Abstract
Purpose
The purpose of this study was to identify early changes in choriocapillaris flow in patients with age-related macular degeneration (AMD) with no history of macular neovascularization (MNV).
Methods
Clinical records of fellow eyes of patients with unilateral neovascular AMD without fundus findings and control eyes of otherwise healthy individuals, except for mild cataract, diagnosed at St. Luke's International Hospital from April 2020 to March 2021, were retrospectively analyzed. Optical coherence tomography (OCT) angiography images of the choriocapillaris slab were binarized using the Phansalkar local thresholding methods to evaluate the choriocapillaris flow area (CCFA) and its coefficient of variation (CV).
Results
The data of 24 AMD fellow eyes (17 for men, 71.7 ± 1.9 years old) and 21 control eyes (11 for men, 69.1 ± 2.0 years old) were analyzed. The mean CCFA ratio was lower in the AMD fellow eyes (58.6 ± 1.2%) than in the control eyes (62.4 ± 1.3%, P = 0.032), and the mean CV of CCFA ratio was greater in the AMD fellow eyes (0.174 ± 0.007) than in the control eyes (0.154 ± 0.007, P = 0.032). Eyes with CCFA ratio <60% and CV of CCFA ratio ≥0.154 had a 4.371-fold higher risk of being AMD fellow eyes (95% confidence interval = 1.029–18.56, P = 0.046). Differences in CV of CCFA ratio between AMD fellow eyes and control eyes were particularly clear in eyes with thick choroids (mean CV of CCFA in control versus AMD fellow eyes with central choroidal thickness ≥220 µm: 0.144 ± 0.005 vs. 0.173 ± 0.007, P = 0.009**).
Conclusions
Neovascular AMD fellow eyes without MNV had reduced, heterogeneous, and imbalanced choriocapillaris flow, which may constitute early changes in neovascular AMD, although further studies are required.
Keywords: choriocapillaris, blood flow, optical coherence tomography (OCT) angiography, age-related macular degeneration (AMD)
Age-related macular degeneration (AMD), a leading cause of visual impairment, gradually progresses to macular neovascularization (MNV) and/or chorioretinal atrophy,1,2 defined as late AMD. Early AMD, represented by drusen and/or pigmentary abnormalities, may be found during the course of late AMD development. However, because early AMD lesions are associated with vision impairment1 and irreversible changes,1,3 identifying subclinical changes before the lesions become visible on fundus examination would be of value to delineate the fundamental pathogenesis and develop a screening method for AMD at the very early stage.
Here, we focused on the choriocapillaris of the fellow eyes of patients with neovascular AMD (hereafter, “AMD fellow eyes”). Although AMD is a disease of the components of the photoreceptor/retinal pigment epithelium (RPE)/Bruch's membrane/choriocapillaris complex, loss of the choriocapillaris reportedly occurs in areas with intact RPE in neovascular AMD,4,5 suggesting that choriocapillaris change is a very early lesion that may not be visible on fundus examination. The choriocapillaris is approximately 10-µm thick at the fovea, where the capillaries are most dense, and is composed of a single-layer, hexagonal (or lobular)-shaped domain of fenestrated capillaries that supply oxygen and nutrients to the outer retina.6–8
Choriocapillaris thinning is observed in pachychoroid diseases,8,9 such as central serous chorioretinopathy,10,11 and may cause relative hypoxia.9,10 Choriocapillaris dropout has been observed in the area surrounding the MNV in AMD,4,8,12–14 and flow deficits were also found outside the MNV lesion and were not consistently correlated with MNV lesions.15 Choriocapillaris dysregulation is also seen in early and intermediate AMD5,16,17 and is considered causative of relative hypoxia. Local hypoxia may increase vascular endothelial growth factor (VEGF) expression to contribute to MNV development.18,19 Therefore, choriocapillaris change, which could cause relative hypoxia, may be one of the earliest changes in neovascularization. However, whether AMD lesions, such as MNV and drusen, or choriocapillaris flow deficits occur earlier remains unclear.
Optical coherence tomography angiography (OCTA) enables the visualization of the choriocapillaris with high resolution and less invasiveness compared to fluorescein and indocyanine green angiographies (FA and IA). Because the signal from the structural tissue remains steady, in contrast to the signal from the red blood cells in the flowing blood that changes over time, temporal changes in the OCT signal in subsequent scans can reflect blood flow.20 Moreover, in three-dimensional scan images, the system can extract signals from a particular layer, including the choriocapillaris.20
We analyzed the data of AMD fellow eyes without particular findings, such as drusen or MNV in the fundus to identify very early changes in the choriocapillaris. As reported by the Age-related Eye Disease Study, fellow eyes are at high risk for developing AMD.21 We have previously reported that AMD fellow eyes have a shorter photoreceptor outer length and lower macular pigment optical density compared with the eyes of age-matched, otherwise healthy individuals, except those with mild cataract,22,23 and have minimal changes that are undetectable on fundus examination. Given that AMD develops due to continuous low-grade inflammation,24–27 pathological changes might have occurred in the AMD fellow eyes as a process of neovascular AMD development.
The current study aimed to analyze choriocapillaris changes using OCTA in the AMD fellow eyes to help elucidate the very early stages of the pathogenesis of AMD and early detection of future AMD risk.
Methods
This retrospective study adhered to the tenets of the Declaration of Helsinki and was approved by the St. Luke's International University Ethics Committee (approval number: 20-R048). Informed consent for research use of the data was obtained from all patients.
Patients
The analyses were based on a detailed medical chart review of patients with no ocular diseases other than mild cataract and unilateral neovascular AMD diagnosed at the Vitreo-Retina Division Clinic of the Department of Ophthalmology at St. Luke's International Hospital in Tokyo, Japan, between April 2020 and March 2021. AMD was diagnosed using FA and IA. The AMD fellow eyes that showed drusen, pigmentary abnormality, RPE detachment, choroidal neovascularization, and chorioretinal atrophy, as confirmed through fundus examination using a slit lamp by a retina specialist (author Y.O.), with fundus photographs, FA, IA, OCT, and OCTA images, were excluded.
Eye Examinations
All patients underwent best-corrected visual acuity (BCVA) measurement based on refraction tests, slit-lamp examinations, and binocular indirect ophthalmoscopy after pupil dilation with 0.5% tropicamide at each time point. The axial length was measured using IOLMaster 500 Zeiss (Carl Zeiss Meditec, AG. Jena, Germany).
OCT and OCTA
The OCT sectional images were obtained using Heidelberg Spectralis OCT (Heidelberg Engineering, Dossenheim, Germany) to evaluate central retinal thickness (CRT) and central choroidal thickness (CCT). OCTA images were recorded with pupil dilation using spectral-domain OCT (CIRRUS 5000; Carl Zeiss Meditec, AG. Jena, Germany). Images of a 3 × 3 mm pattern were assessed after excluding the projection artifacts using built-in software applying a validated semiautomated segmentation algorithm to identify the relevant retinal layers with manual corrections, as necessary, to ensure accurate segmentation. We evaluated en face images of the choriocapillaris slab, which was defined by a layer starting 29 µm below the RPE fit line and ending approximately 49 µm beneath the RPE fit line. The RPE fit line corresponds to the center level of the RPE line. The quality index values evaluated by signal strength of the OCTA images were all over seven. Signals from OCTA images were binarized to measure changes in choriocapillaris flow. Of the established binarization methods, the Phansalkar local binarization threshold can account for variable illumination or contrast of each image data.28 Thus, for all the analyses, image binarization was performed using the Phansalkar local binarization threshold28 to measure the flow signal area by the original ImageJ (National Institutes of Health, Bethesda, MD, USA; available at http://rsb.info.nih.gov/ij/index.html).29 The percentage of the area to the analyzed area was defined as the choriocapillaris flow area (CCFA) ratio. The CCFA ratio was compared between AMD fellow and control eyes in all four locations around the fovea to show whether there were choriocapillaris flow deficits, particularly in a specific area in the AMD fellow eyes. To evaluate the coefficient of variation (CV) of the CCFA ratio that represents the homo- or heterogeneities of the choriocapillaris flow, the binarized images were split into 20 × 20 small images, considering the size of choriocapillaris lobules that reportedly have a polygonal structure with a diameter of 200 to 250 µm.30 The CCFA ratio of each small image was measured, and the CV was calculated by dividing the standard deviation by the average.
Statistical Analyses
Data are expressed as mean ± standard error. The Mann–Whitney U test, Fisher exact test, and multiple logistic regression analysis were performed using SPSS version 27.0 (SPSS Japan, Tokyo, Japan). The P values <0.05 were considered statistically significant.
Results
Twenty-four AMD fellow eyes of 24 patients with unilateral late AMD (17 men, mean age = 71.7 ± 1.9 years, range = 50–88 years) and 21 eyes of 21 control individuals with no particular diseases, such as retinal diseases or glaucoma, except those with mild cataract (11 men, mean age = 69.1 ± 2.0 years, range = 49–82 years) were included in the study (Table 1). There were no significant differences in age, sex, BCVA, axial length, CRT, CCT, or macular volume between the two groups. No patients with high myopia (axial length >26.5 mm) were included. The quality index values of the OCTA images were comparable between the groups.
Table 1.
Participant Characteristics of Control and Age-Related Macular Degeneration (AMD) Fellow Eyes
| Control Eyes | AMD Fellow Eyes | P Value | |
|---|---|---|---|
| n (eyes) | 21 | 24 | |
| Age (years) | 49–82 (69.1 ± 2.0) | 50–88 (71.7 ± 1.9) | 0.820 |
| Sex (men; eyes (%)) | 11 (52.4) | 17 (70.8) | 0.167 |
| BCVA (LogMAR) | −0.18–0.10 (−0.06 ± 0.01) |
−0.08–0.15 (−0.06 ± 0.01) |
0.301 |
| Axial length (mm) | 22.55–26.02 (24.18 ± 0.236) |
21.80–26.20 (23.93 ± 0.279) |
0.225 |
| CRT (µm) | 192−269 (222 ± 4) | 180–264 (227 ± 4) | 0.790 |
| CCT (µm) | 124−578 (240 ± 23) | 75–325 (210 ± 13) | 0.360 |
| Quality index value of OCTA | 8–10 (9.47 ± 0.18) | 7–10 (9.25 ± 0.20) | 0.469 |
Data are presented as mean ± standard error (ranges); Mann–Whitney U test and Fisher exact test.
BCVA, best-corrected visual acuity; CRT, central retinal thickness; CCT, central choroidal thickness.
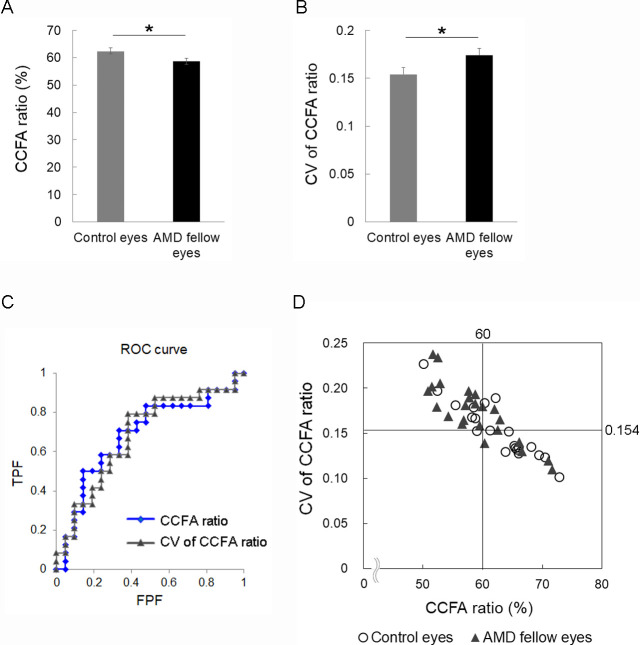
In OCTA images, the respective mean CCFA ratios of the control and AMD fellow eyes were 62.4 ± 1.3% and 58.6 ± 1.2%, respectively; the CCFA ratio was significantly smaller in the AMD fellow eyess (Fig. 1A, P = 0.032), suggesting that choriocapillaris flow deficits were already observed in eyes at AMD risk in the absence of MNV or early AMD findings. Moreover, the respective mean CVs of the CCFA ratio of the control and the AMD fellow eyes were 0.154 ± 0.007 and 0.174 ± 0.007, respectively, thus, the choriocapillaris flow was significantly heterogeneous (or non-homogenous) in the AMD fellow eyes (Fig. 1B, P = 0.032). These results suggested that flow deficits may progress heterogeneously. We analyzed the receiver operating characteristic (ROC) curves (area under the concentration-time curve [AUC] value, 0.6845 for the CCFA ratio and 0.6865 for the CV of the CCFA ratio; Fig. 1C) and showed a scatter plot of the CCFA ratios and the CVs of the CCFA ratio in each patient (Fig. 1D). We found that eyes with a CCFA ratio <60% and a CV of the CCFA ratio ≥0.154 had 4.371-fold higher risks of being AMD fellow eyes after adjusting for age and sex (95% confidence interval = 1.029–18.56, P = 0.046), suggesting that these cutoff values can be utilized to define future AMD risk (Table 2).
Figure 1.
Choriocapillaris flow area (CCFA) ratio and coefficient of variation (CV) of the CCFA ratio in the control eyes and the age-related macular degeneration (AMD) fellow eyes. The mean CCFA ratio (A) was lower and the mean CV of the CCFA ratio (B) was higher in the AMD fellow eyes compared with age-matched control eyes. ROC curve (C) and scatter plot of the CCFA and the CV of the CCFA ratio (D). In total, 16 (66.7%) AMD fellow eyes and 6 (28.6%) control eyes met the definition of eyes with the CCFA <60% and the CV of the CCFA ratio ≥0.154. AUC values: 0.6845 for CCFA ratio and 0.6865 for the CV of the CCFA ratio. *P < 0.05. Mann–Whitney U test.
Table 2.
Risk of Age-Related Macular Degeneration-Fellow Eyes According to the Choriocapillaris Flow Area (CCFA) Ratio and the Coefficient of Variation (CV) of the CCFA Ratio
| OR | P Value | 95% CI | |
|---|---|---|---|
| CCFA ratio < 60% and CV of CCFA ratio ≥ 0.154 | 4.371 | 0.046* | 1.029 to 18.56 |
Multiple logistic regression analysis adjusted for age and sex.
95% CI, 95% confidence interval; CCFA, choriocapillaris flow area; CV, coefficient of variation.
P < 0.05.
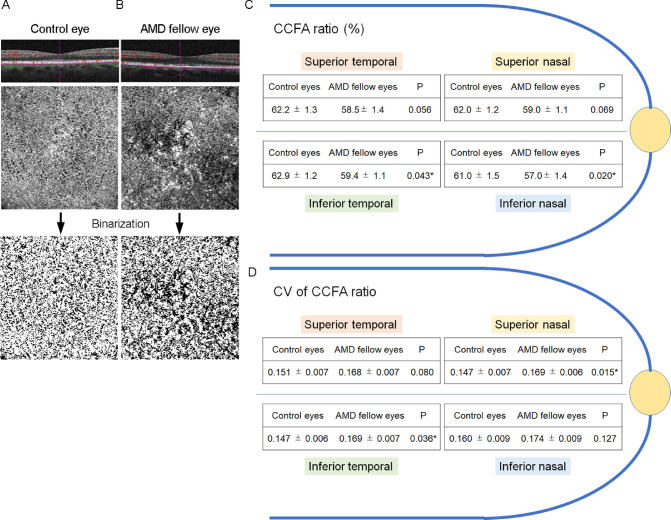
The binarized images of the OCTA (Figs. 2A, 2B, Supplementary Fig. 1) were segmented into four parts: superior temporal, inferior temporal, superior nasal, and inferior nasal to the fovea. The mean CCFA ratios of the AMD fellow eyes were lower than those of the control eyes in the inferior temporal and inferior nasal retina, and similar trends were observed in the other locations (Fig. 2C). The mean CV of the CCFA ratio of the AMD fellow eyes was greater in the inferior temporal and superior nasal retina with similar trends in the other locations (Fig. 2D). In the current study, we did not find any specific location on the retina that had a greater CV of the CCFA ratio. These findings indicated that choriocapillaris flow deficits can occur in every location in the fovea.
Figure 2.
Representative OCTA images of the choriocapillaris in the control eyes (A) and the age-related macular degeneration (AMD) fellow (B) eyes. An OCTA image of the choriocapillaris slab with B-scan and its binarized images from a control eye of a 61-year-old patient, otherwise healthy individual, except for mild cataract, with a choriocapillaris flow area (CCFA) ratio of 72.9%, and a coefficients of variation (CV) of CCFA ratio of 0.101 (A), and those from the AMD fellow eye of a 61-year-old patient with AMD with CCFA ratios of 61.9%, and CV of CCFA ratio of 0.177 (B). The mean CCFA ratio (C) and mean CV of the CCFA ratio (D) in the control eyes and the AMD fellow eyes at the superior, inferior, temporal, and nasal parts to the fovea are shown. Mann–Whitney U test. *P < 0.05.
We further analyzed the data dividing the eyes according to the CCT. The CV of CCFA ratio was greater in the AMD fellow eyes than in the control eyes for eyes with CCT ≥220 µm (mean CV of CCFA in control versus the AMD fellow eyes with CCT ≥220 µm = 0.144 ± 0.005 vs. 0.173 ± 0.007, P = 0.009**, Mann–Whitney U test); this was not observed in eyes with CCT <220 µm (0.161 ± 0.011 vs. 0.174 ± 0.011, P = 0.446). We previously reported that eyes with CCT ≥220 µm are at risk of recurrent exudative changes in AMD and may be associated with a particular pathological condition related to the pachychoroid.31 The CV of the CCFA ratio according to the CCT in individual eyes is shown in a scatter plot (Fig. 3). The data suggest that local CCFA ratio imbalance and heterogeneity in the choriocapillaris particularly clear in eyes with thick choroids in the AMD fellow eyes compared with the control eyes.
Figure 3.
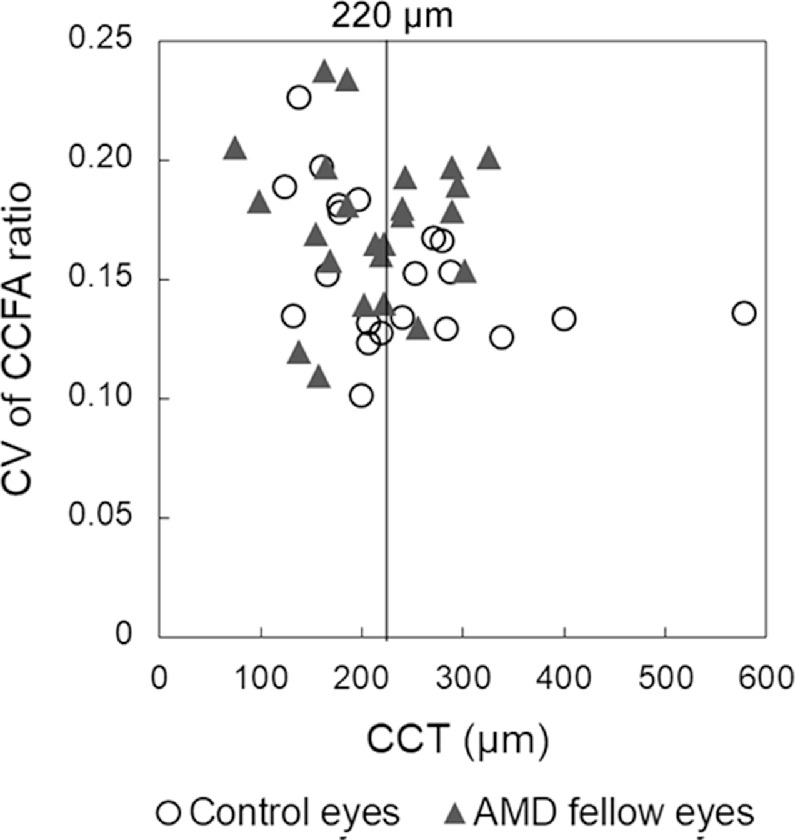
Scatter plot of the coefficient of variation (CV) of the choriocapillaris flow area (CCFA) ratio of the control eyes and the age-related macular degeneration (AMD) fellow eyes according to central choroidal thickness (CCT).
Discussion
In this study, we demonstrated that the mean CCFA ratio of the macular area was smaller and the mean CV of the CCFA ratio was greater in the AMD fellow eyes and AMD high-risk eyes with no particular findings such as drusen or MNV in the fundus than in the control eyes. Eyes with CCFA ratio <60% and CV of CCFA ratio ≥0.154 had a higher risk of being AMD fellow eyes. The overall local imbalance of the CCFA ratio was significant. A greater CV of the CCFA ratio was observed in the AMD fellow eyes than in the control eyes, particularly in eyes with thick choroid.
The CCFA was evaluated by binarizing the OCTA images at the choriocapillaris-slab level. We used a local thresholding method, the Phansalker local method, which assigns different threshold values adapting to the local histograms. Thus, the method would reduce the influence of image recording conditions, and this was emphasized in this study.
The CCFA ratio in the current study was evaluated using the flow signals at the choriocapillaris-slab level. Given that the studied eyes had no particular AMD findings, including RPE irregularity due to RPE detachment and choroidal neovascularization (as we also confirmed by B-scans), the segmentation error would be minimal, if any, and the signals were representative of the choriocapillaris flow both in controls and patients with AMD.
A reduction in the CCFA ratio in the AMD fellow eyes may have represented a reduction in choriocapillaris flow as an early change during AMD development, given that AMD fellow eyes are at high risk for future AMD development.21,22,32,33 This is consistent with the pathological findings from postmortem eyes of patients with clinically documented early AMD, showing choriocapillaris dropout with confocal microscopy.34 It was reported that the reduction in vessel density in the choriocapillaris of the macular area was approximately 20%. Pathological examinations also indicated that hypercellular capillaries that appeared to be “buds” of neovascularization were present in areas of submacular capillary dropout in some eyes with early AMD,34 suggesting that the dropout may cause relative hypoxia in the area, potentially inducing VEGF expression and leading to MNV and late AMD.
Perimacular sections of donor eyes were analyzed using light and electron microscopy, and the analysis showed that eyes with normal aging also had increased choriocapillaris breakdown, which increased with AMD progression.19 The vascular density of the choriocapillaris in human macular sections showed a decreasing trend in association with the AMD status, and vascular density was inversely associated with sub-RPE deposit density, which most likely clinically forms drusen.35 Conversely, recent studies have shown that AMD can be classified according to its estimated etiology into drusen-related AMD, which has a relatively thinner choroid36 and pachychoroid, and thick choroid-related AMD.37 Central serous chorioretinopathy (CSC), which is considered to have a similar background as pachychoroid-related AMD,9,37 involves reduced flow in the choriocapillaris.38 Therefore, choriocapillaris disorder may occur during the progression of both AMD types, theoretically. The current observation that the change was particularly clear in the eyes with thick choroid might involve statistical factor related to the fact that the CV of CCFA varied in the eyes with thin choroid. Further studies are required in the future.
Another new finding of the current study was the greater CV of the CCFA ratio, which represents heterogeneous choriocapillaris flow most likely due to spatial heterogeneity of the onset and progress in the flow deficits. Basal laminar deposits,34 found in early AMD, and cholesterol-rich deposits similar to drusen3,39 are localized in areas with an attenuated choriocapillaris, and reduction in flow could promote increased debris accumulation such as drusen or basal laminar deposits.34 Bruch's membrane thickening may promote relative hypoxia in the retina, in addition to the choriocapillaris flow deficits. Localization of reticular pseudodrusen, a high-risk RPE change in AMD,40,41 is reportedly observed in the choroidal watershed zone. Therefore, the etiology of drusen-related AMD may be related to blood flow imbalance in the choroid and was reflected in the greater CV of the CCFA ratio in the current study. Meanwhile, CSC, a pachychoroid spectrum disease with a thick choroid, can develop because of local imbalance in choroidal flow,11,42–47 and a geographic filling delay in the choriocapillaris is reportedly related to CSC lesions.11 Imbalanced choroidal circulation in CSC is explained by an asymmetric dilated vortex vein; the congestion has been proven using laser speckle flowgraphy48 and is considered pathogenetic. The mechanisms of imbalance of choroidal circulation in each AMD type would be a topic to consider in the future.
The odds ratio of the AMD fellow eyes was as great as 4.371 when the analysis included both the CCFA ratio and the CV of the CCFA ratio. The current results were derived from noninvasive OCTA examinations, which can be repeatedly used in living patients and may reflect the process of AMD development. Whether the parameters may be used as a biomarker to estimate the risk of AMD development, and they can be utilized in future health checks in persons with no AMD lesions in either eye are of research interest. Screening methods, if developed, may help in warning patients that they may develop AMD in the future so that they can undertake preventive approaches, such as avoiding smoking and high-fat diets and using antioxidative nutrient supplements.21,49–51 These promising results should be further validated in future studies.
The limitations of this study include the relatively small sample size, retrospective design, and inclusion of both drusen-related and pachychoroid-related AMD37; however, both eyes with thin and thick choroids had similar changes in the CV of the CCFA ratio. The reduction in the CCFA ratio could have reflected the decreased signal reflection from the choriocapillaris due to the deposits in and around the RPE, such as drusen52 and Bruch's membrane thickening, which may develop before AMD development.24–27 However, we did not include patients who exhibited visible drusen on fundus examination, whereas an AMD animal model study27 had shown that lipid deposits are clearly found using electron microscopy in the absence of particular fundus findings. We did not correct the CCFA value and measurement area using the Littman formula, in which differences in image magnification according to axial length can be corrected,53 however, our results are supported by the ratio of CCFA to the measured area, and the effect of axial length in the ratio was nullified. Nonetheless, correction of the measurement area was not performed, and the evaluated area would have some variation among the individuals if it was corrected before the anayses; this should be further assessed in the future.
Although treatment with anti-VEGF may be curative for many patients with neovascular AMD, the treatment effect varies among individuals,31,54,55 and preventive approaches utilizing micronutrient supplementation may reduce, but not fully arrest, progression to late AMD from early and intermediate AMD.21,49 Very early detection of choriocapillaris changes will help deepen the understanding of the fundamental pathogenesis to develop a screening method for future AMD risk.
In summary, neovascular AMD fellow eyes without MNV had reduced, heterogeneous, and imbalanced choriocapillaris flow, which may constitute an early change in neovascular AMD. Further studies on the choriocapillaris are warranted to fully elucidate the pathogenesis of AMD.
Supplementary Material
Acknowledgments
The authors thank all the clinicians and co-medical staff of the Department of Ophthalmology, St. Luke's International Hospital, for their kind assistance.
Author Contributions: Conception and design: Y.O. Data collection: N.H. and N.N. Analysis and interpretation: N.N. and Y.M. Review of the manuscript: N.H., N.N., and Y.M. Overall responsibility: Y.O.
Disclosure: N. Harada, None; N. Nagai, None; Y. Mushiga, None; Y. Ozawa, None
References
- 1. Bhutto I, Lutty G.. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012; 33: 295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitchell P, Liew G, Gopinath B, Wong TY.. Age-related macular degeneration. Lancet. 2018; 392: 1147–1159. [DOI] [PubMed] [Google Scholar]
- 3. Curcio CA, Presley JB, Millican CL, Medeiros NE.. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005; 80: 761–775. [DOI] [PubMed] [Google Scholar]
- 4. McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA.. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009; 50: 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seddon JM, McLeod DS, Bhutto IA, et al.. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016; 134: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanyuda N, Akiyama H, Shimoda Y, et al.. Different Filling Patterns of the Choriocapillaris in Fluorescein and Indocyanine Green Angiography in Primate Eyes Under Elevated Intraocular Pressure. Invest Ophthalmol Vis Sci. 2017; 58: 5856–5861. [DOI] [PubMed] [Google Scholar]
- 8. Lejoyeux R, Benillouche J, Ong J, et al.. Choriocapillaris: Fundamentals and advancements. Prog Retin Eye Res. 2021:87;100997. [DOI] [PubMed] [Google Scholar]
- 9. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB.. Pachychoroid disease. Eye (Lond). 2019; 33: 14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsumoto H, Hoshino J, Mukai R, Nakamura K, Kishi S, Akiyama H.. Chronic choriocapillaris ischemia in dilated vortex vein region in pachychoroid neovasculopathy. Sci Rep. 2021; 11: 16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kishi S, Matsumoto H, Sonoda S, Hiroe T, Sakamoto T, Akiyama H.. Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS One. 2018; 13: e0206646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moult EM, Alibhai AY, Rebhun C, et al.. Spatial distribution of choriocapillaris impairment in eyes with choroidal neovascularization secondary to age-related macular degeneration: A Quantitative OCT Angiography Study. Retina. 2020; 40: 428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia Y, Bailey ST, Wilson DJ, et al.. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014; 121: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Treister AD, Nesper PL, Fayed AE, Gill MK, Mirza RG, Fawzi AA.. Prevalence of Subclinical CNV and Choriocapillaris Nonperfusion in Fellow Eyes of Unilateral Exudative AMD on OCT Angiography. Transl Vis Sci Technol. 2018; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nesper PL, Ong JX, Fawzi AA.. Exploring the Relationship Between Multilayered Choroidal Neovascularization and Choriocapillaris Flow Deficits in AMD. Invest Ophthalmol Vis Sci. 2021; 62: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borrelli E, Uji A, Sarraf D, Sadda SR.. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 4792–4798. [DOI] [PubMed] [Google Scholar]
- 17. Borrelli E, Shi Y, Uji A, et al.. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am J Ophthalmol. 2018; 196: 34–43. [DOI] [PubMed] [Google Scholar]
- 18. Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S.. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999; 5: 35. [PubMed] [Google Scholar]
- 19. Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U.. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014; 35: 2562–2573. [DOI] [PubMed] [Google Scholar]
- 20. Kashani AH, Chen CL, Gahm JK, et al.. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017; 60: 66–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagai N, Minami S, Suzuki M, et al.. Macular Pigment Optical Density and Photoreceptor Outer Segment Length as Predisease Biomarkers for Age-Related Macular Degeneration. J Clin Med. 2020; 9: 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozawa Y, Shigeno Y, Nagai N, et al.. Absolute and estimated values of macular pigment optical density in young and aged Asian participants with or without age-related macular degeneration. BMC Ophthalmol. 2017; 17: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nita M, Grzybowski A, Ascaso FJ, Huerva V.. Age-related macular degeneration in the aspect of chronic low-grade inflammation (pathophysiological parainflammation). Mediators Inflamm. 2014; 2014: 930671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero-Vazquez S, Llorens V, Soler-Boronat A, Figueras-Roca M, Adan A, Molins B. Interlink between Inflammation and Oxidative Stress in Age-Related Macular Degeneration: Role of Complement Factor H. Biomedicines. 2021; 9: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ozawa Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020; 37: 101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagai N, Kawashima H, Toda E, et al.. Renin-angiotensin system impairs macrophage lipid metabolism to promote age-related macular degeneration in mouse models. Commun Biol. 2020; 3: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta N, Liu K, Alibhai AY, et al.. Impact of Binarization Thresholding and Brightness/Contrast Adjustment Methodology on Optical Coherence Tomography Angiography Image Quantification. Am J Ophthalmol. 2019; 205: 54–65. [DOI] [PubMed] [Google Scholar]
- 29. Schneider CA, Rasband WS, Eliceiri KW.. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Povazay B, Hermann B, Hofer B, et al.. Wide-field optical coherence tomography of the choroid in vivo. Invest Ophthalmol Vis Sci. 2009; 50: 1856–1863. [DOI] [PubMed] [Google Scholar]
- 31. Nagai N, Suzuki M, Minami S, et al.. Dynamic changes in choroidal conditions during anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Sci Rep. 2019; 9: 11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fasler K, Fu DJ, Moraes G, et al.. Moorfields AMD database report 2: fellow eye involvement with neovascular age-related macular degeneration. Br J Ophthalmol. 2020; 104: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amissah-Arthur KN, Panneerselvam S, Narendran N, Yang YC.. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond). 2012; 26: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lutty GA, McLeod DS, Bhutto IA, Edwards MM, Seddon JM.. Choriocapillaris dropout in early age-related macular degeneration. Exp Eye Res. 2020; 192: 107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J.. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyake M, Ooto S, Yamashiro K, et al.. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015; 5: 16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamashiro K, Hosoda Y, Miyake M, Takahashi A, Ooto S, Tsujikawa A.. Hypothetical pathogenesis of age-related macular degeneration and pachychoroid diseases derived from their genetic characteristics. Jpn J Ophthalmol. 2020; 64: 555–567. [DOI] [PubMed] [Google Scholar]
- 38. Kuroda Y, Ooto S, Yamashiro K, et al.. Increased Choroidal Vascularity in Central Serous Chorioretinopathy Quantified Using Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2016; 169: 199–207. [DOI] [PubMed] [Google Scholar]
- 39. Sura AA, Chen L, Messinger JD, et al.. Measuring the Contributions of Basal Laminar Deposit and Bruch's Membrane in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2020; 61: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alten F, Clemens CR, Heiduschka P, Eter N.. Localized reticular pseudodrusen and their topographic relation to choroidal watershed zones and changes in choroidal volumes. Invest Ophthalmol Vis Sci. 2013; 54: 3250–3257. [DOI] [PubMed] [Google Scholar]
- 41. Spaide RF, Ooto S, Curcio CA.. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018; 63: 782–815. [DOI] [PubMed] [Google Scholar]
- 42. Scheider A, Nasemann JE, Lund OE.. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol. 1993; 115: 50–56. [DOI] [PubMed] [Google Scholar]
- 43. Prunte C, Flammer J.. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121: 26–34. [DOI] [PubMed] [Google Scholar]
- 44. Iida T, Kishi S, Hagimura N, Shimizu K.. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999; 19: 508–512. [DOI] [PubMed] [Google Scholar]
- 45. Shinojima A, Ozawa Y, Uchida A, et al.. Assessment of Hypofluorescent Foci on Late-Phase Indocyanine Green Angiography in Central Serous Chorioretinopathy. J Clin Med. 2021; 10: 2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spaide RF, Gemmy Cheung CM, Matsumoto H, et al.. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2021; 86: 100973. [DOI] [PubMed] [Google Scholar]
- 47. Spaide RF, Ledesma-Gil G, Gemmy Cheung CM. Intervortex venous anastomosis in pachychoroid-related disorders. Retina. 2021; 41: 997–1004. [DOI] [PubMed] [Google Scholar]
- 48. Hirooka K, Saito M, Yamashita Y, et al.. Imbalanced choroidal circulation in eyes with asymmetric dilated vortex vein. Jpn J Ophthalmol. 2022; 66: 14–18. [DOI] [PubMed] [Google Scholar]
- 49. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 50. Jager RD, Mieler WF, Miller JW.. Age-related macular degeneration. N Engl J Med. 2008; 358: 2606–2617. [DOI] [PubMed] [Google Scholar]
- 51. Nagai N, Izumi-Nagai K, Suzuki M, et al.. Association of macular pigment optical density with serum concentration of oxidized low-density lipoprotein in healthy adults. Retina. 2015; 35: 820–826. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Q, Zheng F, Motulsky EH, et al.. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Invest Ophthalmol Vis Sci. 2018; 59: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Llanas S, Linderman RE, Chen FK, Carroll J.. Assessing the Use of Incorrectly Scaled Optical Coherence Tomography Angiography Images in Peer-Reviewed Studies: A Systematic Review. JAMA Ophthalmol. 2020; 138: 86–94. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki M, Nagai N, Izumi-Nagai K, et al.. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol. 2014; 98: 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki M, Nagai N, Shinoda H, et al.. Distinct Responsiveness to Intravitreal Ranibizumab Therapy in Polypoidal Choroidal Vasculopathy With Single or Multiple Polyps. Am J Ophthalmol. 2016; 166: 52–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.