Abstract
Objective:
Understanding the minimum infective dose is significant for risk assessment in the performance of suitable infection control strategies in healthcare centers. However, the literature lacks strong evidence regarding this value for severe acute respiratory syndrome coronavirus 2. Therefore, the aim of this study was to investigate the minimum infectious dose of coronavirus disease 2019.
Methods:
We searched the databases of PubMed, Scopus, Web of Science, and Cochrane and retrieved all the relevant literature by 25 July 2021. The records were downloaded into the EndNote software and underwent title/abstract and full-text screenings. A summary of included studies was organized into tables for further analysis, interpretation, and drafting of the results.
Results:
Nineteen studies including the laboratory data on human and animal hosts were selected based on the eligibility criteria. All the literature reported on the infective dose, particularly in humans. The main methods for measurement of infection were through tissue culture infectious dose (TCID50) and counting plaque-forming units. The range of minimum infective was 1.26–7 × 106.25 PFU.
Conclusion:
In this study, we have presented a range of minimum infective doses in humans and various animal species. Such numbers can possibly vary between the individuals based on numerous demographic, immunologic, or other factors.
Keywords: Coronavirus disease 2019, minimum infective dose, severe acute respiratory syndrome coronavirus 2
Introduction
The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) disease as a global public health emergency on 30 January 2020.1,2 COVID-19 started its devastating trajectory into a global pandemic in Wuhan, China.3 –5 COVID-19 spread rapidly around the world. Globally, as of 28 March 2022, more than 480 million confirmed cases and 6.1 million deaths due to COVID-19 were reported to the WHO. 6 Most patients with COVID-19 are asymptomatic or show mild symptoms such as fever, upper respiratory tract symptoms, shortness of breath, and diarrhea, but the more severe cases can lead to pneumonia, multiple organ failure, and death. 7
The newly emerged virus that caused the COVID-19 was named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) on 11 February 2020. 8 Coronaviruses are a large family of single-stranded RNA viruses that infect animals and humans and cause respiratory, gastrointestinal, liver, and neurological diseases.
Coronaviruses are further divided into four genera: alpha-coronavirus, beta-coronavirus, gamma-coronavirus, and delta-coronavirus. 9
There were six most identified coronaviruses before 2019 that affected the humans: (1) human coronavirus 229E (HCoV-229E), (2) human coronavirus OC43 (HCoV-OC43), (3) severe acute respiratory syndrome coronavirus (SARS-CoV), (4) human coronavirus NL63 (HCoV-NL63), (5) human coronavirus HKU1 (HCoV-HKU1), and (6) Middle East respiratory syndrome coronavirus (MERS-CoV).
The first four are recognized as endemic (that is, these are associated with mild and self-limiting diseases), the last two cases lead to severe illness and death. The seventh strain of the corona virus is SARS-CoV-2, also called COVID-19. This strain causes severe disease and respiratory infections in humans and is widespread. 10
The SARS-CoV-2 genome has great sequence similarity (89%–96.3%) with two bat coronaviruses, bat-SLCoVZC45 and bat-SL-CoVZXC21, and 79%–82% with that of human SARS-CoV. The genome consists of 14 kilo bases that encode 27 proteins. The 5′ terminus encoding for 15 nonstructural proteins collectively involved in virus replication and possibly in immune evasion. The 3′ terminus of the genome encodes for structural and accessory proteins. 11 Dose means the number of particles to cause a detectable infection. For understanding viral pathogenicity, determining the number of particles that trigger infection is crucial. Low infectious doses mean that the organism is highly contagious from person to person through contact with infected surfaces. In a US study, isolation of SARS-CoV-2 from a sample of the oropharynx and nasopharynx, one patient and inoculation into Vero cells showed that SARS-CoV-2 proliferated rapidly, reaching 105 TCID50/mL within 24 h. 12
SARS-CoV-2 infection requires a minimal dose of infection because lower doses can be safe. The minimum infectious dose indicates how much virus has entered the body and caused the infection. To determine the pattern of transmission, we need the minimum infectious dose of the virus. Given the importance and necessity of the topic, we aimed to review the literature on the minimum infective dose of SARS-CoV-2 to identify what was the lowest range of SARS-CoV-2 dose that caused the COVID-19 in the related studies.
Methods
This review study was designed to encompass all the evidence related to the infective dose of COVID-19 that has been published by 25 July 2021. The authors aimed to investigate the minimum infective dose of novel coronavirus (SARS-CoV-2) and to ensure the reliability and validity of the results, the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist was applied (Supplemental Material 1).
Data sources
The relevant literature was retrieved by a systematic search of the keywords on the online databases comprising PubMed, Web of Science, Scopus, and Cochrane, we extracted all pertinent records published from December 2019 to 25 July 2021. The search strategy contemplated numerous keyword combinations that were identified through the MeSH (medical subject headings) database or previous articles. All the search strategies were recorded in the Supplemental Material 2, but the final search for PubMed is presented below in the query [C] (all the keywords were searched as title/abstract):
A. “COVID-19” OR “SARS-CoV-2” OR “SARS-CoV2” OR “2019-nCoV” OR “Novel Coronavirus.”
B. “Infective dose” OR “Infectious dose” OR “Minimum infective dose” OR “Minimum infectious dose” OR “Minimal infective dose” OR “minimum viral load” OR “minimum infectious viral load” OR “minimum infective level” OR “minimum infectious level” OR “Tissue culture infectious dose” OR “Plaque forming unit.”
C. [A] AND [B].
Study selection
Three independent researchers screened the retrieved studies and selected that serve the objectives of the present review by titles and abstracts. Later, the full texts of these articles were inspected carefully and based on the eligibility criteria, the most relevant studies were included in the qualitative synthesis. We included the original studies (including laboratory, animal, and human studied) that assessed the COVID-19 minimum infective dose. We also included the pre-prints as the number of studies on this matter is limited.
As part of the eligibility protocol, we deemed the exclusion criteria as below:
Non-original studies, including review articles and meta-analyses.
Publications with inaccessible full texts, conference abstracts, and abstract articles.
Ongoing clinical trials that lack published results.
Suspicion of data duplication, for example, two studies reporting the results of a single research
Data extraction
A summary of findings and minimum infective dose of COVID-19 were prepared and included in two tables by five researchers. The first table describes the characteristics of the studies and participants including first author information, type of study (e.g. experimental), publication year, country of origin, study population, age and gender, signs and symptoms, comorbidities, and laboratory data. The second table addresses host type, sampling site and method, mode of transmission, minimum infective dose, symptomatic cases (%), and clinical outcomes (%). Ultimately, other co-authors double-checked each of the selected publications to avoid potential duplications or overlaps, if any exist in the results.
Quality/risk of bias assessment
We used the Newcastle–Ottawa scale (NOS) to assess the quality of the included studies. 13 A maximum score of 9 will be allocated to the studies based on three categories of selection, comparability, and exposure/outcome.
Statistical analysis
As this study is a systematic review and not a meta-analysis, we did not aim to conduct any statistical analysis.
Results
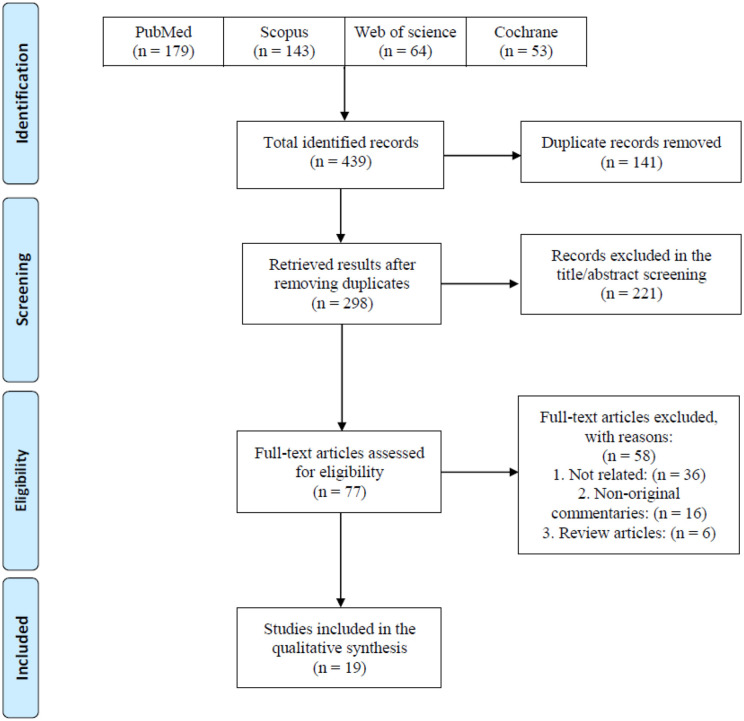
Following a systematic search on online databases, 439 total records were identified. After duplicate removal and title/abstract screening, 298 and 77 of them remained, respectively. Finally, 19 eligible studies including human and animal subjects were included to assess the minimum infective dose, route of transmission, signs and symptoms, and outcomes of COVID-19 infection (Figure 1). Studies were conducted in six countries. Most of the studies were from the United States (n = 8), followed by China (n = 6) and Canada (n = 2). The majority of studies were experimental (n = 13), and some of them were human studies (n = 6). Experimental studies were conducted on golden Syrian hamsters (n = 5), mice (n = 3), African green monkeys (n = 3), Rhesus macaques (n = 1), and ferrets (n = 1) (Table 1).
Figure 1.
PRISMA flow diagram of the study selection process.
Table 1.
Characteristics of the studies and participants.
| ID | First author (reference) | Type of study | Publication year | Country | Study population | Age | Gender (%) | Sign and symptoms | Laboratory data |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bao et al. 14 | Experimental (animal study) | 2020 | China | 34 transgenic mice | 6–11 months old | Male/female | Weight loss, interstitial pneumonia, and virus replication in the lungs | N/A |
| 2 | Bao et al. 15 | Experimental (animal study) | 2020 | China | 23 transgenic mice | 4–6 months old | Male/female | Weight loss | Based on serological analyses, 10 out of 23 mice were infected after direct contact and respiratory droplets exposure |
| 3 | Basu 16 | Cross-sectional | 2021 | USA | 2 SARS-CoV-2 positive | 49 (37 and 61 years old) | Female (100) | N/A | N/A |
| 4 | Bullard et al. 17 | Cross-sectional | 2021 | Canada | 90 SARS-CoV-2-positive samples | 45 | Male (49) Female (51) |
N/A | N/A |
| 5 | Bullard et al. 18 | Case series | 2021 | Canada | 305 COVID-19 patients (97 children aged ⩽ 10 years, 78 children aged 11-17 years, 130 adults) |
N/A | N/A | N/A | Culture growth in 31% of samples was seen; 19% in young children, 23% in older children, and 44% in adults |
| 6 | Chan et al. 19 | Case series | 2020 | China | 273 Specimens from 15 SARS-CoV-2-positive patients | N/A | N/A | N/A | 28.2% were COVID-19-RdRp/Hel and RdRp-P2 positive. All of them were positive for COVID-19-RdRp/Hel assay |
| 7 | Cross et al. 20 | Experimental (animal study) | 2020 | USA | 6 African green monkeys | N/A | N/A | Declined appetite, increased temperature, hypercapnia (3/6), gas distinction of small intestines. All monkeys had pulmonary consolidation with hyperemia and hemorrhage. Three of them had multifocal neutrophilicbroncho-interstitial pneumonia | Lymphocytopenia (5/6), thrombocytopenia (3/6 animals), granulocytosis (6/6), mild increase in ALT (2/6), a moderate increase in CRP (5/6), prolonged aPTT (6/6). SARS-CoV-2 vRNA was detected in the nasal swabs of all animals |
| 8 | Deng et al. 21 | Experimental (animal study) | 2020 | China | 5 Rhesus macaques | 3-5 years old | Male (100) | Opaque glass signs, obscure lung marking, severe progressive pulmonary infiltration, patchy lesions, increased density, and interstitial pneumonia appeared in the lungs | Viral load detected in nasal and throat swabs of CJ- and IT-inoculated animals from 1 to 6 days’ post-inoculation. Only in CJ-inoculated animals, viral load detected in conjunctival swabs and only in IT-inoculated animals is viral load detected in anal swabs |
| 9 | Dhakal et al. 22 | Experimental (animal study) | 2021 | USA | Golden Syrian hamsters | 8–10 week | Male/female | Bodyweight loss, lung injury | Females had two-fold greater IgM, IgG, and IgA responses and significantly greater IgG antibodies against the virus |
| 10 | Johnston et al. 23 | Experimental (animal study) | 2021 | USA | 3 naïve African green monkeys 4rhesus Macaques 4cynomolgus macaques |
N/A | N/A | Fever, lung opacity, mild hypoxia and erythema around the eyes, rectal bleeding | Lymphocyte and neutrophil fluctuations were noted for most animals on the study and were likely a result of the inflammatory response to infection |
| 11 | Kumar et al. 24 | Experimental (animal study) | 2021 | USA | Male Syrian golden hamsters | N/A | N/A | N/A | N/A |
| 12 | Rathnasinghe et al. 25 | Experimental (animal study) | 2020 | USA | 2 hACE2 mice | 6 weeks old | Female (100) | In K18-hACE2 mice: 75% body weight loss, lethargy, ruffled fur, hunched posture, and labored breathing | Rathnasinghe et al.
25
In K18-hACE2 mice: viral replication in the spleen and the lungs. Viral titers in the lung were 2–3 log higher than Ad-HaCE2 mice |
| 13 | Rosenke et al. 26 | Experimental (animal study) | 2020 | USA | 24 Syrian hamsters | 4–6 weeks old and >27 weeks old | Male/female | Moderate broncho-interstitial pneumonia, along with high viral load in lungs and extensive virus shedding. Minor changes in respiratory pattern, lung lesions, loss of <10% body weight | In oral and rectal swabs high levels of viral gRNA were detected. On day 11 after infection, all oral swabs were positive and suggested that viral replication in upper respiratory areas was still ongoing |
| 14 | Ryan et al. 27 | Experimental (animal study) | 2021 | UK | 18 ferrets (three groups, every 6 ferrets) | N/A | Male/Female | In all ferrets of medium and high group: mild multifocal bronchopneumonia | In all ferrets of the medium and high group and one ferret of low group: Viral RNA shedding in the upper respiratory tract |
| 15 | Sia et al. 28 | Experimental (animal study) | 2020 | China | Golden hamsters | 4–5 weeks | Male (100) | Pneumocyte hyperplasia and weight loss | N/A |
| 16 | Song et al. 29 | Experimental (animal study) | 2021 | China | 18 hamsters | 12 weeks | Male/female | N/A | N/A |
| 17 | Van der Moeren et al. 30 | Cross-sectional | 2021 | Netherland | 248 (74 qRT-PCR positives, 174 qRT-PCR negatives) clinical combined oro-nasopharyngeal samples of individuals with COVID-19-like symptoms | N/A | N/A | Rhinitis, cough, elevated temperature, shortness of breath, or sudden loss of sense of taste or smell | DAA result: positive = 54 (Ct-value 12.2–27.7), negative = 20 (Ct-value 22.6–39.5) |
| 18 | Woolsey et al. 31 | Experimental (animal study) | 2020 | USA | 6 adult African green monkeys | N/A | N/A | Decreased appetite, fever, and hyperemia multifocal lesions of each lobe. The small intestine was somewhat loose and dilated with gas and yellow fluid. Mild lymphoid enlargement. Pulmonary edema and pulmonary hemorrhage. Pneumonia. | N/A |
| 19 | Yamayoshi et al. 32 | Case series | 2020 | Japan | SARS-CoV-2-positive samples | N/A | N/A | N/A | N/A |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; qRT-PCR: quantitative real-time polymerase chain reaction; ALT: alanine aminotransferase; CRP: C-reactive protein; aPTT: activated partial thromboplastin time; CJ: conjunctivally; IT: intratracheally.
The main methods for reporting the infective dose were through tissue culture infectious dose (TCID50) and by counting plaque-forming units (PFU). 33
In TCID50, the viral dose in 5% of inoculated tissue culture made pathological changes or cell death. PFU is estimated of viral concentration in plaque-forming units by measuring the number of particles that form a plaque. 34 The minimum infective doses have been summarized in Table 2.
Table 2.
Minimum infective dose of the subjects and related details of the studies.
| ID | First author (reference) | Host | Sampling site and method | Mode of transmission | Minimum infective dose | Symptomatic cases (%) | Clinical outcome (%) |
|---|---|---|---|---|---|---|---|
| 1 | Bao et al. 14 | tgMice | RT-qPCR, heart, liver, spleen, lung, kidney, brain, intestine, and testis | IN | 70,000 PFU | 3 | All were alive at the end of the study |
| 2 | Bao et al. 15 | hACE2 mice | Throat and anal swab | Aerosol | 630 PFU | 39 | All were alive at the end of the study |
| 3 | Basu 16 | Human | Nasopharyngeal swab. Computed tomography |
Droplet | 330 PFU | 100 | Both of them were alive |
| 4 | Bullard et al. 17 | Human | RT-PCR Nasopharyngeal (NP) or endotracheal (ETT) |
Tissue culture | 197 PFU | 28.9 | N/A |
| 5 | Bullard et al. 18 | Human | RT-PCR Nasopharyngeal swabs |
N/A | Children aged ⩽ 10 years = 221 PFU Children 11–17 years = 125 PFU Adults = 820 PFU |
75.4 | All were alive at the end of the study. Children had lower live virus growth, higher cycle thresholds, and lower viral concentration in comparison with adults, so children are not the main carriers of infection. Children aged ⩽10 years were more likely to be asymptomatic than others. |
| 6 | Chan et al. 19 | Human | RT-qPCR Nasopharyngeal aspirate/swabs, throat swab, a sputum specimen |
N/A | 1.26 PFU in vitro in the COVID-19-RdRp/Hel assay | N/A | N/A |
| 7 | Cross et al. 20 | African green monkey | Blood and mucosal swabs | IN | 2,800,000 PFU | 100 | All alive |
| 8 | Deng et al. 21 | Rhesus macaques | Nasal, throat, conjunctival, and anal swabs | CJ, IT, IG | 700,000 PFU | 100 | All alive. All of them are infected via CJ and IT routes but not the IG route. |
| 9 | Dhakal et al. 22 | Golden Syrian hamsters | Blood, nasal turbinate, trachea, and lung samples. Antibody and cytokine ELISA, computed tomography (CT), and qPCR | IN | 70,000 PFU | 100 | All were alive at the end of the study. Male experienced greater morbidity, greater body mass loss, more extensive pneumonia, and recovery slower than females |
| 10 | Johnston et al. 23 | Monkey Rhesus Macaques Cynomolgus macaques |
qRT-PCR Oropharyngeal (OP) and nasopharyngeal (NP), and rectal swabs |
Airborne | 38,400 PFU | 100 | N/A |
| 11 | Kumar et al. 24 | Syrian golden hamsters | N/A | IN | 10,000,000 PFU | 100 | N/A |
| 12 | Rathnasinghe et al. 25 | Mice | qRT-PCR: Lung samples |
Injection | 10,000 PFU | 50 | In K18-hACE2 mice: dead |
| 13 | Rosenke et al. 26 | Hamsters | qRT-PCR: Blood samples and oral and rectal swabs |
IN | 700 PFU | 100 | All were alive at the end of the study |
| 14 | Ryan et al. 27 | Ferrets | RT-qPCR: Nasal washes, throat, and rectal swabs |
IN | Low: 500 PFU Medium: 50,000 PFU High: 5,000,000 PFU |
72 | All were alive at the end of the study |
| 15 | Sia et al. 28 | Hamster | RT-PCR: Nasopharyngeal aspirate and throat swab |
Aerosols | 7 × 106.25 PFU | 50 | N/A |
| 16 | Song et al. 29 | Hamster | qRT-PCR: Throat swabs |
IN | 700,000 PFU | SARS-CoV-2-infected but not mock-infected animals exhibited progressively body weight loss from 1 to 9 dpi. The infected animals exhibited severe weight loss at 5 days dpi (8.91%), which peaked at 9 dpi (18.02%), then gradually regained their weight by 14 dpi (5.04%) | Alveolar damage, involvement of the spleen, lymph nodes, different segments of the alimentary tract, kidney, adrenal gland, ovary, vesicular gland and prostate damage, gallbladder, myocardium, and lymph nodes. All the infected hamsters displayed severe systemic inflammatory responses |
| 17 | Van der Moeren et al. 30 | Human | qRT-PCR: Combined oropharyngeal and nasopharyngeal flocked swab |
N/A | 364 PFU | 29.9 | N/A |
| 18 | Woolsey et al. 31 | Monkey | RT-qPCR mucosal swabs: BAL whole blood or plaque titration of plasma |
IN, IT | 500,000 PFU | N/A | N/A |
| 19 | Yamayoshi et al. 32 | Human calf | PCR (RT-qPCR): Nasal vestibule swab, nasopharyngeal, tracheal aspirate |
N/A | 75–7500 PFU | N/A | Alveolar damage, involvement of the spleen, lymph nodes, different segments of the alimentary tract, kidney, adrenal gland, ovary, vesicular gland and prostate damage, gallbladder, myocardium, and lymph nodes. All the infected hamsters displayed severe systemic inflammatory responses |
TCID50: tissue culture infectious dose 50; PFU: plaque-forming unit; tgMice: transgenic mice; hACE2: human angiotensin converting enzyme 2; IN: intranasal; IG: intragastric; IO: intraocular; IT: intrathecal; IC: intracerebral; IP: intraperitoneal; CJ: conjunctivally; NR: not reported; DAA: Diasorin SARS-CoV-2 antigen detection assay; BAL: bronchoalveolar lavage.
Human studies on infective dose of SARS-CoV-2
We found no experimental studies that assess the infective dose in human, so we included observational human studies. The minimum infective dose of SARS-CoV-2 causing COVID-19 in humans in assessed cross-sectional and case-series studies was low; in a case-series study that investigated infective dose in 273 specimens from 15 SARS-CoV-2-positive patients, detected minimum infective dose was 1.26 PFU in vitro in the COVID-19-RdRp/Hel assay. 1 In another study, 248 oro-nasopharyngeal samples of COVID-19 individuals were assessed, and infective dose was reported to be 364 PFU. 2 In a case-series study which assessed 97 children 10 years and lower, 78 children aged 11–17 years, and 130 adults, the infective dose in 11–17 years children was lower than two other groups (125 PFU). Children had lower live virus growth, higher cycle thresholds, and lower viral concentration in comparison with adults, so children are not the main carriers of infection. Children aged ⩽10 years were more likely to be asymptomatic than others. 3
Animal studies
Tables 1 and 2 summarize experimental studies of different animals on SARS-CoV-2.
Ferret
Intranasal inoculation of SARS-CoV-2 virus in three level of low (500 PFU), medium (50,000 PFU), and high (5,000,000 PFU) caused symptoms in 72% of ferrets. 27 In all ferrets of medium and high group (n = 12; 66%) that were infected at a dose of 50 000 PFU, and 5,000,000 PFU, respectively, mild multifocal bronchopneumonia was observed. Also, in medium and high groups and one ferret of low group, viral RNA shedding in the upper respiratory tract was observed. At the end of study, all of the ferrets were alive.
Mice
A study on 23 transgenic mice after aerosol inoculation at a dose of 630 PFU of SARS-CoV-2 showed weight loss and 43.5% (n = 10) of them were infected based on serological analyses. 15 Another study on 34 transgenic mice after infection at a dose of 70,000 PFU by intranasal route showed weight loss, interstitial pneumonia, and virus replication in the lungs. 14 In K18-hACE2 mice infected by the intraperitoneal injection at a dose of 10,000 PFU of SARS-CoV-2, 75% body weight loss, lethargy, ruffled fur, hunched posture, and labored breathing. Also, at the end of study, the mice died. In Ad-hACE2 Mice, infected with same route and dose, viral titers at the day 2 post-infection were seen in the lungs. 25
African green monkey
All six African green monkeys exposed to 280,000 PFU by the intranasal route had pulmonary consolidation with hyperemia and hemorrhage. Three of them had multifocal neutrophilicbroncho-interstitial pneumonia. Also, declined appetite, increased temperature, hypercapnia, gas distinction of small intestines showed in three of them. 20 African green monkeys inoculated by combined intrathecal and intranasal routes at a dose of 5.0 × 105 PFU showed decreased appetite, fever, hyperemia multifocal lesions of each lobe, mild lymphoid enlargement, pulmonary edema and pulmonary hemorrhage, pneumonia, and the small intestine was somewhat loose and dilated with gas and yellow fluid. 31 At a dose of 38,400 PFU, they showed fever, lung opacity, and mild hypoxia. 23
Rhesus macaques
After airborne inoculation at a dose of 38,400 PFU macaques showed inflammatory responses like lymphocyte and neutrophil fluctuations, fever, erythema around the eyes, and rectal bleeding. 23 Exposure to higher dose leads to moderate pulmonary symptoms in a group of rhesus macaques infected at a dose of 700,000 PFU by combined intranasal, intrathecal, and conjunctivally routs. 21
Cynomolgus macaques
All cynomolgus macaques infected with SARS-CoV-2 at a dose of 38,400 PFU via airborne presented fever, mild hypoxia, rectal bleeding, and lung opacity. 23
Hamsters
In two groups of hamsters infected with SARS-CoV-2 by intranasal routes at a dose of 700 PFU and 70,000 PFU, respectively, weight loss was shown.22,26 Also, higher dose–infected hamsters presented lung injury, and morbidity, body mass loss, and pneumonia were more severe in male hamsters. 22 All of Syrian golden hamsters infected via intranasal route at a high dose of 10,000,000 PFU showed clinical presentation. 24
The risk of bias assessment also demonstrated an acceptable quality (score of 5 or more) for all the included studies (Table 3).
Table 3.
Newcastle–Ottawa scale (NOS) quality assessment of the study.
| First author | Selection (out of 4) | Comparability (out of 2) | Exposure/outcome (out of 3) | Total (out of 9) |
|---|---|---|---|---|
| Bao et al. 14 | *** | - | *** | 6 |
| Bao et al. 15 | *** | - | *** | 6 |
| Basu 16 | **** | * | *** | 8 |
| Bullard et al. 17 | **** | * | *** | 8 |
| Bullard et al. 18 | *** | * | *** | 7 |
| Chan et al. 19 | *** | * | *** | 7 |
| Cross et al. 20 | *** | - | ** | 5 |
| Deng et al. 21 | **** | - | *** | 7 |
| Dhakal et al. 22 | *** | - | *** | 6 |
| Johnston et al. 23 | *** | - | *** | 6 |
| Kumar et al. 24 | **** | - | ** | 6 |
| Rathnasinghe et al. 25 | *** | - | *** | 6 |
| Rosenke et al. 26 | *** | - | *** | 6 |
| Ryan et al. 27 | **** | - | ** | 6 |
| Sia et al. 28 | *** | - | *** | 6 |
| Song et al. 29 | *** | - | *** | 6 |
| Van der Moeren et al. 30 | *** | * | ** | 6 |
| Woolsey et al. 31 | *** | - | *** | 6 |
| Yamayoshi et al. 32 | *** | * | ** | 6 |
Discussion
SARS-CoV-2, a lately found coronavirus accountable for COVID-19, was first reported in China in late 2019 and then extends rapidly worldwide. 35 In this systematic review, we have aimed to discover the average minimum infective dose of SARS-CoV-2. The range of minimum infective was found to be 1.26–7 × 106.25 PFU. Many of the findings that illustrate the reaction of the immune system against the virus have been experimented on animals such as mice, and the studies on minimum infective dose yield consistent results.36,37 It is vital to study the factors affecting transmissibility and mortality of the COVID-19 to curb the current pandemic, in order to predict the virus behavior in the host body and inform the preventive measure to adjust accordingly, it is essential that we find the average minimum infective dose of SARS-CoV-2. 12 Although, the infective dose differs significantly by virus and the way of administration, for coronavirus mostly hundreds or even more virus particles are needed to begin an infection. 12 Due to the rapid spread of the COVID-19 across the world, there are now more infected cases. Many people have recovered from this viral infection; however, more apprehension of virus biological behaviors is needed to control the virus. 38
One of the factors that determine the spread of the virus is its transmissibility. Finding the minimum infective dose of the virus is one of the steps which bring us closer to measuring the virus transmission power. To our knowledge, minimum infective dose is required to start an infection, because very low dosages of virus can be harmless. To substantiate this theory, the study by Bao et al. 15 showed that SARS-CoV-2 can be experimentally spread among hACE2 mice by close contact or through respiratory droplets, but it is hardly transmitted through aerosol inoculation. This could be justified by the amount of virus in the aerosol, which is less than droplets, in some cases is not even enough to cause infection; hence, a cut-off point for causing an infection known as minimum infective dose for the virus is a very useful indicator for virus infectivity in different exposure occasions. According to Basu, 16 based on a new computational strategy to quantify the infectious dose, it is estimated that the particles needed to infect humans is possibly in the order of hundreds.
A minimum infective dose indicates the amount of the virus that causes infection and reflects on the probability of infection. In line with this hypothesis, Ryan et al. show that there is a relationship between the viral load and the severity of the disease. These findings suggest that the low dose of the virus could only cause mild infection, and even in five of six ferrets did not cause infection. 27 Another method that indicated the number of virus titers is TCID50/mL, particularly it measures the amount of virus needed to kill 50% of tissue culture cells. Bullard et al. stated that the cycle threshold value was highly prognostic of culture positivity. As opposed to this statement, symptoms to test time were not capable of discriminating between cases with positive and negative cultures. 17 This dose-response relationship between the mild and high viral dose group could reflect the relationship between minimum infective dose and disease severity.
Finding the minimum infective dose of the virus can be extremely useful in determining the transmission pattern. This represents itself in inconsistent results across the included studies; similar viral load did not cause the same outcome. This indicates that despite having a similar minimum infective dose, the infection rate could differ so this minimum is not the same across the same population. On the other hand, there are some human studies which have shown some hypothetical infective viral dosages. One of the most well discussed one is the study done by Basu et al., the main goal of which was to evaluate the size of the droplets which have high probability of causing infection. But besides this finding, they also had some points related to the viral load which can cause the infection. They found that the number of virions placing at a closely situated individual’s nasopharynx over the 2.5 h duration approximates to (11/5) virions per minute × 60 min × 2.5 h = 330. They also mentioned some related past studies done on ferrets (which showed 500 as the lower tested limit for the number of virions needed to launch an infection) and on humans but with SARS-CoV (the SARS-CoV dose that correlated to 10% and 50% adverse responses (i.e. illness) was estimated, respectively at 43 and 280 PFU). 16
This controversial point should be discussed in further studies, to find whether there can be a period of minimum infective dose or not, so the idea of minimum infective dose would be useful in determining the transmission of the disease.
This study has several limitations. First, we did not aim to conduct statistical analysis as the articles were limited, and the data and their ways of presentation were heterogeneous. Moreover, we had to include the pre-print studies (e.g. on bioRxiv) because of the limited number of studies and the existing evidence, and this problem could cause some studies that are not peer-reviewed and may have some errors in methodology and results, to leak into our data. Many of the studies were also laboratory or animal studies so the average minimum infective dose could vary from the actual human circumstances. Therefore, future studies are required to address the shortcomings of our study and to compensate for the lack of information regarding human models.
Conclusion
The results of this review suggest that one of the key factors to control the pandemic could be the study of virus transmission. The minimum infective dose is one of the main components of virus transmission. In this study, we have presented a range of minimum infective doses in humans and various animal species, yet such numbers can possibly vary between the individuals based on numerous factors. Measuring the minimum infective dose can provide a clearer overall understanding of the disease and its transmissibility and help better halt its spreading.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221115053 for Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review by SeyedAhmad SeyedAlinaghi, Amirali Karimi, Hengameh Mojdeganlou, Zahra Pashaei, Pegah Mirzapour, Ahmadreza Shamsabadi, Alireza Barzegary, Fatemeh Afroughi, Soheil Dehghani, Nazanin Janfaza, Amirata Fakhfouri, Sepideh Khodaei, Esmaeil Mehraeen and Omid Dadras in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121221115053 for Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review by SeyedAhmad SeyedAlinaghi, Amirali Karimi, Hengameh Mojdeganlou, Zahra Pashaei, Pegah Mirzapour, Ahmadreza Shamsabadi, Alireza Barzegary, Fatemeh Afroughi, Soheil Dehghani, Nazanin Janfaza, Amirata Fakhfouri, Sepideh Khodaei, Esmaeil Mehraeen and Omid Dadras in SAGE Open Medicine
Acknowledgments
This study was conducted in collaboration with Khalkhal University of Medical Sciences, and Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences.
Footnotes
Author contributions: All the authors have read and approved the final version of the article. The conception and design of the study were performed by E.M. and S.S. Methodology was given by E.M., S.S., and A.K. Screening of the articles and acquisition of data were done by Z.P., P.M., N.J., H.M., S.D., F.A. Writing—original draft preparation was by A.B., A.F., S.K., A.K., and A.S. Writing—review & editing was by S.S. and O.D. Validation E.M., O.D., and S.S.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nazanin Janfaza  https://orcid.org/0000-0002-0990-620X
https://orcid.org/0000-0002-0990-620X
Esmaeil Mehraeen  https://orcid.org/0000-0003-4108-2973
https://orcid.org/0000-0003-4108-2973
Omid Dadras  https://orcid.org/0000-0001-9385-2170
https://orcid.org/0000-0001-9385-2170
Supplemental material: Supplemental material for this article is available online.
References
- 1. Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis 2020; 96: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliaei S, SeyedAlinaghi S, Mehrtak M, et al. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur J Med Res 2021; 26(1): 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. SeyedAlinaghi S, Mehrtak M, MohsseniPour M, et al. Genetic susceptibility of COVID-19: a systematic review of current evidence. Eur J Med Res 2021; 26(1): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehraeen E, Dadras O, Afsahi AM, et al. Vaccines for COVID-19: a systematic review of feasibility and effectiveness. Infect Dis Drug Targ 2022; 22(2): e230921196758. [DOI] [PubMed] [Google Scholar]
- 5. Sheikhbahaei E, Mirghaderi SP, Moharrami A, et al. Incidence of symptomatic COVID-19 in unvaccinated patients within one month after elective total joint arthroplasty: a multicenter study. Arthroplast Today 2022; 14: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO coronavirus (COVID-19) dashboard, 2021, https://covid19.who.int/
- 7. Park M, Cook AR, Lim JT, et al. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med 2020; 9(4): 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2021; 54(1): 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu D, Wu T, Liu Q, et al. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis 2020; 94: 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu K, Gu Z, Islam MS, et al. Global landscape of patents related to human coronaviruses. Int J Biol Sci 2021; 17(6): 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abduljalil JM, Abduljalil BM. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes New Infect 2020; 35: 100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karimzadeh S, Bhopal R, Nguyen Tien H. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-COV-2: comparison with other respiratory viruses. Epidemiol Infect 2021; 149: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perera RA, Mok CK, Tsang OT, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill 2020; 25(16): 2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bao L, Deng W, Huang B, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020; 583(7818): 830–833. [DOI] [PubMed] [Google Scholar]
- 15. Bao L, Gao H, Deng W, et al. Transmission of severe acute respiratory syndrome coronavirus 2 via close contact and respiratory droplets among human angiotensin-converting enzyme 2 mice. J Infect Dis 2020; 222(4): 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basu S. Computational characterization of inhaled droplet transport to the nasopharynx. Sci Rep 2021; 11(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; 71(10): 2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bullard J, Funk D, Dust K, et al. Infectivity of severe acute respiratory syndrome coronavirus 2 in children compared with adults. CMAJ 2021; 193(17): E601–E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan JFW, Yip CCY, To KKW, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 2020; 58(5): e00310–e00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cross RW, Agans KN, Prasad AN, et al. Intranasal exposure of African green monkeys to SARS-CoV-2 results in acute phase pneumonia with shedding and lung injury still present in the early convalescence phase. Virol J 2020; 17(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng W, Bao L, Gao H, et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 2020; 11(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhakal S, Ruiz-Bedoya CA, Zhou R, et al. Sex differences in lung imaging and SARS-CoV-2 antibody responses in a COVID-19 golden Syrian hamster model, 2021, https://www.biorxiv.org/content/10.1101/2021.04.02.438292v1.full.pdf+html [DOI] [PMC free article] [PubMed]
- 23. Johnston SC, Ricks KM, Jay A, et al. Development of a coronavirus disease 2019 nonhuman primate model using airborne exposure. PLoS ONE 2021; 16(2): e0246366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar R, Kolloli A, Subbian S. Inactivation and elimination of SARS-CoV-2 in biosamples using simple fixatives and ultrafiltration. Meth Protoc 2021; 4(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rathnasinghe R, Strohmeier S, Amanat F, et al. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 2020; 9(1): 2433–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenke K, Meade-White K, Letko M, et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg Microbes Infect 2020; 9(1): 2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan KA, Bewley KR, Fotheringham SA, et al. Dose-dependent response to infection with SARS-CoV-2 in the ferret model: evidence of protection to re-challenge, 2020, https://www.biorxiv.org/content/10.1101/2020.05.29.123810v1 [DOI] [PMC free article] [PubMed]
- 28. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020; 583(7818): 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song Z, Bao L, Yu P, et al. SARS-CoV-2 causes a systemically multiple organs damages and dissemination in hamsters. Front Microbiol 2021; 11: 618891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Moeren N, Zwart VF, Goderski G, et al. Performance of the Diasorin SARS-CoV-2 antigen detection assay on the LIAISON XL. J Clin Virol 2021; 141: 104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woolsey C, Borisevich V, Prasad AN, et al. Establishment of an African green monkey model for COVID-19, 2020, https://pubmed.ncbi.nlm.nih.gov/32511377/ [DOI] [PMC free article] [PubMed]
- 32. Yamayoshi S, Sakai-Tagawa Y, Koga M, et al. Comparison of rapid antigen tests for covid-19. Viruses 2020; 12(12): 1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ward RL, Akin EW, D’Alessio DJ. Minimum infective dose of animal viruses. Crit Rev Environ Control 1984; 14(4): 297–310. [Google Scholar]
- 34. Carter J, Saunders V, Saunders VA. Virology: principles and applications. Hoboken, NJ: John Wiley & Sons, 2007. [Google Scholar]
- 35. SeyedAlinaghi S, Karimi A, MohsseniPour M, et al. The clinical outcomes of COVID-19 in HIV-positive patients: a systematic review of current evidence. Immun Inflamm Dis 2021; 9(4): 1160–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. SeyedAlinaghi S, Mirzapour P, Dadras O, et al. Characterization of SARS-CoV-2 different variants and related morbidity and mortality: a systematic review. Eur J Med Res 2021; 26(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karimi A, Shobeiri P, Kulasinghe A, et al. Novel systemic inflammation markers to predict COVID-19 prognosis. Front Immunol 2021; 12: 741061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dadras O, Alinaghi SAS, Karimi A, et al. Effects of COVID-19 prevention procedures on other common infections: a systematic review. Eur J Med Res 2021; 26(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221115053 for Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review by SeyedAhmad SeyedAlinaghi, Amirali Karimi, Hengameh Mojdeganlou, Zahra Pashaei, Pegah Mirzapour, Ahmadreza Shamsabadi, Alireza Barzegary, Fatemeh Afroughi, Soheil Dehghani, Nazanin Janfaza, Amirata Fakhfouri, Sepideh Khodaei, Esmaeil Mehraeen and Omid Dadras in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121221115053 for Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review by SeyedAhmad SeyedAlinaghi, Amirali Karimi, Hengameh Mojdeganlou, Zahra Pashaei, Pegah Mirzapour, Ahmadreza Shamsabadi, Alireza Barzegary, Fatemeh Afroughi, Soheil Dehghani, Nazanin Janfaza, Amirata Fakhfouri, Sepideh Khodaei, Esmaeil Mehraeen and Omid Dadras in SAGE Open Medicine