Abstract
Primary ciliary dyskinesia (PCD) is a rare genetic disorder characterised by impaired mucociliary clearance leading to irreversible lung damage. In contrast to other rare lung diseases like cystic fibrosis (CF), there are only few clinical trials and limited evidence-based treatments. Management is mainly based on expert opinions and treatment is challenging due to a wide range of clinical manifestations and disease severity. To improve clinical and translational research and facilitate development of new treatments, the clinical trial network for PCD (PCD-CTN) was founded in 2020 under the framework of the European Reference Network (ERN)-LUNG PCD Core. Applications from European PCD sites interested in participating in the PCD-CTN were requested. Inclusion criteria consisted of patient numbers, membership of ERN-LUNG PCD Core, use of associated standards of care, experience in PCD and/or CF clinical research, resources to run clinical trials, good clinical practice (GCP) certifications and institutional support. So far, applications from 22 trial sites in 18 European countries have been approved, including >1400 adult and >1600 paediatric individuals with PCD. The PCD-CTN is headed by a coordinating centre and consists of a steering and executive committee, a data safety monitoring board and committees for protocol review, training and standardisation. A strong association with patient organisations and industrial companies are further cornerstones. All participating trial sites agreed on a code of conduct. As CTNs from other diseases have demonstrated successfully, this newly formed PCD-CTN operates to establish evidence-based treatments for this orphan disease and to bring new personalised treatment approaches to patients.
Short abstract
The disease-specific clinical trial network for primary ciliary dyskinesia (PCD-CTN) was built under the framework of the European Reference Network (ERN)-LUNG PCD Core, and operates to establish evidence-based and new personalised treatment for PCD https://bit.ly/3sLtC8o
Introduction
Primary ciliary dyskinesia (PCD, MIM 242650) is a rare, genetic, multisystem disorder mainly characterised by impaired mucociliary clearance, which might result in irreversible destructive airway disease. Available data on the epidemiology of PCD are limited, but the estimated prevalence is 1:10 000 individuals [1] with at least 74 000 Europeans estimated to be affected. The reported prevalence of PCD varies widely between countries because only a fraction of individuals are correctly diagnosed and registered, and access to diagnostic facilities differs regionally. In addition, occurrence varies in distinct populations [1], while the recorded prevalence in national registries remains markedly lower in adults than in children [2]. PCD is both genetically and clinically heterogeneous. To date, there are more than 50 genes reported to be associated with PCD [1]. There is limited information on the natural history and the long-term course of the disease, but it is clear that the disease is already a serious threat to lung function at preschool age and that the course of lung function after diagnosis shows a high degree of variation [3]. Furthermore, it has been shown that the majority of individuals with PCD presents with chronic respiratory infections, bronchiectasis (already evolving in childhood), progressive decline in lung function and chronic rhinosinusitis [1, 4]. Some individuals even progress to respiratory failure with the need for lung transplantation [5]. There seems to be a genotype/phenotype correlation in PCD, with subjects carrying mutations, e.g. in the genes CCDC40 [6] or CCNO [7], displaying worse lung function outcomes and individuals with mutations in, for example, DNAH9, RSPH1 and RSPH9 showing milder respiratory phenotypes, as reported in small cohorts [1, 8–10].
There is no cure for this chronic disorder and treatment modalities are mainly symptomatic and supportive, aiming to reduce secondary effects of dysfunctional motile cilia such as mucostasis, bacterial infections and destructive inflammation. Evidence-based treatment is very limited, and management strategies are based on expert opinions and experiences [11–13]. Most of the strategies are extrapolated from other respiratory diseases like cystic fibrosis (CF) or non-CF bronchiectasis. Daily airway clearance regimens are of utmost importance to prevent mucostasis, along with consequent antibiotic treatment of recurrent infections. The treatment of non-respiratory manifestations, such as fertility problems, congenital heart defects or brain malformations, are organ-specific. The management strategies are individualised, because no standardised treatment methods exist and experts from different disciplines need to be involved.
Recently, the first randomised, placebo-controlled trial on pharmacotherapy in PCD was published [14]. This was a multicentre study of maintenance treatment with azithromycin for 6 months in 90 individuals with PCD aged 7 to 50 years showing a reduced frequency of respiratory exacerbations and positive airway cultures compared to placebo. At the same time, it is well known that a wide range of other medicines is used empirically in individuals with PCD but without supportive evidence from randomised controlled trials [11–13]. Consequently, there is an urgent need to generate knowledge from clinical trials for the effective management of individuals with PCD using a collaborative, multicentre approach.
Especially in the field of rare diseases such as PCD, i.e. with small patient populations, collaboration between specialised centres is extremely important to obtain sufficient sample sizes for the assessment of outcome parameters with adequate statistical power. Thus, a CTN provides a centralised infrastructure for the successful implementation of clinical trials. The purpose of this publication is to present an overview of important and coherent activities within the European Reference Network (ERN)-LUNG PCD Core Network (https://ern-lung.eu) with a special focus on the newly established PCD-CTN (https://ern-lung.eu/portfolio-items/clinical-trial-network-for-primary-ciliary-dyskinesia-established/), including its interaction with the ERN-LUNG International PCD Registry [15] (ClinicalTrials.gov NCT02419365).
Clinical trial networks
The first CTNs were established in the field of cancer research in the late 1950s, followed by several disease-specific CTNs in the 1960s. Over the years, CTNs have successfully established new treatment approaches for specific groups of patients. In the field of respiratory diseases, the Cystic Fibrosis Foundation (CFF) in the USA founded the first specific CTN in 1998 and thereby pioneered CF treatment, having played an important role in the development and use of specific cystic fibrosis transmembrane conductance regulator (CFTR) modulators, which in the meantime have been approved for use in up to 90% of the CF population (https://www.cff.org/about-us/our-history). The European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) was founded in 2009 and stimulated the development of other disease-specific CTNs in Europe [21]. This network currently provides access to 57 large and experienced CF centres, located in 17 different countries in Europe, caring for 21 500 children/adolescents and adults with CF. Since 2009 more than 80 protocols have been reviewed, with up to 10 studies running at the same time. Furthermore, the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) was established in 2012 to facilitate multidisciplinary collaborative research in non-CF bronchiectasis. The initiative's goals are, among others, to create a European bronchiectasis registry facilitating research and quality improvement initiatives across healthcare systems, to build a network of researchers and clinical experts in bronchiectasis to guide future research and clinical priorities, and to facilitate applications to industry and EU funding sources to build bronchiectasis research capacity in Europe [22]. EMBARC has been supported by a large community of more than 150 centres in more than 40 countries. It is therefore one of the most successful Clinical Research Collaborations (CRCs) within the European Respiratory Society (ERS) [23], involved in the publication of more than 10 original papers in less than 4 years and the development of several consensus documents as well as supporting several clinical trials. A second funding period (2017–2020) of EMBARC as an ERS CRC has been approved, EMBARC2. The core of the ongoing study Bronchiectasis Research Integrating Datasets, Genomics and Endotyping (BRIDGE) is to develop an international bioresource (blood, DNA, sputum and other biological materials) based on the registry for use in translational research, but it also aims to generate recommendations for the design and conduct of bronchiectasis trials including study end-points and research priorities in each area [23].
ERN-LUNG PCD Core Network
ERN-LUNG is a network of European healthcare providers (HCPs) dedicated to ensuring and promoting excellence in care and research for the benefit of patients affected by rare respiratory diseases. The vision of ERN-LUNG is to be a European knowledge hub for rare respiratory diseases with the aim of decreasing morbidity and mortality from such diseases in people of all ages. ERN-LUNG consists of nine core networks representing the diversity of diseases and conditions affecting the lungs (https://ern-lung.eu/governance). The network's current set-up has evolved since its creation in 2017 and will continue to do so with better and more inclusive geographical coverage.
The ERN-LUNG PCD Core Network currently consists of 28 participating centres and eight affiliated partners in 23 countries (https://ern-lung.eu/reference-centers-2/). The ERN-LUNG PCD Core Network aims to optimise current efforts to improve PCD patient care and quality of life. The exchange of biological materials for diagnostic purposes is the current main means in PCD to improve and formalise cross-border patient care. This is especially important, as diagnosing PCD is complex and not all centres can provide all recommended methods needed to detect the whole range of PCD variants.
Members of the ERN-LUNG PCD Core Network continuously update diagnostic and clinical PCD guidelines. There is a close cooperation with, for example, the CRC BEAT-PCD funded by the ERS, which is a recently established network of researchers and healthcare professionals aiming to improve patient diagnosis and care [24].
ERN-LUNG PCD Core is headed by a coordinator and supported by a coordinating team. All members participate in the ERN-LUNG International PCD Registry (https://www.pcdregistry.eu/ [15]) to facilitate evaluation of clinical outcomes and to enable large clinical studies. The ERN-LUNG Population Registry (https://www.popreg.ern-lung.eu) has also been implemented, an initiative whereby patients interested in participating in clinical trials, disease-specific registries and research projects can register in the system and are contacted by experts from the specific ERN-LUNG Core that covers the patient's disease area, such as PCD. This population registry is open to all interested patients and not restricted to specific countries; registered patients have the opportunity to become registered in the ERN-LUNG International PCD Registry, with measures in place that prevent double entries.
ERN-LUNG International PCD Registry
The ERN-LUNG International PCD Registry was initially launched in 2014 within the BESTCILIA project [15]. It has lately been expanded through the REGISTRY WAREHOUSE project of ERN-LUNG [2]. The registry aims to facilitate the recruitment of patients for clinical and research studies. Data on family history, symptomatology, socioeconomic status, clinical manifestations, disease course, treatments, outcomes and natural history, as well as diagnostic data, are collected. Patient data are continuously contributed by participating centres [2]. To participate in the ERN-LUNG International PCD Registry the centres need to meet the necessary legal and ethical requirements, which are dependent on national conditions. The registry management team assists with all necessary processes. Participation in the registry is mandatory to become a member or associated partner of ERN-LUNG PCD Core. The ERN-LUNG International PCD Registry is registered in the European Rare Disease Registries Infrastructure (https://eu-rd-platform.jrc.ec.europa.eu/erdri_en).
Establishment of the PCD-CTN
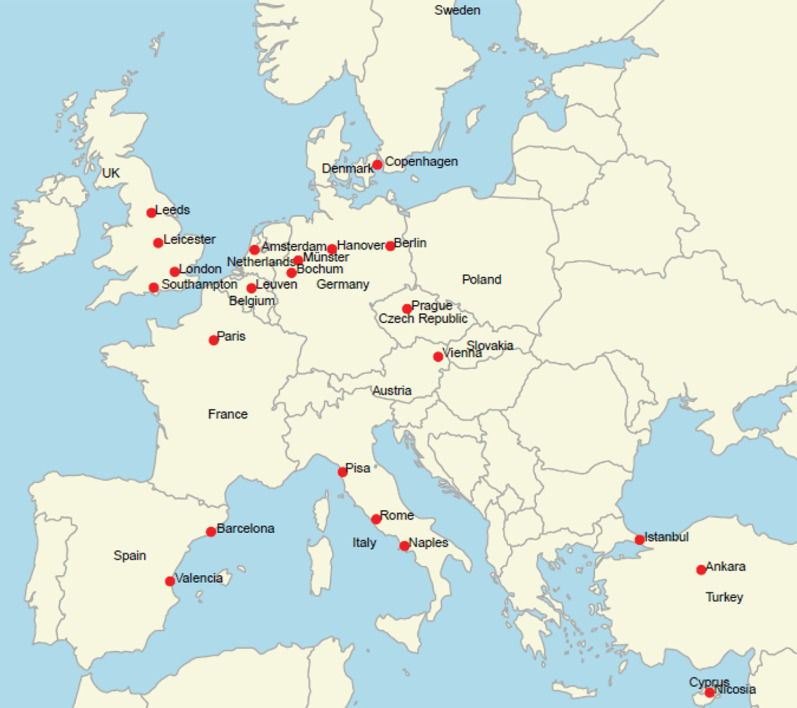
The disease-specific CTN for PCD was founded in 2020 under the framework of ERN-LUNG. It initially consisted of 18 clinical trial sites in 12 countries in Europe and included >1100 adult and >1100 paediatric individuals with PCD. However, four more sites from three countries were recently accepted to the CTN, resulting in a CTN with 22 clinical trial sites and access to >1600 adults and >1400 children with PCD that are potentially available for participation in trials (figure 1). More centres are expected to apply for membership. All centres fulfilled the participation criteria including patient numbers (n>30: adults, children or both), experience in PCD and/or CF clinical research, membership of ERN-LUNG PCD Core with the implementation of associated standards of care, human and material resources to run clinical trials and certification of good clinical practice (GCP). An annual update is required, in the form of a feasibility survey, because several fundamental and important characteristics (such as the number of patients, age distribution, genetic characteristics, new methods or services within diagnostics, monitoring and treatment) are under constant development (table 1). All centres provided confirmation of local institutional support, agreed on a code of conduct and are all equal partners in the PCD-CTN (table 1). The PCD-CTN is managed on a daily basis by a director of the coordinating centre and is additionally staffed by a part-time academic secretary and several committees: a steering and executive committee, a committee in charge of data safety monitoring and committees for protocol review, training and standardisations of diagnostic procedures and important outcome measures (figure 2).
FIGURE 1.
Map showing the participating centres of the clinical trial network for primary ciliary dyskinesia (PCD-CTN) (red dots). The PCD-CTN consists of 22 trial sites from 12 European countries including >3000 children and adults with PCD. The map was created with R [16] and RStudio [17], the packages “maps” [18], “ggplot2” [19] and “dplyr” [20] were used.
TABLE 1.
Details on available information from all participating centres of the PCD-CTN
| Information requested | At application for membership | At yearly overall feasibility survey |
| Patients with PCD: overview | ||
| Paediatric, adult or mixed centre | x | |
| Number of patients currently under direct care at the centre (adults/children) | x | x |
| Number of patients within different age groups (0–2 years/ 2–6 years/6–12 years/12–18 years/>18 years) | x | |
| Number of patients with genetically confirmed diagnosis | x | |
| Number of patients with specific bi-allelic mutations | x | |
| Experience | ||
| Industry-sponsored trials (PCD or CF-related; past 5 years) | x | x |
| Investigator-initiated trials (PCD or CF-related; past 5 years) | x | x |
| GCP certifications | x | x |
| Inspection of a regulatory authority | x | x |
| Publications (PCD, non-CF bronchiectasis and CF-related publications (past 5 years)) | x | |
| Site information | ||
| Ethic committee (local, central) | x | x |
| Monitoring regulations | x | x |
| Experience with the use of EDC systems | x | x |
| Resources for clinical research (personnel, database, available outcome parameters, available clinical research facility) | x | |
| Institutional support | x | |
| Commitment to work within PCD-CTN (hours dedicated to work for the CTN/month) | x | |
| Diagnostics and follow-up | ||
| Frequency of outpatient visits per patient | x | x |
| SOPs for diagnostic methods and monitoring tools for patient follow-up and for study outcome parameters on site | x | x |
| Participation in a national programme to improve quality in PCD care | x | |
| Participation and entering patients in a registry | x | |
| Recruitment strategies typically used at your centre | x | |
| Treatment practice | ||
| Fraction of patients with distinct medication (e.g. hydrator therapy, mucolytics, chronic antibiotic therapy, bronchodilators, inhaled corticosteroids, physiotherapy) | x | |
| Multidisciplinary team (PCD/CF dedicated) | x |
CF: cystic fibrosis; CTN: clinical trial network; GCP: good clinical practice; EDC: electronic data capture; PCD: primary ciliary dyskinesia; SOP: standard operating procedure
FIGURE 2.
Organisational diagram representing the structure of the clinical trial network for primary ciliary dyskinesia (PCD-CTN). The PCD-CTN was founded under the framework of the European Respiratory Network (ERN)-LUNG PCD Core in 2020. Core structures are the steering committee (SC), the executive committee (EC) and the coordinating centre. The coordinating centre provides a director and an academic secretary. The EC includes the PCD-CTN director and both deputies, the ERN-LUNG PCD Core coordinator and one additional ERN-LUNG PCD Core member representing three different European trial sites. The SC is composed of one principal investigator from each PCD-CTN member site, all members of the EC, the chair of the additional committees, and all appointed patient–parent representatives. The PCD-CTN includes several subcommittees: a committee in charge of data safety monitoring and committees for protocol review, training and standardisations of diagnostic procedures and important outcome measures. Important cornerstones of the PCD-CTN are a strong association with the ERN-LUNG International PCD Registry, the patient organisations and partnering networks, like the PCD Foundation in the USA.
Aims
The overall aims of the PCD-CTN are to intensify clinical research, primarily by encouraging and contributing to the initiation of randomised controlled trials (RCTs) in PCD patients, and to bring new medicines to patients as quickly as possible. New medicines with a direct effect on the underlying defect naturally have a very high priority, while the development of an evidence base for medicines that are already widely used for patients with PCD also deserves a great deal of attention. Thus, we aim to increase clinical and translational research and to facilitate the development of evidence-based management including novel treatments for PCD. To achieve these aims, improved access to patient populations is ensured by the network of participating clinical trial sites, including the ERN-LUNG International PCD Registry. Through the structures of the established PCD-CTN, clinical trials can be successfully pursued, planned and executed in a sufficient number of patients. The network also promotes a strong collaboration with patient organisations and pharmaceutical companies.
Function and agreements
The PCD-CTN Code of Conduct is a document that all site members have agreed to, which contains guidelines for cooperation between the members, but also between the members and pharmaceutical companies. The following aspects on procedures to follow are discussed: 1) when sites are contacted by pharmaceutical companies with enquiries for interest or feasibility of pharma-initiated interventional drug trials; 2) in case of investigator-initiated interventional drug trials (IIT) involving at least one CTN site; and 3) registry-based studies initiated by pharma or investigators involving the ERN-LUNG International PCD Registry [15].
Aspects concerning confidentiality, conflicts of interest, GCP compliance, quality management, publication policy, financial agreements, relationships with sponsors, communication, responsibilities of membership, as well as failure to comply with these codes of conduct, are all covered by this document.
Patient involvement
Since the beginning of the PCD networks, a major concern was to include patient/parent representatives. The ECFS-CTN also reports that active involvement of patient organisations was crucial for its success [21]. Therefore, it was undoubtedly important to involve patient/parent representatives in the network. We feel that it is mandatory to keep patient organisations informed about ongoing trials and activities of the network, which is ensured by inclusion of all patient representatives in the steering committee and three in the protocol review committee, and by regular meetings with patient representatives and through a PCD-CTN newsletter.
Partnership with pharmaceutical/industrial organisations
Although the national patient organisations support the network intellectually, little financial support will be brought in, because these organisations raise money mainly for educational tasks on a national level.
The PCD-CTN would not be financially dependent on pharmaceutical or commercial companies, but the CTN will facilitate their work and as such the CTN will need some funding and support to organise the network. We therefore decided to write the aforementioned code of conduct to provide guidance for our partnership with the pharmaceutical organisations. The close collaboration between PCD sites enables the CTN to provide pharmaceutical companies with an updated number of patients in the network, as well as updated number of sites that meet specific study inclusion criteria. Because the PCD-CTN holds experienced PCD physicians and researchers, the CTN can also assist pharmaceutical companies in building study designs.
The PCD-CTN will also offer a variety of services to pharmaceutical companies (description and requested updated information of the different clinical sites as presented in table 1, commentaries about study design, possibility of inclusion rate, capabilities to deliver outcome measures adhering to quality-assured standardised operating procedures (SOPs)) for which a fee will be charged. The pharmaceutical companies will be assured that high standards will be maintained in all centres. The CTN is not a contract research organisation (CRO) and the conduct, responsibility and sponsorship of the study remain in the hands of the pharmaceutical companies.
Structure of the PCD-CTN
Figure 2 and the following paragraphs describe the composition of the PCD-CTN and associated structures.
Coordinating centre
The CTN director and the coordinating centre have been appointed by the ERN-LUNG PCD Core Network and will act as contact for the network, interacting with pharmaceutical/industrial organisations, patient–parent organisations, collaborators as well as potential partners. The director is in continuous contact with all sites of the CTN and is responsible for the everyday activities of the network. He or she also supervises the decisions of the distinct committees and coordinates workflows such as the protocol review process. The CTN is co-chaired by two deputy coordinators from other member sites in two different countries within the EU, and an academic secretary at the coordinating centre.
Executive committee
The executive committee (EC) consists of the PCD-CTN director and both deputies, as well as the coordinator of the ERN-LUNG PCD Core and one additional member of PCD Core. They represent three PCD trial sites from three different European countries. The EC is responsible for the development and adaption of the global strategies of the CTN and it meets once or twice per month. The members of the EC are elected for 3 years with a possibility of re-election for another 3 years. Membership is limited to 6 years in total.
Steering committee
The steering committee (SC) is composed of one principal investigator from each PCD-CTN member site, all members of the EC, the chair of the additional committees, and all appointed patient–parent representatives. The SC meets four times a year, which for 2020/2021 was on a virtual basis due to the COVID pandemic restrictions. The election of members to the other committees was carried out during the SC meeting in April 2021. At each SC meeting, updates are presented on all network activities, including news from the individual subcommittees. Any new policies or future action plans are drawn up beforehand. These are then discussed, if necessary, and amended and agreed. A representative from the partnering network is also invited to these meetings. In addition, a work and financial plan will be discussed and agreed for the upcoming year. Depending on the situation, the meetings will be held face-to-face, digitally or as hybrid meetings.
Other committees
Protocol review committee
The protocol review committee (PRC) includes one chair among five physicians (four pulmonologists and one ENT (ear/nose/throat) physician, all with expertise in conducting clinical trials), three patient–parent representatives from three different countries in the EU and an epidemiologist. As required, additional experts are available ad hoc, e.g. pharmacologists, microbiologists or radiologists. The protocol review process includes evaluation of the study design, safety and ethical aspects, feasibility and the scientific value of the proposed study. This also includes the patient–parent perspective on the trial. Before protocol review, a contract is made between PCD-CTN and the pharmaceutical/industrial company or collaborator. The PRC writes a summary report and gives feedback about the study design with advice on potential adjustments. On the basis of this information, the EC votes on whether the trial will be conducted within the network and prioritises its level of performance in relation to other reviewed studies. The PCD-CTN director supervises the whole process and is responsible for communication between the PRC and potential collaborating partners. Depending on specific characteristics of the site, such as the total number of patients, the number of patients with specific genetic profiles, care of adult or paediatric patients and the location, the PCD-CTN can inform individual pharmaceutical companies about the potential for participation among member sites and will be able to propose distinct trial sites as suitable for particular studies, so that interested companies can select between potential participation of sites. Until now, the PCD-CTN has been contacted by diverse companies with potential trial protocols to review.
Standardisation committee
The standardisation committee is composed of one chair and five other clinical experts in PCD research, pulmonologists from six trial sites in six different European countries. This committee will establish SOPs, defining and harmonising the diagnostic methods to assure correct and high standard PCD diagnosis at sites as well as SOPs for monitoring tools for main study outcome parameters. By using harmonised SOPs at the various trial sites for clinical trials, study outcome parameters will be less variable and more comparable, which will thereby strengthen and facilitate the interpretation of the outcome data. The standardisation committee will work on specific topics forming distinct subgroups and will prepare consensus documents to create high-quality SOPs.
Training committee
This committee is responsible for keeping all participating PCD-CTN members up to date in relation to clinical research tasks. Through annual training schools, preferably alongside major scientific meetings such as ERS conferences, PCD-CTN site staff (research coordinators, study nurses and investigators) will have the opportunity to improve their knowledge and skills in the conduct of PCD-related clinical research including RCTs. In addition, online GCP training for the members is projected. Training schools with theoretical and practical topics are organised once a year. This committee includes four experienced physicians from four trial sites in four different European countries.
Data safety monitoring board
A data safety monitoring board (DSMB) of independent experts from outside the PCD-CTN will be appointed and instructed by a subcommittee of two experts from two trial sites in two different European countries. The DSMB will work independently from the network, and will be available upon request of pharma companies. The DSMB, an independent group of experts that advises the PCD-CTN, will include 1) two experts in PCD from two different European countries and 2) at least one statistician/epidemiologist (also a member of the PRC).
The primary responsibilities of DSMB are to: 1) periodically review and evaluate the accumulated study data for participant safety, study conduct and progress, and, when appropriate, efficacy; 2) make recommendations to the PCD-CTN concerning the continuation, modification or termination of the trial.
The DSMB should review each protocol for any major concerns prior to implementation. During the trial, the DSMB should review cumulative study data to evaluate safety, study conduct, and the scientific validity and integrity of the trial.
Evaluation of the PCD-CTN – performance metrics
The PCD-CTN will ensure ongoing reporting of performance metrics, defined as figures and data representative of the organisation's actions, abilities and overall quality to be presented on its home page. In an annual report, among other data will be included: the number of centres, the number of patients covered by the network, the number of projects, feasibility checks and protocol reviews for pharma companies, scientific publications, trial results, number of patients recruited to trials and the number of active and completed trials.
The strengths of the PCD-CTN
The PCD-CTN has emerged from the ERN-LUNG PCD Core Network and is thus strongly rooted in a well-functioning, Europe-wide organisation with great ambitions to promote the provision of health care for patients with rare diseases. The main strength of the network is its access to >3000 clinically well-characterised adults and children with PCD, the majority of them bearing a genetic diagnosis and having a high standard of care already defined by the accreditation of the sites through membership of ERN-LUNG.
Furthermore, the close association with the ERN-LUNG International PCD Registry will ensure an ongoing registration of new patients in various studies in addition to the obvious possibility of further understanding the longitudinal natural history of the disease, genotype–phenotype associations and possibly additional recruitment opportunities from centres not covered by the network. In addition, there is a close interconnection between networks caring for individuals with non-CF bronchiectasis such as EMBARC or ERN-LUNG nCF-BE (non-CF bronchiectasis) as some investigators participate in the networks of both diseases. Individuals with non-CF bronchiectasis also have the possibility to register themselves in the ERN-LUNG Population Registry and thus get in contact with centres participating in the different networks helping to find a specific diagnosis. During its set-up, the PCD-CTN has secured inspiration and support from ECFS-CTN [21] and will continue in the future to learn from their experiences, obtaining advice and guidance where needed. The PCD-CTN will also work closely with the US PCD Foundation (US PCDF) to strengthen the development of the overall global research effort within PCD.
The establishment of a committee for ongoing systematic evaluation of methods for diagnostics and adoption of modern outcome parameters will ensure implementation of technological advances and scientific strength in future trials.
Finally, the diverse representation of patients and family members in the PRC provides a strong opportunity for highly relevant constructive criticism and guidance to pharmaceutical companies in the development process of the protocols.
Conclusion
With 22 trial sites from 12 European countries, the PCD-CTN provides outstanding expertise and sufficient patient numbers, including both children/adolescents and adults with PCD, for the successful conduct of clinical trials. The network has already grown since its establishment in February 2020 and is expected to grow even further.
Centres with smaller numbers of PCD individuals (n<30) already have the opportunity to enter their patients into the International PCD Registry, who are then managed under the ERN-LUNG PCD Core coordinating centre or the neighbouring local ERN-LUNG PCD Core and/or PCD-CTN centre. Thus, PCD individuals supervised at smaller centres do already have the opportunity to participate in clinical or research trials.
The agreed code of conduct between all trial sites defines interaction between the network sites and with pharmaceutical companies. The established committees ensure a well-functioning process in the development of a clinical trial. Information on the PCD-CTN has been provided to potential collaborators, pharmaceutical and industrial companies and is available on request. Patient–parent representatives are both actively involved and highly supportive of this initiative and will increase the awareness of specific clinical trials and facilitate access to participation in the studies. Furthermore, protocol review will lead to improvement in the feasibility of clinical trials and established SOPs will facilitate evaluation/interpretation of study outcome parameters. For PCD, as a rare disease, this CTN builds a strong basis to implement high-quality clinical research in order to identify new therapies potentially restoring ciliary function, increase evidence for already used therapies and facilitate that evidence-based targeted drug treatment progress from clinical trials to patients.
Acknowledgements
The authors thank the individuals with PCD and their families for their continuous support and the willingness to participate in clinical and research trials. We especially acknowledge the patient support groups in Europe as well as the US PCDF. For excellent organisational assistance with the ERN-LUNG International PCD Registry, the authors thank the study nurses S. Helms and M. Tekaat. For maintenance of the International PCD Registry, we thank J. Varghese and S. Riepenhausen (University Muenster, Institute of Medical Informatics, Muenster, Germany), who also supported the creation of the figures. All participating centres are HCPs in ERN-LUNG. The authors thank the ERN-LUNG coordinating centre in Frankfurt (coordinator T.O.F. Wagner and team). In particular, we acknowledge ECFS-CTN for its valuable guidance and support in building the PCD-CTN.
Provenance: Submitted article, peer reviewed.
Conflict of interest: J. Raidt declares grants for Clinical Research Unit “Male Germ Cells: from Genes to Function” (CRU 326) – Subproject RA3522/1-1 from the Deutsche Forschungsgemeinschaft, in connection with the present manuscript; that they are a holder of patent WO 2020/165352 A1 (Treatment of ciliopathies); and an unpaid role as a Deputy Director of PCD-CTN.
Conflict of interest: B. Maitre declares unpaid roles as a Deputy Director of PCD-CTN and as a member of the scientific committee of RADICO PCD.
Conflict of interest: P. Pennekamp declares research grants to their institution from NEOCYST (BMBF, 01GM1903A); and that they are a holder of patent WO 2020/165352 A1 (Treatment of ciliopathies).
Conflict of interest: M. Armengot declares research grant FIS PI19/00949 from Instituto de Salud Carlos III, in connection with the present manuscript.
Conflict of interest: M. Boon declares Forton grant 2020-J1810150-217926 from the King Baudouin Foundation, in the 36 months prior to manuscript submission.
Conflict of interest: S.B. Carr declares grants to their institution from the National Institute of Health Research (Programme Development Grant 202952; Health Technology Assessment Grant NIHR121889; HTA grant 14/22/23; NIHR RfPb QOL-PCD; NIHR RfPb VALU- CF); consulting fees paid to their institution by Vertex Pharmaceuticals; personal and institutional honoraria from Chiesi Pharmaceuticals; payment to their institution for participation on a Data Safety Monitoring Board or Advisory Board from Profile Pharma, Pharmaxis and Vertex Pharmaceuticals, all in the 36 months prior to manuscript submission; and that they are Chair of the UK CF Registry Steering Committee (payment to their institution) and of the European CF Society Patient Registry Scientific Committee (unpaid).
Conflict of interest: M.A. Mall declares research grant 82DZL009B1 from the German Federal Ministry of Education and Research, in connection with the present manuscript.
Conflict of interest: M. Narayanan declares that they have been a co-applicant on the National Institute for Health Research – Research for patient benefit (NIHR –RfPB) grant for ‘Parent reported quality of life measures for young children with primary ciliary dyskinesia (QOL-PCD study)’ in the 36 months prior to manuscript submission; and that they are a member of the British Paediatric Respiratory Society executive committee and the British Thoracic Society standards of care committee.
Conflict of interest: P. Pohunek declares research grants from the Ministry of Health of the Czech Republic (NV19-07-00210) and Charles University Grant Agency (670119P) in connection with the present manuscript.
Conflict of interest: S. Range declares an unpaid role as medical advisor to PCD Support.
Conflict of interest: F.C. Ringshausen declares grants to their institution from the German Center for Lung Research (DZL), the German Center for Infection Research (DZIF), IMI (EU/EFPIA), iABC Consortium (including Alaxia, Basilea, Novartis and Polyphor), Mukoviszidose Institute, Novartis, Insmed Germany, Grifols, Bayer and InfectoPharm; consulting fees from Parion, Grifols, Zambon, Insmed and the Helmholtz-Zentrum für Infektionsforschung; payments or honoraria from I!DE Werbeagentur GmbH; Interkongress GmbH; AstraZeneca; Insmed; Grifols and Universitätsklinikum Frankfurt am Main; payment to their institution for expert testimony at the Social Court Cologne; support for attending meetings from German Kartagener Syndrome and PCD PAG and Mukoviszidose e.V; participation on a Data Safety Monitoring Board or Advisory Board for Insmed, Grifols and Shionogi; fees for clinical trial participation paid to their institution by Abbvie, AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon; and honorary roles as Coordinator of the ERN-LUNG Bronchiectasis Core Network, Chair of the German Bronchiectasis Registry PROGNOSIS, Member of the SteerCo of the European Bronchiectasis Registry EMBARC, Member of the SteerCo of the European NTM Registry EMBARC-NTM, Co-Speaker of the Medical Advisory Board of the German Kartagener Syndrome and PCD PAG, Speaker of the Respiratory Infections and TB group of the German Respiratory Society (DGP), Speaker of the Cystic Fibrosis group of German Respiratory Society (DGP), PI of the DZL, member of the protocol review committee of the PCD-CTN and Member of Physician Association of the German Cystic Fibrosis PAG.
Conflict of interest: J. Roehmel declares payments or honoraria from Vertex Pharmaceuticals, in the 36 months prior to manuscript submission.
Conflict of interest: G. Thouvenin declares unpaid membership of the RADICO PCD scientific committee.
Conflict of interest: H. Omran declares grants for Clinical Research Unit “Male Germ Cells: from Genes to Function” (CRU 326) – Subproject RA3522/1-1 from the Deutsche Forschungsgemeinschaft, from Interdisziplinaeres Zentrum für Klinische Forschung Muenster (Om2/009/12; Om2/015/16; Om2/010/20), from BESTCILIA (EU FP7 GA 305404) and from Registry Warehouse (Horizon2020 GA 777295), all in connection with the present manuscript; in addition to grants to their institution from LYSOCIL (Horizon2020 GA n°811087) NEOCYST (BMBF, 01GM1903A) and Transkripttherapie für primäre ziliäre Dyskinesien (Zentrales Innovationsprojekt Mittelstand (ZIM), BMWi; ZF-4610102SK8), in the 36 months prior to manuscript submission; that they are a holder of patent WO 2020/165352 A1 (Treatment of ciliopathies); and unpaid roles as Head of the ERN-LUNG PCD Core and as a member of the PCD Family Support Group medical advisory board.
Conflict of interest: K.G. Nielsen declares funding for the present manuscript from the Danish Children's Lung Foundation (Børnelungefonden); and an unpaid role as a Director of the PCD-CTN.
Conflict of interest: All other authors declare no competing interests.
Support statement: This work was supported in part by grants from the Danish “Children's Lung Foundation” (Børnelungefonden, K.G. Nielsen), the German Federal Ministry of Education and Research (82DZL009B1, M.A. Mall), the “Deutsche Forschungsgemeinschaft” (DFG OM6/7, OM6/8, OM6/10, OM6/14, CRU 326 (subprojects OM6/11 (H. Omran), RA3522/1-1 (J. Raidt)), the “Interdisziplinaeres Zentrum für Klinische Forschung Muenster” (Om2/009/12, Om2/015/16 and Om2/010/20), Registry Warehouse (Horizon2020 GA 777295)) and BESTCILIA (EU FP7 GA 305404). It was also supported by the Ministry of Health of the Czech Republic (NV19-07-00210, P. Pohunek) and Charles University Grant Agency (670119P, P. Pohunek) as well as the Spanish Instituto de Salud Carlos III (FIS PI19/00949, M. Armengot). B. Maitre, A. Coste, G. Thouvenin are members of the RadiCONetwork (Inserm, France). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Wallmeier J, Nielsen KG, Kuehni CE, et al. Motile ciliopathies. Nat Rev Dis Primers 2020; 6: 77. doi: 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- 2.Ardura-Garcia C, Goutaki M, Carr SB, et al. Registries and collaborative studies for primary ciliary dyskinesia in Europe. ERJ Open Res 2020; 6: 00005-2020. doi: 10.1183/23120541.00005-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marthin JK, Petersen N, Skovgaard LT, et al. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med 2010; 181: 1262–1268. doi: 10.1164/rccm.200811-1731OC [DOI] [PubMed] [Google Scholar]
- 4.Frija-Masson J, Bassinet L, Honoré I, et al. Clinical characteristics, functional respiratory decline and follow-up in adult patients with primary ciliary dyskinesia. Thorax 2017; 72: 154–160. doi: 10.1136/thoraxjnl-2015-207891 [DOI] [PubMed] [Google Scholar]
- 5.Hayes D, Reynolds SD, Tumin D. Outcomes of lung transplantation for primary ciliary dyskinesia and Kartagener syndrome. J Heart Lung Transpl 2016; 35: 1377–1378. doi: 10.1016/j.healun.2016.08.025 [DOI] [PubMed] [Google Scholar]
- 6.Davis SD, Rosenfeld M, Lee HS, et al. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am J Respir Crit Care Med 2019; 199: 190–198. doi: 10.1164/rccm.201803-0548OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallmeier J, Al-Mutairi DA, Chen CT, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet 2014; 46: 646–651. doi: 10.1038/ng.2961 [DOI] [PubMed] [Google Scholar]
- 8.Loges NT, Antony D, Maver A, et al. Recessive DNAH9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am J Hum Genet 2018; 103: 995–1008. doi: 10.1016/j.ajhg.2018.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles MR, Ostrowski LE, Leigh MW, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med 2014; 189: 707–717. doi: 10.1164/rccm.201311-2047OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yiallouros PK, Kouis P, Kyriacou K, et al. Implementation of multigene panel NGS diagnosis in the national primary ciliary dyskinesia cohort of Cyprus: an island with a high disease prevalence. Hum Mutat 2021; 42: e62–e77. doi: 10.1002/humu.24196 [DOI] [PubMed] [Google Scholar]
- 11.Strippoli MP, Frischer T, Barbato A, et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J 2012; 39: 1482–1491. doi: 10.1183/09031936.00073911 [DOI] [PubMed] [Google Scholar]
- 12.Lucas JS, Burgess A, Mitchison HM, et al. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child 2014; 99: 850–856. doi: 10.1136/archdischild-2013-304831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirra V, Werner C, Santamaria F. Primary ciliary dyskinesia: an update on clinical aspects, genetics, diagnosis, and future treatment strategies. Front Pediatr 2017; 5: 135. doi: 10.3389/fped.2017.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobbernagel HE, Buchvald FF, Haarman EG, et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (BESTCILIA): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2020; 8: 493–505. doi: 10.1016/S2213-2600(20)30058-8 [DOI] [PubMed] [Google Scholar]
- 15.Werner C, Lablans M, Ataian M, et al. An international registry for primary ciliary dyskinesia. Eur Respir J 2016; 47: 849–859. doi: 10.1183/13993003.00776-2015 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/.
- 17.RStudio Team (2020). RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. www.rstudio.com/.
- 18.Original S code by Richard A. Becker, Allan R. Wilks. R version by Ray Brownrigg. Enhancements by Thomas P Minka and Alex Deckmyn. (2021). maps: Draw Geographical Maps. R package version 3.4.0. https://CRAN.R-project.org/package=maps.
- 19.Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, 2016. [Google Scholar]
- 20.Wickham H, François R, Henry L,et al. (2021). dplyr: A Grammar of Data Manipulation. R package version 1.0.7. https://CRAN.R-project.org/package=dplyr.
- 21.De Boeck K, Bulteel V, Tiddens H, et al. Guideline on the design and conduct of cystic fibrosis clinical trials: the European Cystic Fibrosis Society-Clinical Trials Network (ECFS-CTN). J Cyst Fibros 2011; 10: Suppl 2, S67–S74. doi: 10.1016/S1569-1993(11)60010-6 [DOI] [PubMed] [Google Scholar]
- 22.Aliberti S, Polverino E, Chalmers JD, et al. The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) ERS clinical research collaboration. Eur Respir J 2018; 52: 1802074. doi: 10.1183/13993003.02074-2018 [DOI] [PubMed] [Google Scholar]
- 23.Brightling C, Genton C, Bill W, et al. ERS clinical research collaborations: underpinning research excellence. Eur Respir J 2018; 52: 1801534. doi: 10.1183/13993003.01534-2018 [DOI] [PubMed] [Google Scholar]
- 24.Goutaki M, Crowley S, Dehlink E, et al. BEAT-PCD clinical research collaboration. The BEAT-PCD (Better Experimental Approaches to Treat Primary Ciliary Dyskinesia) clinical research collaboration. Eur Respir J 2021; 57: 2004601. doi: 10.1183/13993003.04601-2020 [DOI] [PubMed] [Google Scholar]