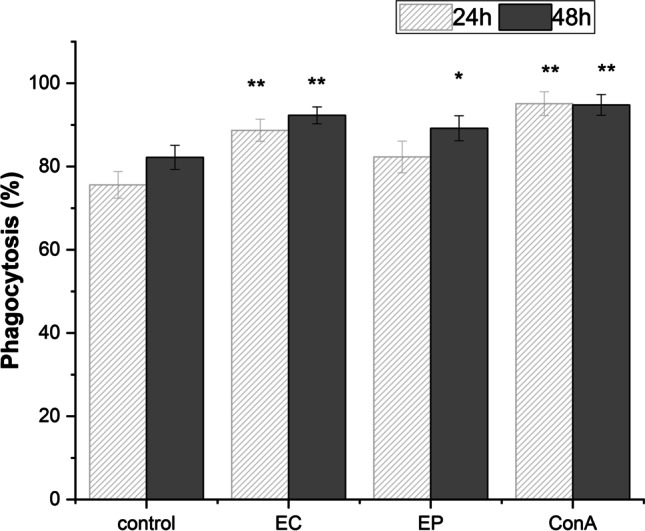
Phagocytosis of RAW 264.7 macrophages following E. canadensis (EC) and E. purpurea (EP) 24 h and 48 h cell exposure with 100 µg/mL. Untreated RAW 264.7 cells were used as negative control (Control). Cell mitogen Concanavalin A (Con A, 10 µg/mL) was used as positive control. All data are presented as Mean ± SD values from two biological and three technical replicates. Statistical significance of differences between untreated and stimulated cells using one-way ANOVA and post hoc Bonferroni tests is expressed as: *** P < 0.001, ** 0.001 < P < 0.01, * 0.01 < P < 0.05
Presented data are suggestive of moderately enhanced effectiveness of Erigeron exposure over Echinacea exposure, as Erigeron and Echinacea ratio at 24 h has been 1.077 and at 48 h 1.03. The differences between 24 and 48 h revealed 1.04-fold increase for Erigeron and 1.08 for Echinacea (p < 0.01). Evidently, the longer cell exposure with Echinacea potentiated the phagocytic macrophages activity.
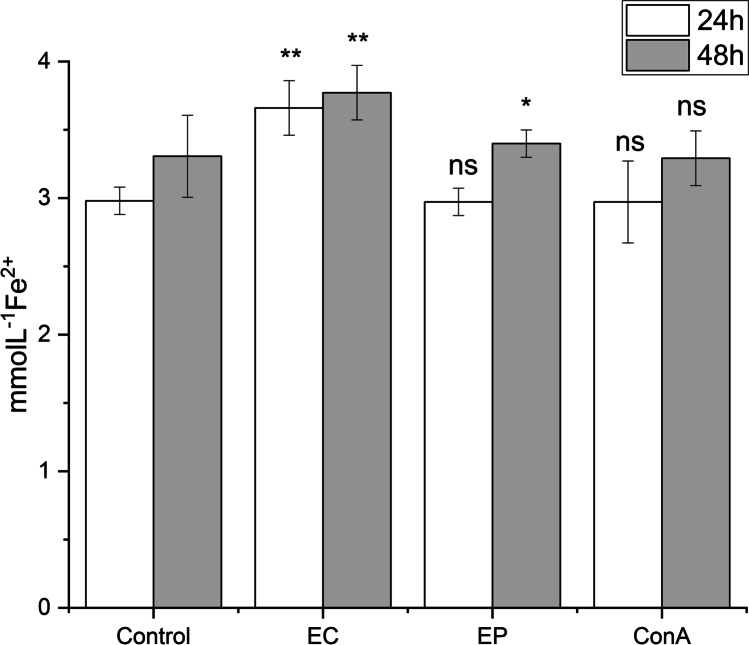
The effect of exposure of RAW 264.7 macrophages with E. canadensis and E. purpurea PPPs on free radicals released by the cells
The exposure of RAW 264.7 macrophages to Erigeron and Echinacea PPPs had revealed the significant induction of free radicals released by the cells, especially following Erigeron exposure: 1.22- fold after 24 h compared to untreated control and 1.14-fold after 48 h (p < 0.01) (Fig. 5). With Echinacea exposure for 24 h, the ratio between control and exposed cells was lower, represented 81.1% of EC values and significantly increased up to 90.17% after prolonged 48 h treatment. The ratio between Erigeron and Echinacea values of inductive release of free radicals has been 1.23-fold after 24 h and 1.11- fold after 48 h exposure. Evidently, the Erigeron is more effective to accelerate the production free radicals than Echinacea. As concerned prolonged treatment, 48 h values overcame 24 h values, more for Echinacea i.e. 1.14- fold and for Erigeron i.e.1.03- fold increases.
Fig. 5.
Time–dependent pattern of total free radicals release. The release was measured after 24 and 48 h stimulation of RAW 264.7 macrophages in response to the exposure with 100 μg/mL of Erigeron (EC) and Echinacea (EP), and Con A (10 μg/mL). Control release represents the levels reached by untreated cells (Control). All data are presented as Mean ± SD values from two biological and three technical replicates. Statistical significance of differences between untreated and stimulated cells using one-way ANOVA and post hoc Bonferroni tests is expressed as: *** P < 0.001, ** 0.001 < P < 0.01, * 0.01 < P < 0.05
Discussion
As chemical analyses have shown, PPPs isolated from the flowering parts of E. purpurea (Ep) and E. canadensis (Ec) differ chemically in the content of the individual components, i.e. phenols, carbohydrates and proteins. Gel chromatography of PPP complexes indicated a macromolecular character of studied biopolymers. Both the polysaccharide and the polyphenolic components were found to have the same mobility, indicating that they are interconnected. The carbohydrate moieties showed differences in the content of the individual monosaccharides involved in their structures. In addition, differences in the content of arabinogalactans and acidic heteropolysaccharides were determined in PPPs. Therefore, the question is whether these structural differences in their polysaccharide moieties may also reflect differences in their immunobiological effects.
According this, the selected assessment of the immunobiological activity of Echinacea and Erigeron PPP complexes in RAW 264.7 macrophage cells comprised determination of cell proliferation, cell phagocytosis, free radicals release, and the production of inflammatory and anti-inflammatory interleukins as well as growth factors.
Our results (Fig. 1) are in agreement with the observation of Roesler et al. (1991) demonstrating the increase of cell proliferation based on 3H-thymidine incorporation into C3H/HeJ and BALB/c mice monocytes following exposure with xyloglucan and arabinogalactan polysaccharides purified from Echinacea. Classen et al. (2006) observed reduction of Echinacea arabinogalactan induced proliferation in comparison with ConA in mouse spleen cells C3H/HeJ (Classen et al. 2006). Cell exposition with both PPPs exerted the comparable pattern, decreased cell proliferation compared to that induced by ConA (Fig. 1). Yang et al.(2018) showed that concentrations of 0.0039–0.5 mg/mL of tetraploid and diploid Echinacea crude polysaccharides could promote the proliferation of normal mouse spleen lymphocytes ConA stimulated. Moreover, the stimulation of the proliferation of T-lymphocytes by the Echinacea polysaccharide fraction (M 5 000–50 000) had been demonstrated. Echinacea herb and root powders enhanced the human PBMC proliferation in vitro (Rininger et al. 2000). Echinacea anti-proliferative effect has been reported with MCF-7, HeLa and HCT-15 cell lines (Aarland et al. 2017). The anti-proliferative activity and cell growth – inhibitory activity of Erigeron was studied against cancer cell lines as HeLa, A431 and MCF7 (Kozuharova et al. 2019).
Generally, interleukins and growth factors are essential modulators of immune responses engaged in various immune system processes, as cell activation and proliferation, signalization, polarization, phagocytosis, and inflammation in a complex network of target immune cell interactions. Consequently, these multipurpose indicators of cell polarization reflect the mode and effectivity of immunocompetent cell reactivity.
Immunobiological evaluation of Echinacea and Erigeron PPPs impact on RAW 264.7 revealed effective induction of Th1 (TNFα, IL-10, IL-12) and Th2 (IL-6) responses and release of pro-inflammatory (TNFα, IL-6, IL-12), as well as the anti-inflammatory (IL-10) cytokines. The cytokines highest release following EP and EC exposure has been determined for TNFα and IL-6, extremely potent inflammatory molecules, primary cytokines mediating acute inflammation (Fig. 2.). This fact, together with induced free radical release supported the effective cell immunomodulation following EP and EC PPPs exposure. Moreover, regulatory effects of IL-6 involve inhibition of TNFα cell release, thus providing negative feedback for limiting the acute inflammatory response. IL-6 is frequently used as a marker for systemic activation of next pro-inflammatory cytokines.
The next, highly induced cytokine IL-12 (Fig. 2.) is produced by antigen-presenting cells and plays a critical role in host defense against intracellular microbial infection via its ability to stimulate both innate and adaptive immune effector cells. In most infections IL-12 regulates the extent of the IFN-γ response at the initiation of infection, thus potentiating natural resistance, favoring Th1-cell development, and inhibiting Th2 responses. Therefore, the effective time-dependent induction of IL-12 release by Echinacea and Erigeron PPPs (p < 0.001) is an important observation, suggesting acceleration of cellular immunity. Our results with Echinacea PPPs are consistent with previously published findings demonstrated, that the acceleration of in vitro release of TNF-α by RAW 264.7 macrophages corresponded with Echinacea extracts concentration (Lee et al. 2010). Arabinogalactan represented the main polysaccharide of Echinacea PPP (Table 1). Mouse peripheral macrophages treated with arabinogalactan (10–100 µg/mL) exhibited induction TNFα and IL-6, and arabinogalactan had been shown to be mitogenic in mouse splenocytes and peripheral macrophages (Choi et al. 2005).The increased production of TNF-α, IL-6, and IL-1α by human monocytes subjected to polysaccharides xyloglucan and arabinogalactan (2:1) isolated from cell cultures of EP complex was demonstrated (Roesler et.al. 1991). Interestingly, arabinogalactan from Echinacea cell culture demonstrated weak stimulation of IL-6 production in alveolar mouse macrophage culture (Roesler et.al.1991, Yang et al. 2018).The induction of macrophages, and increased production of TNF-α, IL-1 and interferon-ß2 by Echinacea acidic arabinogalactan was described (Luettig et al. 1989). Significant increases in circulating IL-2 were found in rats after treatment with aerial parts of Echinacea (Cundell et al. 2003). Several studies of Echinacea extracts cultured with mouse and human macrophages reported significantly augmented production of IL-1, TNF-α, IL-6 and IL-10 compared to untreated control (Aarland et al. 2017). The increase of 24 h production of GM-CSF and M-CSF induced by Echinacea overcoming that of Erigeron has been revealed (Fig. 2). We have observed induced production of IL-17 (p < 0.001, after the prolonged Echinacea cell 48 h treatment. Park et al.(2018) described, that Echinacea formulae (EFLA®894) induces mRNA expression of IL-17, IL-6 and IL-10 in spleen of Balb/c mice with restraint stress-induced immunosuppression and these cytokines might have beneficial effects. The induction of IL-17 following EP and EC exposure of RAW 264.7 cells (Fig. 2.) is important, as interleukin-17 is a pro-inflammatory cytokine with various effects on innate immune cells. IL-17 induces the production of granulocyte colony-stimulating factor (G-CSF) and CXCL1 and CXCL2 chemokines. During the infection, IL-17 is engaged in elimination of extracellular bacteria and fungi, by inducing antimicrobial peptides. The anti-inflammatory mechanisms of Erigeron extract on TNFα, IL-4 and IL-1β-induced A549 cell stimulation were studied (Sohn et al. 2009). They revealed that inflammatory-related genes such as NOS1, NOS2A, IL-1 ß, IL-8 and CSF2 and cell adhesion-related genes such as SELE, MMP3, VCAM1, ICAM1, ITGA7 and ITGB2 were downregulated in Erigeron treated A549 cells that had been pretreated with TNF-alpha, IL-4 and IL-1ß. (Sohn et al. 2009).
Our experiments revealed, that the treatment of RAW 264.7 cells with PPP complex of Erigeron induced media release of TNFα, and IL-1ß in lower extent compared to treatment with PPP complex of Echinacea. (Fig. 2).
Principally, cell phagocytosis represents the functional complex process for the engulfment, ingestion and elimination of pathogens, apoptotic cells, and foreign large particles > 0.5 μm. Phagocytosis has been executed by professional phagocytes as macrophages, neutrophils, dendritic cells etc. and is fundamental for overall tissue homeostasis, engaged in several aspects of inflammatory and the immune responses, comprising induction of generation of reactive oxygen and nitrogen species, and pro-inflammatory cytokines. Therefore, the mode of modulation of phagocytosis process by various immunomodulators is important. The stimulation of human granulocytes phagocytosis (Borchers et al.) and peripheral blood mononuclear cells phagocytosis (Rininger et al. 2000) by the Echinacea raw materials has been documented (Borchers et al. 2000; Rininger et al. 2000). Next, the stimulative effect of Echinacea crude polysaccharides on cell simulated digestion has been observed also in murine macrophages (Yang et al. 2018), these results are in agreement with resulting data of direct RAW cell exposure to Echinacea (Fig. 3). The enhanced phagocytosis of murine bone-marrow macrophages with Echinacea polysaccharide enriched extract (100 μg/mL) has been revealed (Fu et al. 2017). Macrophages treated with 10–100 μg/mL of arabinogalactan exhibited increased phagocytosis (Choi et al. 2005). The stimulation of phagocytosis with aqueous polysaccharide components as arabinogalactan of Echinacea sp. has been reported (Luettig et al. 1989; Baueret al. 1989;Roesler et al. 1991). Thus, we could suggest, that the arabinogalactan fraction of EP and EC PPPs supported the immunostimulation of RAW 264.7 macrophages phagocytosis following EP and EC PPPs exposure (Fig. 3.)
The generation of reactive oxygen species, reactive nitrogen species, and free radicals throughout the different pathophysiological conditions is responsible for the overall oxidative stress. The oxidative stress represented complex process characterizing the imbalance between the formation of free radicals and capability of organism to eliminate these reactive species by endogenous and exogenous antioxidants. Reactive oxygen species and free radicals can cause large chain chemical reactions in the body and are potentially inducers of tissue damage (Bauer et al. 1989). Thus, the evaluation of oxidative stress and the mode, how antioxidants are able to prevent tissue damage by regulation of signal transduction pathways and gene expression at the cellular and tissue levels is of importance. The antioxidant activity of Echinacea polysaccharides has been supported by study of Lee et al. (2009). Next, the antioxidant effect of polyphenolic-polysaccharide conjugates from Erigeron and other selected medicinal plants was demonstrated by Saluk-Juszczak et al. (2010a and 2010b).
The secretion of oxygen radicals by macrophages activated with Echinacea purified polysaccharides was increased (Stimpel et al. 1984; Choi et al. 2005).
In our experiments the significant increase of free radicals over physiological value (p < 0.01) has been observed with Echinacea PPP complex (Fig. 4). This observation together with increase of phagocytic activity and stimulation of pro-inflammatory cytokines represents the promising role of EP and EC PPPs in immune defense. Hou et al. (2020) revealed in vitro and in vivo reduction of the oxidative stress by a polysaccharide from Echinacea (2020). Mishima et al. (2004) observed in the case of radiation-induced leukopenia increase of mouse peripheral blood antioxidant activity by Echinacea suggesting relationship between the suppressive effect on radiation-induced leukopenia and the antioxidant effect of Echinacea.
Conclusions
Polyphenolic polysaccharide-protein complexes have been isolated from the flowering parts of E. purpurea and E. canadensis under strong alkaline conditions to obtain polymeric materials with a high content of phenolics and uronic acids. Arabinogalactans associated with acidic polysaccharides were the major polysaccharides in both PPP complexes. It seems that the relatively high charge in the polymer chains is responsible for their biological effects such as anticoagulant or antiplatelet. It could also affect the immunological properties of the complexes. In vitro immunobiological evaluation of both PPP complexes in RAW 264.7 cells demonstrated effective immunomodulation with respect to the induction of interleukins and growth factors release, cell proliferation, phagocytosis, and free radicals release. In vitro RAW 264.7 cell exposure to Echinacea and Erigeron PPP complexes revealed effective induction of Th1 (TNFα, IL-10, IL-12) and Th2 (IL-6) responses and release of pro-inflammatory (TNFα, IL-6, IL-12), as well as the anti-inflammatory (IL-10) cytokines. Next, both formulas reflected after 24 h and.
48 h cell exposure the antiproliferative pattern. Time-dependent pattern of RAW 264.7 macrophages phagocytic effectivity had revealed the significant acceleration of number of phagocytic cells especially following Erigeron PPP exposure. Time-dependent significant induction of free radicals released by the cells, especially following Erigeron exposure has been revealed. According to these results, the isolated herbal complexes are promising immunomodulatory compounds for further in vitro and in vivo immunobiological and immunotoxicological studies. According to the cytokine immunomodulation, induction of phagocytosis and anti-proliferative activity, E. purpurea and E. canadensis PPP complexes represent interesting natural macromolecules with a potential therapeutic or prophylactic application as immunomodulating herbal remedy.
Acknowledgements
This work was supported by the Slovak Grant Agency VEGA (Grant No. 2/0054/22), Wrocław University of Science and Technology, Wrocław (Internal grant K24 No. 8211104160) and Cost action CA 18238 (Ocean4Biotech) and by Slovak Research and Development Agency grant APVV-15-0161. This article/publication is based upon work from COST Action CA16231 ENOVA (European Network of Vaccine Adjuvants). This publication was created with the support of the Operational Program Integrated Infrastructure for the project: Study of structural changes of complex glycoconjugates in the process of inherited metabolic and civilization diseases, ITMS: 313021Y920, co-financed by the European Regional Development Fund.
CRediT authorship contribution statement
Ema Paulovicova: Conception and design of the study, Immunobiological research, Data analyses, Original draft preparing.
Lucia Paulovicova: Immunobiological research, Data analyses.
Izabela Pawlaczyk-Graja: Methodology, Review & editing, Formal analysis.
Roman Gancarz: Project administration, Formal analysis, Review & editing.
Peter Capek: Conceptualization, Writing – review & editing, Project administration, Funding acquisition.
Mária Kopáčová: Chemical analyses.
All authors contributed to manuscript revision, red and approved the submitted version.
Declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ema Paulovičová, Email: ema.paulovicova@savba.sk.
Peter Capek, Email: chemcape@savba.sk.
References
- Aarland RC, Bañuelos-Hernández AE, Fragoso-Serrano M, Sierra-Palacios EC, Díaz de León-Sánchez F, Pérez-Flores LJ, Rivera-Cabrera F, Mendoza- Espinoza JA. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm Biol. 2017;55:649–656. doi: 10.1080/13880209.2016.1265989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A. Pharmacological and therapeutic importance of Erigeron Canadensis (Syn: Conyza canadensis) IAJPS. 2017;4:248–256. doi: 10.5281/zenado.344938. [DOI] [Google Scholar]
- Aucoin M, Cooley K, Saunders PR, Carè J, Anheyer D, Medina DN, Cardozo V, Remy D, Hannan N, Garber A. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv Integr Med. 2020;7:203–217. doi: 10.1016/j.aimed.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Remiger P, Jurcic K, Wagner H. Influence of Echinacea extracts on phagocytic ctivity. Z Phytother. 1989;10(2):43–48. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Analyt Biochem. 1973;54(2):484–489. doi: 10.1016/0003-2697(73)9037. [DOI] [PubMed] [Google Scholar]
- Borchers AT, Keen CL, Stern JS, Gershwin ME. Inflammation and native American medicine: the role of botanicals. Am J Clin Nutr. 2000;72:339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- Capek P, Šutovská M, Kocmálová M, Fraňová S, Pawlaczyk I, Gancarz R. Chemical and pharmacological profiles of Echinacea complex. Int J Biol Macromol. 2015;79:388–391. doi: 10.1016/j.ijbiomac.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Choi EM, Kim AJ, Kim YO, Hwang V. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food. 2005;8:446–453. doi: 10.1089/jmf.2005.8.446. [DOI] [PubMed] [Google Scholar]
- Cundell DR, Matrone MA, Ratajczak P, Pierce JD. The effect of aerial parts of Echinacea on the circulating white cell levels and selected immune functions of the aging male Sprague-Dawley rat. Int Immunopharmacol. 2003;7:1041–1048. doi: 10.1016/S1567-5769(03)00114-0. [DOI] [PubMed] [Google Scholar]
- Dobrange E, Peshev D, Loedolff B, Van den Ende W. Fructans as Immunomodulatory and Antiviral Agents: The Case of Echinacea. Biomolecules. 2019;9:615–626. doi: 10.3390/biom9100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analyt Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- El-Ashmawy NE, El-Zamarany EA, Salem ML, El-Bahrawy HA, Al-Ashmawy GM. In vitro and in vivo studies of immunomodulatory effect of Echinacea purpurea on dendritic cells. J Genet Eng Biotechnol. 2015;132015:185–192. doi: 10.1016/j.jgeb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Wang Y, Wu Y, Chen H, Zheng S, Li Y, Xu X, Li W. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J Cell Biochem. 2017;118:2664–2671. doi: 10.1002/jcb.25875. [DOI] [PubMed] [Google Scholar]
- Hou R, Xu T, Li Q, Yang F, Wang C, Huang T, Hao Z. Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo. Int J Biol Macromol. 2020;149:41–50. doi: 10.1016/j.ijbiomac.2020.01.129. [DOI] [PubMed] [Google Scholar]
- Hudson JB (2012) Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases. J Biomed Biotechnol 769896. 10.1155/2012/769896 [DOI] [PMC free article] [PubMed]
- Khalifa SAM, Yosri N, El-Mallah MF, Ghonaim R, Guo Z, Musharraf SG, Du M, Khatib A, Xiao J, Saeed A, El-Seedi H, Zhao C, Efferth T, El-Seedi HR. Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Int J Phytother. 2021;85:153311 . doi: 10.1016/j.phymed.2020.153311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuharova E, Ionkova I, Raimondo FM. Invasive alien species: potential cheap resources of plant substances for medicinal use. Fl Medit. 2019;29:13–25. doi: 10.7320/FlMedit29.013. [DOI] [Google Scholar]
- Lee TT, Huang CC, Shieh XH, Chen CL, Chen LJ, Yu B. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. and it’s immunostimulant capacity in vitro. Int J Environ Sci Dev. 2010;1:5–9 . doi: 10.7763/IJESD.2010.V1.2. [DOI] [Google Scholar]
- Liun J, Willfor S, Xun CU. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact Carbohydr and Diet Fibre. 2015;5:31–61. doi: 10.1016/j.bcdf.2014.12.001. [DOI] [Google Scholar]
- Luettig B, Steinmüller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. JNCI. 1989;81(9):669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mahla RS, Reddy MC, Prasad DVR, Kumar H (2013) Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol 4(248) 10.3389/fimmu.2013.00248 [DOI] [PMC free article] [PubMed]
- Manayi A, Vazirian M, Saeidnia S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn Rev. 2015;9(17):63–72. doi: 10.4103/0973-7847.156353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Saito K, Maruyama H, Inoue M, Yamashita T, Ishida T, Gu Y. Antioxidant and immuno-enhancing effects of Echinacea purpurea. Biol Pharm Bull. 2004;27:1004–1009. doi: 10.1248/bpb.27.1004. [DOI] [PubMed] [Google Scholar]
- Muñoz-Carrillo JL, Castro-García FP, Chávez-Rubalcaba F, Chávez-Rubalcaba I, Martínez- Rodríguez JL, Hernández-Ruiz ME (2018) Immune System Disorders: Hypersensitivity and Autoimmunity, Immunoregulatory Aspects of Immunotherapy. In: Shamsadin S (ed) Immunoregulatory Aspects of Immunotherapy, Athari. 10.5772/intechopen.75794.
- Nagoor Meeran MF, Javed H, Sharma C, Goyal SN, Kumar S, Jha NK, Ojha S (2019) Can Echinacea be a potential candidate to target immunity, inflammation, and infection - The trinity of coronavirus disease. Heliyon 7(2). 10.1016/j.heliyon.2021.e05990 [DOI] [PMC free article] [PubMed]
- Nugraha RV, Ridwansyah R, Ghozali M, Khairani AF, Atik N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: A Review of Their Mechanisms, Pros and Cons. Evid Based Complement Alternat Med. 2020;1–12:2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee MS, Jung S, Lee S, Kwon O, Kreuter MH, Perrinjaquet-Moccetti T, Min B, Yun SH, Kim Y. Echinacea purpurea protects against restraint stress-induced immunosuppression in BALB/c mice. J Med Food. 2018;2:261–268. doi: 10.1089/jmf.2017.4073. [DOI] [PubMed] [Google Scholar]
- Pawlaczyk I, Czerchawski L, Kuliczkowski W, Karolko B, Pilecki W, Witkiewicz W, Gancarz R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb Res. 2011;127(4):328–340. doi: 10.1016/j.thromres.2010.11.031.11.031. [DOI] [PubMed] [Google Scholar]
- Percival SS. Use of Echinacea in Medicine. Biochem Pharmacol. 2000;60:155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- Rininger JA, Kickner S, Chigurupati P, McLean A, Franck Z. Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J Leukoc Biol. 2000;68(4):503–510. doi: 10.1189/jlb.68.4.503. [DOI] [PubMed] [Google Scholar]
- Saluk-Juszczak J, Pawlaczyk I, Olas B, Kołodziejczyk J, Ponczek M, Nowak P, Tsirigotis-Wołoszczak M, Wachowicz B, Gancarz R. The effect of polyphenolic-polysaccharide conjugates from selected medicinal plants of Asteraceae family on the peroxynitrite-induced changes in blood platelet proteins. Int J Biol Macromol. 2010;47:700–705. doi: 10.1016/j.ijbiomac.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Saluk-Juszczak J, Olas B, Nowak P, Wachowicz B, Bald E, Głowacki R, Pawlaczyk I, Gancarz R. Extract from Conyza canadensis as a modulator of plasma protein oxidation induced by peroxynitrite in vitro. Cent Eur J Biol. 2010;5:800–807. doi: 10.2478/s11535-010-0065-6. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;14:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006;6(3):317–333. doi: 10.1016/j.inti.mp.2005. [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad M, Mnayer D, Morais-Braga MFB, Carneiro JNP, Bezerra CF, Coutinho HDM, Salehi B, Martorell M, del Mar CM, Soltani-Nejad A, Uribe YAH, Yousaf Z, Iriti M, Sharifi-Rad J. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phyther Res. 2018;32(9):1653–1663. doi: 10.1002/ptr.6101. [DOI] [PubMed] [Google Scholar]
- Sohn SH, Ko E, Oh BG, Kim J, Choi E, Kim SH, Kim Y, Shin M, Hong M, Bae H. Global gene analysis of Erigeron canadensis-treated TNF-alpha-, IL-4- and IL-1beta-stimulated A549 human epithelial cells. Ann Nutr Metab. 2009;54(3):227–235. doi: 10.1159/000225378. [DOI] [PubMed] [Google Scholar]
- Stimpel M, Proksch A, Wagner H, Lohmann-Matthes ML. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun. 1984;46(3):845–849. doi: 10.1128/IAI.46.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka H, Glinkowska G. Studies on the chemistry of Erigeron canadensis. Part 1. Herba Pol. 1981;27:201–212 . [Google Scholar]
- Šutovská M, Capek P, Kazimierová I, Pappová L, Jošková M, Matulová M, Fraňová S, Pawlaczyk I, Gancarz R. Echinacea complex-chemical view and anti-asthmatic profile. J Ethnopharmacol. 2015;175:163–171. doi: 10.1016/j.jep.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Šutovská M, Kocmálová M, Mažerik J, Pawlaczyk-Graja I, Gancarz R, Capek P. Chemical characteristics and significant antitussive effect of the Erigeron canadensis polyphenolic polysaccharide-protein complex. J Ethnopharmacol. 2022;284:114754 . doi: 10.1016/j.jep.2021.114754. [DOI] [PubMed] [Google Scholar]
- Veres K, Csupor-Löffler B, Lázár A, Hohmann J (2012) Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Scientific World Journal 489646. 10.1100/2012/489646 [DOI] [PMC free article] [PubMed]
- Weaver SE. The biology of Canadian weeds. 115. Conyza canadensis. Can J Plant Sci. 2001;81(4):867–875. doi: 10.4141/P00-196. [DOI] [Google Scholar]
- Wiesner J, Knöss W. Herbal medicinal products in pregnancy - which data are available? Reprod Toxicol. 2017;72:142–152. doi: 10.1016/j.reprotox.2017.06.046. [DOI] [PubMed] [Google Scholar]
- Yang G, Li K, Liu C, Peng P, Bai M, Sun J, Li Q, Yang Z, Yang Y, Wu H (2018) A Comparison of the Immunostimulatory Effects of Polysaccharides from Tetraploid and Diploid Echinacea purpurea. BioMed Res Intern ID 8628531, 12 pages. 10.1155/2018/8628531 [DOI] [PMC free article] [PubMed]
- Zbikowska HM, Szejk M, Saluk J, Pawlaczyk-Graja I, Gancarz R, Olejnik AK. Polyphenolic-polysaccharide conjugates from plants of Rosaceae/Asteraceae family as potential radioprotectors. Int J Biol Macromol. 2016;86:329–337. doi: 10.1016/j.ijbiomac.2016.01.090. [DOI] [PubMed] [Google Scholar]