Abstract
MarR negatively regulates expression of the multiple antibiotic resistance (mar) locus in Escherichia coli. Superrepressor mutants, generated in order to study regions of MarR required for function, exhibited altered inducer recognition properties in whole cells and increased DNA binding to marO in vitro. Mutations occurred in three areas of the relatively small MarR protein (144 amino acids). It is surmised that superrepression results from increased DNA binding activities of these mutant proteins.
The chromosomal multiple antibiotic resistance (mar) locus of Escherichia coli controls an adaptational response to antibiotics and other environmental hazards (1). The expression of multiple genes on the E. coli chromosome is regulated by MarA, a transcriptional activator encoded within the marRAB operon (1).
MarR negatively regulates expression of the marRAB operon (6, 15, 20). DNA footprinting experiments suggest that MarR dimerizes at two locations, sites I and II, within the mar operator (marO) (15); site I is positioned among the −35 and −10 hexamers, and site II spans the putative MarR ribosome binding site (see Fig. 1A) (reviewed in reference 1). Many structurally dissimilar chemicals affect MarR activity in whole cells (4, 7, 15, 20). Experiments in vitro demonstrate that MarR binds salicylic acid and, through the use of gel mobility shift assays, that sodium salicylate inhibits the formation of MarR-marO complexes (15). We have extended these initial findings by demonstrating that the DNA binding activity of MarR in vitro is antagonized by several other chemicals (2). Thus, MarR possesses DNA binding and effector molecule recognition properties. In order to study MarR structure and function, we have generated and characterized MarR superrepressor (MarRS) mutants.
FIG. 1.
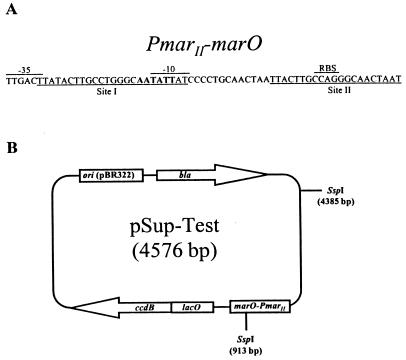
(A) Sequence of PmarII-marO. The locations of the −35 and −10 hexamer sequences and MarR ribosome binding site are indicated, and the SspI restriction enzyme recognition sequence in site I is in boldface. (B) Map of plasmid pSup-Test. Expression of ccdB is positively controlled by the marRAB promoter (PmarII) and negatively regulated by the lac (lacO) and marRAB (marO) operators in the presence of their cognate proteins, LacI and MarR. The positions of the SspI sites within the plasmid are indicated.
Bacterial strains, plasmids, and genetic techniques.
The bacterial strains and plasmids used are listed in Table 1. A low-copy-number wild-type MarR expression vector was constructed by using a modified version of pACT7 (14). marR was amplified by PCR from E. coli AG100 (8) chromosomal DNA by using Taq DNA polymerase in accordance with the manufacturer’s protocols (Life Technologies, Gaithersburg, Md.). EcoRI and PstI restriction sites were incorporated into the forward and reverse primers to facilitate directional cloning into pACT7 in place of the T7 RNA polymerase gene following digestion with EcoRI and PstI. In pAC-MarR (WT), transcription of marR is regulated by the lacP1 promoter and protein synthesis is governed by the wild-type MarR ribosome binding site (AGGG) and translational initiation (GTG) signals (6).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR (φ80lacZ ΔM15) | Laboratory collection |
| BL21(DE3) | F−ompT hsdSB(rB−mB−) gal dcm (DE3) | Novagen |
| SPC105 | MC4100 (ΔlacU169 araD rpsL relA thi flbB) containing a chromosomal PmarII::lacZ fusion at the λ attachment site and a wild-type mar locus | 7 |
| Plasmids | ||
| pACT7 | Low-copy-number T7 RNA polymerase expression vector | 14 |
| pET11d | High-level expression vector (bla lacI) | Novagen |
| pET13a | Kanamycin-resistant version of pET11a | 22 |
| pAC-MarR (WT) | Low-copy-number wild-type MarR expression vector derived from pACT7 | This study |
| pmarO | pET11d derivative lacking lacI and containing PmarII-marO | This study |
| pSup-Test | pET11d derivative lacking lacI and containing a PmarII-marO::-ccdB fusion | This study |
A high-copy-number wild-type MarR expression vector was constructed in pET13a (22), a kanamycin-resistant version of pET11a (Novagen, Madison, Wis.). PCR amplification of marR was performed as described above by using forward and reverse primers containing VspI and BamHI restriction sites to facilitate directional cloning into NdeI/BamHI-digested pET13a. In the resulting plasmid, pMarR-WT, expression of MarR is under the control of the T7 RNA polymerase promoter and a near-consensus ribosome binding site.
For functional analysis of MarR in whole cells, a PmarII-marO::ccdB fusion was created in pET11d (Novagen). After digestion with EcoRV to remove the majority of lacI, pET11d was purified with the Qiagen (Santa Clarita, Calif.) gel purification kit and religated. PmarII-marO, containing the marRAB promoter (PmarII) and operator (marO) sequences (Fig. 1A), was amplified by PCR with primers containing EagI/BsmI restriction sites and blunt-end cloned into EagI/BsmI-digested pET11d (lacking lacI), creating plasmid pmarO. Subsequently, the lacO-ccdB portion of pKIL 18 (5) was amplified by PCR to exclude the tac promoter sequences. XhoI and BsmI restriction sites were incorporated into the forward and reverse primers to facilitate directional cloning downstream of PmarII-marO, into AvaI-digested (mixed cohesive and blunt ends) pmarO. The resulting plasmid was designated pSup-Test (Fig. 1B).
DNA sequence analysis was performed (in-house) with an ABI automated DNA sequencer. Hydroxylamine and nitrosoguanidine mutagenesis of pAC-MarR (WT) were performed in accordance with established protocols (17).
Selection of MarR superrepressors.
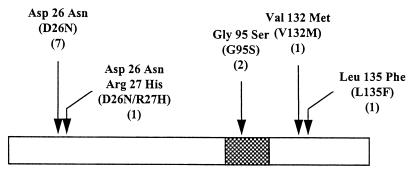
To identify MarR superrepressors, expression of the lethal ccdB gene product on pSup-Test was exploited. Plasmid pAC-MarR (WT) was mutagenized in vitro and transformed into DH5α containing pSup-Test. Transformants were selected in the presence of sodium salicylate, a known marRAB operon inducer (7). Growth of DH5α bearing pET11d or pmarO (pSup-Test lacking ccdB) (Table 1) was unaffected by the highest concentration of this and other inducers tested (Table 2). DH5α cells containing pSup-Test in the absence or presence of plasmid-encoded wild-type marR were nonviable in the presence of sodium salicylate and other inducers (Table 2). However, cells containing a putative MarR superrepressor survived higher concentrations of known marRAB operon inducers, presumably by binding of the mutant protein to marO in front of ccdB on pSup-Test and preventing expression of the lethal gene product (Table 2). From a total of 276 transformants, 12 putative MarR superrepressor mutants were independently isolated (Fig. 2).
TABLE 2.
Survival of DH5α containing pSup-Test and a plasmid bearing wild-type MarR or putative superrepressor MarR mutant on gradient plates containing inducing compounds
| Inducer | Concn (mM)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pET11db | pmarOb | pSup-Testb | Wild-type MarR | MarR mutant
|
|||||
| D26N | D26N/R27H | G95S | V132M | L135F | |||||
| Plumbagin | >0.4 | >0.4 | ≤0.01 | 0.01 | 0.2 | 0.1 | 0.1 | 0.2 | 0.06 |
| 2,4-Dinitrophenol | >0.4 | >0.4 | 0.04 | 0.05 | 0.4 | 0.4 | 0.4 | 0.2 | 0.08 |
| Sodium salicylate | >10 | >10 | ≤0.01 | 0.5 | 7.5 | 6.9 | 6.7 | 5.8 | 2.6 |
| Sodium benzoate | >30 | >30 | 1.4 | 1.4 | 18.9 | 16.7 | 15.8 | 17.8 | 4.4 |
| Menadione sodium metabisulfite | >5 | >5 | 1.6 | 2.8 | 3.5 | 3.6 | 3.5 | 3.1 | 2.7 |
Concentration of the inducer at which growth was inhibited in gradient plates. These values were calculated by measuring the point where growth stopped and dividing it by the total length (90 mm) of the gradient plate. This number was then multiplied by the highest concentration of inducer in the gradient plates, which were as follows: plumbagin, 0.4 mM; 2,4-dinitrophenol, 0.4 mM; sodium salicylate, 10 mM; sodium benzoate, 50 mM; menadione sodium metabisulfite, 5 mM. Results are representative of experiments performed in triplicate.
For details, see Table 1.
FIG. 2.
Locations of the MarR superrepressor mutations identified in this study. The designations in parentheses consist of the single-letter code for the wild-type residue followed by the location of that amino acid in the full-length MarR and the single-letter code for the mutation isolated at this point. The numbers in parentheses represent the number of independent isolates at that site. The cross-hatched box indicates the region of amino acid homology among the MarR family members.
Assay of repressor activity by β-galactosidase.
The trans-dominant nature of the mutant plasmids was then independently retested in E. coli SPC105, a marR+ host which contains a chromosomally located PmarII::lacZ fusion at the λ attachment site (7).
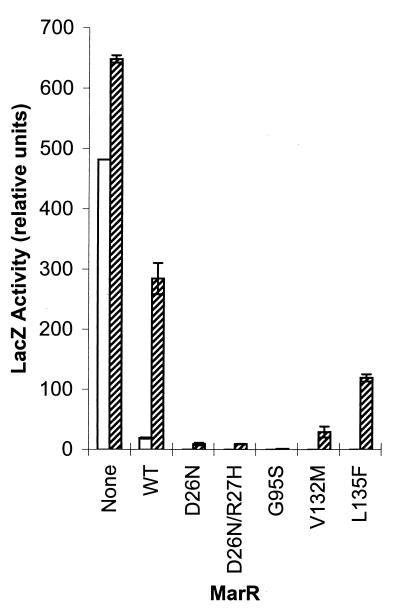
In the absence of exogenously provided wild-type MarR, SPC105 exhibited an easily detectable basal level of LacZ expression (Fig. 3). LacZ expression in cells bearing wild-type marR in trans was minimal and was virtually undetectable in cells containing any of the five putative MarRS mutants (Fig. 3). Sodium salicylate caused a ≥15-fold increase in LacZ expression in cells bearing plasmid-encoded wild-type MarR, while those containing a MarRS mutant displayed greatly reduced responses to this inducer (Fig. 3). The G95S, D26N, D26N/R27H, and V132M mutants showed little if any response, while cells containing the L135F MarRS mutation showed partial responsiveness to the inducer (Fig. 3).
FIG. 3.
MarR repressor activity assayed in the PmarII::lacZ reporter strain E. coli SPC105 (marR+). Cells were grown at 37°C to mid-logarithmic phase in LB broth, without glucose, containing the appropriate antibiotics and IPTG (50 μM) and sodium salicylate (5 mM) where appropriate. β-Galactosidase assays were performed with cells permeabilized with chloroform-SDS as previously described (17, 20). The relative β-galactosidase activities (±standard deviations) in the absence (open bars) or presence (hatched bars) of 5 mM sodium salicylate are presented. Activities were determined for the host strain alone (None), the wild-type MarR (WT), and other MarR proteins that show a superrepressor phenotype (mutations are indicated below each bar).
Properties of MarRS mutants.
DH5α bearing the D26N, D26N/R27H, G95S, or V132M MarR mutant proteins displayed similar decreased susceptibilities to the chemically induced expression of the lethal ccdB gene product as assayed by gradient plates (Table 2). The inducer responsiveness of the L135F MarR mutant-containing cells was less than that of the wild-type control cells but greater than that of cells bearing the other superrepressor mutants (Table 2).
Western blot analysis using MarR polyclonal antibodies, generated in rabbits by using purified MarR (Covance Research Products Inc., Denver, Pa.), showed that cells bearing the D26N, D26N/R27H, and L135F mutants expressed 1.3- to 2.0-fold more protein than did those with the wild-type MarR (data not shown). The intracellular levels of the G95S and V132M mutant proteins were less, 20 and 40%, respectively, of wild-type MarR (data not shown). These results demonstrated that superrepression was not likely attributable to overexpression of the mutant proteins. This point was addressed more clearly by an in vitro DNA binding assay.
The wild-type and marR superrepressor genes were cloned into pET13a and transformed into E. coli BL21(DE3) for overexpression, and the MarR proteins were purified. Cells, grown in Luria-Bertani (LB) broth at 37°C to mid-logarithmic phase, were induced for 3 h with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), collected, washed, and frozen at −70°C. The frozen cell pellet was resuspended in 10 ml of buffer A [50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 2.5 mM 4-(2-aminoethyl)-benzenesulfonylfluoride hydrochloride (AEBSF; serine protease inhibitor) (Sigma, St. Louis, Mo.)], and lysed by sonication. After removal of insoluble matter by centrifugation at 30,000 × g for 1 h, the supernatant was loaded onto a sulphopropyl (SP)-Sepharose HiTrap column (Pharmacia Biotech, Piscataway, N.J.) equilibrated with 50 mM Tris-HCl (pH 7.4). Following a 50 mM Tris-HCl (pH 7.4) wash, MarR was eluted with a linear gradient of 0 to 1 M NaCl in 50 mM Tris-HCl (pH 7.4). Eluting at 0.2 to 0.3 mM NaCl, MarR was dialyzed against 100 volumes of 50 mM Tris-HCl (pH 7.4)–100 mM NaCl–10% glycerol–1 mM phenylmethylsulfonyl fluoride (serine protease inhibitor) overnight at 4°C. Judged to be >90% pure on a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis Coomassie blue-stained gel, MarR was stored in aliquots at −70°C until further use.
A unique SspI recognition sequence within one of two MarR binding sites in marO (Fig. 1A) formed the basis of a restriction enzyme site protection assay (10, 16, 21) to assess MarR binding to marO. Serial dilutions of purified wild-type or MarR superrepressor proteins were added to a final volume of 20 μl containing 0.2 μg of pSup-Test (target DNA, 3.4 nM), 10 mM Tris-HCl (pH 7.5), 5 mM NaCl, 1 mM MgCl2, and 0.0025% Triton X-100. After incubation at room temperature for 10 min, 5 U of SspI (New England Biolabs, Beverly, Mass.) was added and the reaction mixture was incubated for 30 min at 37°C. The incubation was terminated by the addition of 1.5 μl of stop buffer (0.25M EDTA [pH 8.0], 1% SDS) and 5 μl of 6× agarose gel loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol, 30% glycerol) and analyzed on 0.7% agarose (Life Technologies) gels. The point of 50% protection, determined by visual inspection of ethidium bromide-stained DNA in these gels, was assigned a value of 5 U of activity. The specific activity was then calculated from this value as previously described (10).
The D26N, G95S, and L135F superrepressor mutant proteins displayed at least a ∼9-fold-greater DNA binding activity than the wild-type repressor (Table 3). Although the inducer susceptibility profiles of these three mutants were similar in intact E. coli DH5α (Fig. 3 and Table 2), their in vitro DNA binding properties were quite different (Table 3). The DNA binding level of the G95S MarRS mutant was ∼2- and 3.5-fold greater than that of the D26N and L135F mutants, respectively (Table 3).
TABLE 3.
Assay of MarR DNA binding activity by SspI restriction enzyme site protection
| Purified MarR | MarR activity (U/mga) | Fold increase in DNA bindingb |
|---|---|---|
| Wild type | 5,495 | 1 |
| D26N mutant | 89,286 | 16.2 |
| G95S mutant | 166,666 | 30.3 |
| L135F mutant | 48,544 | 8.8 |
Specific activity was determined based on 50% protection of the SspI recognition enzyme sequence in site I of the mar operator (designated as 5 U of activity). Results are representative of experiments performed at least twice.
Relative to DNA binding of the wild-type protein.
The G95S MarRS mutation occurred within a region that is conserved among all members of the MarR family of proteins (Fig. 2), and the mutant protein is 30-fold more active than wild-type MarR. trans-dominant negative complementing MarR mutants that are in proximity to this residue (3, 6, 20) suggest that it may play a more direct role in DNA binding.
The D26N superrepressor mutation results in a charged amino acid being substituted for an uncharged residue. A decrease in electrostatic interactions between the protein and the DNA backbone may be the basis for this superrepressor activity. It is also possible that new hydrogen bonds between the asparagine side chain and the DNA backbone contribute to an increased affinity for DNA. Thus, nonspecific DNA binding is expected to form the basis of superrepression. In the D26N/R27H double mutant, the latter mutation is probably not required since this mutant produced data similar to that of the protein bearing the single D26N change (Fig. 3 and Table 2).
The V132M MarRS mutant showed inducer responses like those of the D26N and G95S mutants in whole cells (Table 2). Since both mutations are expected to lie outside of the putative DNA binding domain of MarR (3), it is speculated that each plays an accessory role in DNA binding. With respect to the L135F mutant, the phenylalanine residue may increase DNA binding through newly acquired interactions with the phosphate backbone (19). The lesion in each mutant may also reside in a region required for proper protein folding, MarR oligomer assembly, or an inducer recognition domain. It is also possible that the superrepressor mutation in these or the other proteins affects transmission of the signal to the DNA binding domain following inducer recognition.
That the MarR superrepressor mutations are scattered throughout the protein suggests that amino acid changes in several regions (Fig. 2) can enhance the DNA binding activity of the repressor (Table 3). Four interspersed missense mutations in TrpR resulted in superrepressor proteins (9, 11, 12), and none displayed altered binding affinities for the corepressor tryptophan (9). Whether enhanced DNA binding is attributable to an increased association or decreased dissociation rate of repressor-operator complex formation or altered DNA complex stoichiometry, as was demonstrated for particular TrpR superrepressors (9, 13), is currently unknown. It is of interest that only one of the TrpR superrepressor mutations lay within the protein’s helix-turn-helix DNA binding domain (18). This finding demonstrates that there is no a priori reason to suspect that a mutation must be confined to the DNA binding domain of MarR in order to result in a superrepressor phenotype.
Acknowledgments
We are grateful to Philippe Bernard for supplying the pKIL vectors.
This work was supported by NIH grant GM 51661.
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;10:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., and S. B. Levy. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli mar locus, by multiple chemicals in vitro. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 3.Alekshun, M. N., and S. B. Levy. Unpublished data.
- 4.Ariza R R, Cohen S P, Bachhawat N, Levy S B, Demple B. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1994;176:143–148. doi: 10.1128/jb.176.1.143-148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard P, Gabant P, Bhassi E M, Couturier M. Positive-selection vectors using the F plasmid ccdB killer gene. Gene. 1994;148:71–74. doi: 10.1016/0378-1119(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S P, Hächler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S P, Levy S B, Foulds J, Rosner J L. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George A M, Levy S B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983;155:531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurlburt B K, Yanofsky C. Enhanced operator binding by trp superrepressors of Escherichia coli. J Biol Chem. 1990;265:7853–7858. [PubMed] [Google Scholar]
- 10.Joachimiak A J, Kelley R L, Gunsalus R P, Yanofsky C, Sigler P B. Purification and characterization of trp aporepressor. Proc Natl Acad Sci USA. 1983;80:668–672. doi: 10.1073/pnas.80.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley R L, Yanofsky C. Mutational studies with the trp repressor of Escherichia coli support the helix-turn-helix model of repressor recognition of operator DNA. Proc Natl Acad Sci USA. 1985;82:483–487. doi: 10.1073/pnas.82.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klig L S, Yanofsky C. Increased binding of operator DNA by trp superrepressor EK49. J Biol Chem. 1988;263:243–246. [PubMed] [Google Scholar]
- 13.Liu Y-C, Matthews K S. trp repressor mutations alter DNA complex stoichiometry. J Biol Chem. 1994;269:1692–1698. [PubMed] [Google Scholar]
- 14.Maneewannakul K, Maneewannakul S, Ippen-Ihler K. Sequence alterations affecting F plasmid transfer gene expression: a conjugation system dependent on transcription by the RNA polymerase of phage T7. Mol Microbiol. 1992;6:2961–2973. doi: 10.1111/j.1365-2958.1992.tb01755.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin R G, Rosner J L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci USA. 1995;92:5456–5460. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melville S B, Gunsalus R P. Isolation of an oxygen-sensitive FNR protein of Escherichia coli: interaction at an activator and repressor sites of FNR-controlled genes. Proc Natl Acad Sci USA. 1996;93:1226–1231. doi: 10.1073/pnas.93.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratories; 1972. [Google Scholar]
- 18.Otwinowski Z, Schevitz R W, Zhang R G, Lawson C L, Joachimiak A, Marmorstein R Q, Luisi B F, Sigler P B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988;335:321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- 19.Schildbach J F, Karzai A W, Raumann B E, Sauer R T. Origins of DNA-binding specificity: role of protein contacts with the DNA backbone. Proc Natl Acad Sci USA. 1999;96:811–817. doi: 10.1073/pnas.96.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seoane A S, Levy S B. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon of Escherichia coli. J Bacteriol. 1995;177:3414–3419. doi: 10.1128/jb.177.12.3414-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith H Q, Somerville R L. The tpl promoter of Citrobacter freundii is activated by the TyrR protein. J Bacteriol. 1997;179:5914–5921. doi: 10.1128/jb.179.18.5914-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]