Abstract
Orthobiologics never cease to cause popularity within the medical science field, distinctly in regenerative medicine. Recently, adipose tissue has been an object of interest for many researchers and medical experts due to the fact that it represents a novel and potential cell source for tissue engineering and regenerative medicine purposes. Stromal vascular fraction (SVF), for instance, which is an adipose tissue-derivative, has generated optimistic results in many scenarios. Its biological potential can be harnessed and administered into injured tissues, particularly areas in which standard healing is disrupted. This is a typical feature of osteoarthritis (OA), a common degenerative joint disease which is outlined by persistent inflammation and destruction of surrounding tissues. SVF is known to carry a large amount of stem and progenitor cells, which are able to perform self-renewal, differentiation, and proliferation. Furthermore, they also secrete several cytokines and several growth factors, effectively sustaining immune modulatory effects and halting the escalated pro-inflammatory status of OA. Although SVF has shown interesting results throughout the medical community, additional research is still highly desirable in order to further elucidate its potential regarding musculoskeletal disorders, especially OA.
Keywords: Stromal vascular fraction, Orthobiologics, Osteoarthritis, Regenerative medicine, Adipose tissue
Introduction
Osteoarthritis (OA) has long affected many individuals. This orthopedic condition remains the most common degenerative and progressive joint disease and is a major cause of pain and disability in adult populations, taking hold of approximately 7% of the global population [1]. According to the Global Burden of Disease (GBD) 2019 paper results, the number of people affected by OA rose 48% globally in between 1990 and 2019, which put OA at the 15thplace for highest cause long-term with disability in the same year [2]. The increase in OA cases is likely attributed to factors such as aging and manifestation of poor metabolic health, especially incidences such as obesity [3–5].
OA is highly influenced by the exchanges between local, systemic and external factors, which consequently dictate the disease's progression and the way patients respond to its treatment processes [6]. Typical observations which characterize OA encompasses a continuous loss of articular cartilage, formation of osteophytes, thickening of the subchondral bone, exasperated synovial inflammation, degeneration of ligaments and menisci as well as joint hypertrophy [4]. Several handling strategies have been proposed. Conservative methods such as administration of pharmacological agents only lead to temporary pain alleviation, rather than targeting the problem root cause [7,8].
Usually, health care providers may prescribe a course of multiple combined drugs for different OA stages, with the objective of controlling inflammatory nociceptive pain. Non-steroidal antiinflammatory drugs (NSAIDs), other analgesics and corticosteroids, for example, may be commonly prescribed to aid in pain management. However, chronic NSAID use is of great concern as reported. Although NSAIDs effectively mitigate pain, they are also responsible for the increased risk of several adverse events, such as peptic ulcer disease, acute renal failure, and myocardial infarction [9]. Non-pharmacological strategies are usually limited to physical therapy, low impact exercise, weight loss, physical aids, and nerve ablation. In severe cases, however, such as grade IV OA, surgical intervention with joint replacement procedures may be unavoidable and therefore extremely detrimental to the patient [6,7].
These obstacles have led researchers to explore non-surgical alternatives, such as prescribing orthobiologics in particular. Orthobiologics are biologic products derived from substances that are naturally found in the human body which can mitigate the healing process of orthopedic injuries. Popular examples include platelet-rich plasma (PRP), hyaluronic acid (HA) and bone marrow aspirate/concentrate (BMA/BMAC), as well as adipose tissue-derived stem cells (ADSCs)[10,11]. According to the literature, these biological materials contain cytokines, mesenchymal, and stem/progenitor cells which have demonstrated an ability to modulate OA pathogenesis. Such materials offer considerable optimism to medical experts regarding the ever expanding field of regenerative medicine [12].
Among popular orthobiologic approaches, adipose tissue (including its derivatives) has proven to be a novel and attractive cell source for tissue engineering and regenerative medicine purposes. Stromal vascular fraction (SVF), for instance, has revealed interesting results. This biologic material may establish evidence in regards to being quite feasible, since it provides easy access to a rich source of stem and progenitor cell populations [13], which may effectively target OA physiopathology. Furthermore, diverse cell populations present in the SVF may further modulate exacerbated inflammation which aggravates degenerative processes in osteoarthritic joints via secretory mechanisms.
Adipose tissue-derived mesenchymal stem cells may not only undergo differentiation into specific cell lineages, but secrete a variety of cytokines and growth factors as well [13]. These bioactive molecules can trigger anti-inflammatory effects and additional biologic responses that can redirect uncontrolled inflammation towards a more regenerative pathway in the joint microenvironment[13,14]. Since one of this pathology's hallmarks is the overall predominance of catabolic and pro-inflammatory status [5], implementation of such therapeutic tools could lead to multiple positive outcomes in clinical settings.
The objective of this article is to review the prospective uses of the stromal vascular fraction as an orthobiologic tool for the treatment of osteoarthritis, further elucidating its utilities and possible biological mechanisms that target the OA pathophysiology.
Stromal Vascular Fraction
The stromal vascular fraction is a biologic material which can be obtained from the adipose tissue that presents rich contents of heterogeneous cell populations. Examples include: endothelial cells, preadipocytes, type 2 macrophages, T cells, and pericytes, as well as mesenchymal stem and progenitor cells [13]. Table 1 provides a more comprehensive set of cells typically found in the SVF and describes their biological roles. Indeed, evidence from medical literature indicates that adipose tissue appears to be the most abundant source of adult stem cells and the one which can be isolated with greater ease when compared to other alternatives. For instance, when compared to bone marrow, which has been highly praised for its biologic value and benefits, adipose tissue is capable of generating a total stem cell yield approximately 40 times greater [15–17].
Table 1. SVF Cell Content Isolated from the Aqueous Portion.
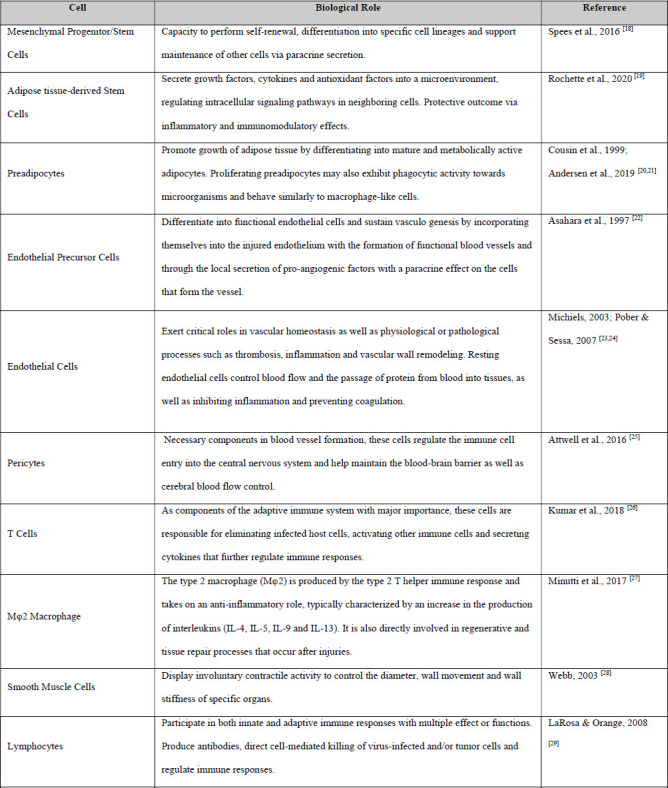
Taking these data into consideration, there is greater motivation for the clinical application of this material towards the treatment and mitigation of musculoskeletal diseases. SVF can be cultured and expanded in vitro in order to meet these expectations; however, it must be brought out that there are certain risks associated with such laboratory operations, which include possible sample contamination and even erroneous cell differentiation. Moreover, the requirements of serum protein-free culture mediums to avoid bovine spongiform encephalopathy and other xenogenic infections from mycoplasma, viruses, and other uncovered pathogens represent some of the obstacles that must be taken into account, if this method is chosen [30,31].
In order to overcome these constraints, administration of fresh SVF may be an advantageous alternative within the field of regenerative medicine.
Obtaining SVF
SVF may be obtained via liposuction procedures and the subsequent processing of adipose tissue (figure 1).The first method which describes the isolation of SVF from adipose tissue were initially documented in the 1960s by Martin Rodbell [32]. This method consisted of grounding fat pads and subsequently isolating adipocytes and stromal cells through collagenase. Enzymatic digestion methods which are known to disaggregate adipose tissue may be widely employed when the goal is mesenchymal cell culture and expansion. Collagenase easily and effectively separates fat into 2 distinct layers: the floating fraction of mature adipocytes and the cellular components in the lower aqueous portion (Figure 1), which can then be further separated by centrifugation [33].
Figure 1. Processing Adipose Tissue. A) Aspirating adipose tissue; B) Method of enzymatic processing with collagenase or lecithin to disaggregate components; C) Mechanical disaggregation techniques via nanofiltration and centrifugation.
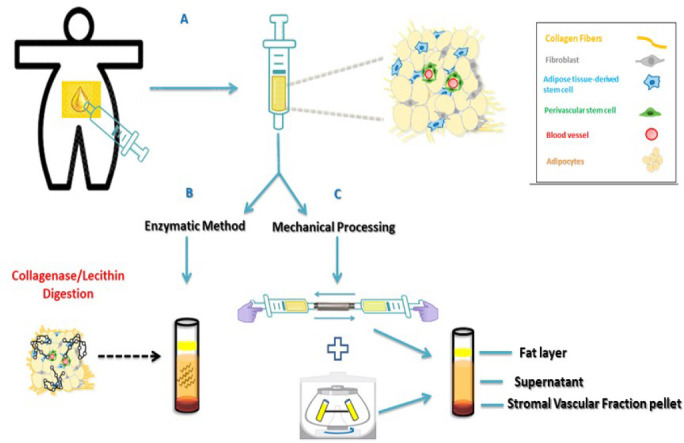
Although an effective tool for SVF extraction, the potential trace amounts of residual collagenase in injectable products is extremely detrimental to the patient, since they are known to induce articular degeneration both by digesting cartilage collagen and by causing articular instability. This brings to light some of the main consequences associated with OA development [34]. Therefore, this method is better suited for research on cell culture and expansion.
In order to find alternative solutions and comply with regulatory bodies, researchers employed slightly different protocols. Most of these techniques rely on the mechanical disaggregation and microfragmentation of adipose tissue in order to obtain SVF. The only drawback, however, is that this method does not allow for high cell yields when compared to enzymatic digestion protocols [35]. This is due to the fact that adipocytes establish strong bonds with collagen, which are not easily released by mechanical means [35]. However, adipose tissue can be filtered and emulsified through what are sometimes referred to as “nano-filters”(Figure 2), thus generating “nano-fragmented” fat [36]. Adipose tissue can be drawn out and immediately nano-filtered in order to gradually emulsify and break its components down.
Figure 2. Adipose tissue filters: A) 1.2 millimeter transfer; B) 1.4 millimetertransfer; C) 2.4 millimetertransfer; D) Mesh screen chamber (600 microns/400 microns).

According to a highly detailed article [37], this process can be achieved through three distinct steps: 1-multiple passages (at least 30) between syringes via a sterile standard 2.4 millimeter luer-to-luer transfer; 2-multiple passages (at least 30) between syringes via a sterile standard 1.2 millimeter luer-to-luer transfer; 3- one single passage through a sterile, non-aligned mesh screen chamber (600 microns/400 microns) into a new syringe or suitable container for further application. There is also an extra mechanical disaggregation alternative which is well-suited for avoiding complex regulatory concerns related to good manufacturing practice (GMP) guidelines.
In their recent paper Xu et al. [38] attempted to process adipose tissue samples with gentle mechanical force using a neoteric and completely sealed device, in which the tissue is washed, emulsified and subsequently micro-fragmented, thus removing residual impurities, which include blood and oil [39,40]. According to their research, this specific system reduces adipose cluster dimensions, which proves to be a technical constraint, providing a ready-to-use micro-fragmented adipose tissue sample without the need to resort to cell expansion and enzymatic digestion treatments. Furthermore, this method maintains an intact stromal vascular niche and preserves a reservoir of heterogeneous cellular populations, including pericytes, which are responsible for the maintenance of ADSCs [41]. These authors had the goal of determining whether this technique would allow them to effectively promote the repair of damaged cartilage with specific components found in micro-fragmented fat, including the stem and progenitor cell populations as well as their associated secretomes (cytokines and growth factors) and the natural scaffold properties pertaining to adipose tissue itself, which collectively support the regenerative cascade in a rat model of full-thickness osteochondral defects [38]. With the proper isolation and characterization of ADSCs, Xu et al conducted corresponding assays and successfully identified notable features such as enhanced chondrocyte migration effects and the overall repair of osteochondral defects.
The fat tissue derivative generated through micro-fragmentation, employing mild mechanical force, is comparably safer since it is processed in a closed system, which limits contact with external environment and eliminates requirements for enzymes and other chemicals, thus remaining under minimal manipulation. This non-enzymatic method also allows the preservation of the vascular stromal niche and respective components highly enriched in pericyte-like elements and perivascular identity, which make up high percentages of mesenchymal stem cells (MSCs), thus proving to be of great interest in regenerative medicine [42]. Other researchers confirmed that this orthobiologic product is also a direct source of ADSCs, as they characterized corresponding surface markers and the multi lineage differentiation potential displayed by these cells, specifically collected from human lipoaspirates [43].
SVF Clinical Utility
SVF-derived mesenchymal stem and progenitor cells have the ability to differentiate into several different cell lines, which is a well-established fact [44,45]. This feature makes them a particularly attractive tool for the targeting of many degenerative musculoskeletal disorders, such as OA. In addition to creating new cells and replenishing damaged tissue, MSCs also elicit paracrine and autocrine effects, which allow them to regulate inflammation and attenuate tissue destruction, as it is observed in OA [46]. These cells may be more advantageous not only due to the fact that they are easily obtained in abundance, but also because research has demonstrated the significant efficacy these cells provide as an alternative treatment [47–51].
The first reported use of SVF in clinical scenarios appears to have occurred as early as 2007, specifically applied towards cosmetic purposes and treatment of radiation injuries associated with postradiotherapy in breast cancer patients [33]. In 2008, Yoshimura et al proposed cell-assisted lipotransfer (CAL), thus demonstrating improved fat graft retention with SVF enrichment in soft tissues [52]. Since then, further research has started to emerge in the literature regarding the management of several diseases. A search conducted by ClinicalTrials.gov using specific keywords such as “autologous stromal vascular fraction” and “osteoarthritis” revealed that there are 14 currently registered clinical trials evaluating SVF for OA treatment, of which only 7 have reached completion.
In 2017, a Japanese study [53] had the objective of evaluating clinical outcomes following the intra-articular administration of SVF in 13 patients with knee osteoarthritis. Based on the Kellgren and Lawrence OA classification, 11 patients presented grade IV knee OA, whereas 2 others had grade III. Researchers manually collected approximately 200 ml of autologous subcutaneous adipose tissue from the lower abdomen via tumescent liposuction techniques. A large volume of a very dilute solution of local anesthesia was injected into the fat beneath the skin, leading to tumescence. Tumescent liposuction is exceptionally safe and eliminates the need for general anesthesia and blood transfusions. This method was safer than liposuction under general anesthesia and leads to fewer complications [54,55]. The biological material was then processed using a sterile single-use functionally-closed system (Celution Centrifuge IV) and the disaggregated SVF cells were directly delivered into the articular cavity of the knee. Despite a small sample size, one month after the application of SVF the authors reassessed the patients and concluded that all scores for Japanese Knee Osteoarthritis Measure (JKOM), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and Visual Analogue Scale (VAS) showed statistically significant improvement (P < 0.01) compared to the baseline. In addition to being free of serious adverse events in this particular scenario, the therapeutic approach boosted overall scores by an average of 35%, 32%, and 40% for JKOM, WOMAC, and VAS, respectively.
Other researchers have conducted slightly different approach procedures, obtaining even more optimistic results with larger sample sizes. A multi-centric prospective non-randomized case control study designed by Michalek et al [56] had the objective of evaluating the safety and effectiveness of SVF applications in the treatments of 1,128 patients with moderate to severe OA, with the goal of improving quality of life without critical adverse side effects. Patients with chronic or degenerative joint OA ranging from grade II–IV (Kellgren-Lawrence) regarding weight bearing joints and other joints with significant functional disability were selected after the confirmation of validated medical imaging evidence and failure of conservative treatment alternatives such as anti-inflammatory drugs and physical therapy, for example. The research team also performed the standard tumescent liposuction technique under local anesthesia in order to collect 20-200 ml of lipoaspirate. The enzymatic method was employed in order to retrieve nucleated cells from SVF, which were then isolated and prepared for a single injection either intra-articularly or peri-articularly. Based on the scores of modified Knee injury and Osteoarthritis Outcome Score/Hip disability, and Osteoarthritis Outcome Score (KOOS/HOOS), results revealed at least a 50% improvement of major joints (at least one knee or hip joint) concerning treatment in 80.6% of patients after 3 months, which continued to improve up to 12 months, reaching a rate of 91%. In this particular SVF cell therapy article, a total of 1,856 joints were treated in 1,128 patients with documented safety and a relatively long term clinical effect with a median follow-up time of 17.2 months for the majority of patients, leading to no serious side effects from 1-4.5 years of follow-ups.
It is interesting to note that Michalek et al. [56] found that SVF isolation via collagenase digestion generates maximum cell yields and better short term results in clinical outcomes after 3 months. However, comparable numbers of viable SVF cells may be obtained through non-enzymatic methods if larger amounts of adipose tissue are processed. On the other hand, it appears that patients with elevated body mass indexes (BMI), especially obese individuals, experience slower cartilage regeneration during the first 3 to 6 months. The same is valid for patients who display severe OA progression [56].
Obese individuals are more susceptible to joint degeneration as the mechanical stress applied to the cartilage of weight-bearing joints is significantly higher and may, therefore, impede the standard cellular processes that take place in the joint microenvironment [5,56]. Moreover, since obese and overweight patients may exhibit a larger distribution of adipose tissue, it would be logical to assume that they would provide more biological material to work with; therefore, a higher number of SVF cells with which to further enhance the healing processes. However, this may not be as beneficial as it seems.
Considering the fact that obesity is a central component of metabolic syndrome, the adipose tissues from these patients may undergo a shift in their secretory pattern [5,57]. Under standard metabolic conditions, adipose tissue-resident cells are known to have fine control over the secretion of several molecules. Conversely, whilst under chronic stress brought on by metabolic syndrome, they tend to shift their activity towards a more pro-inflammatory profile. The type 1 macrophage, for instance, can be found in abdominal adipose tissue and is known to be associated with pro-inflammatory activity and production of higher amounts of “inflammokines”, including TNF-α and interleukin 6 [5]. This also contributes to low-grade systemic inflammation as seen in many chronic inflammatory disorders associated with metabolic syndrome, such as OA [5,57]. Therefore practitioners should be wary of possible pitfalls when working with adipose tissue.
SVF Biological Mechanisms
The major advantages regarding the use of SVF on OA are perhaps mostly attributed to cell potential and their associated secretomes when it comes to halting degenerative processes. Normal joint fluid does possess MSCs; however, these cell quantities are quite limited. Moreover, whilst these cells may differentiate into new chondrocytes, the cartilage that is consequentially formed is rather fragile and very susceptible to damage, even when under minimal stress conditions [58]. This led many researchers to take the utilization of SVF into consideration in order to compensate for such drawbacks. As previously mentioned, SVF can elicit a wide set of functions, such as the enhancement of angiogenesis, immunomodulation, cell differentiation, and proliferation. Additionally, there is much discussion regarding the paracrine signaling functions and cellular crosstalk between cells present in SVF and the host microenvironment, which is highly relevant in regeneration [59,60].
Angiogenesis
SVF displays great capacity at promoting angiogenesis and neovascularization, two processes which are essential for tissue homeostasis. These properties appear to be attributed to the heterogeneous cell populations found in this biologic material, which, when combined, enables the formation of new blood vessels. For instance, one in vitro study suggests that endothelial progenitor cells (EPCs) and ADSCs can cooperate to promote greater neovascularization when compared to their individual administration [61]. The paracrine effect of stromal cells, in turn, can boost angiogenesis even further via the release of growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and transforming growth factor beta (TGF-β), whilst macrophages dictate the structural organization of newly formed blood vessels as a result of the direct contribution of pericytes and endothelial cells [59,62–65]. Increased expressions of VEGF, in particular, have been shown to influence migration of more endothelial and stromal cells to the neovascularized region [66]. Interestingly, freshly isolated SVF appears to induce more distinct vasculogenic properties when compared to its cultured form [67].
Immunomodulation
In terms of immunomodulation, diverse SVF cellular components are capable of promoting significant decreases in inflammation and attenuation of escalated immune responses at the injection site [68]. Stem and progenitor cells, for instance, are known to perform antiinflammatory and anti-apoptotic roles that contribute to the regeneration of host tissue.
The Mφ2 macrophage is known to possess anti-inflammatory properties, typically characterized by an increase in the production of interleukins (IL-4, IL-5, IL-9, and IL-13). It is also directly involved in regenerative and tissue repair processes that occur after injuries, which makes it a pivotal agent regarding chronic inflammatory diseases [27,69,70]. T regulatory (Treg) cells are also found in SVF and are known to secrete high concentrations of immune suppressive cytokines [71,72]. Moreover, it has further been reported that these cells can help the macrophages maintain their Mφ2 phenotype [71]. SVF administration can therefore adequately decrease the overproduction of certain inflammatory cytokines and growth factors such as TNF-α, IL-6 and -12, and interferon-γin many disease models [73,74]. For reasons such as these, this orthobiologic product is presented as an excellent tool in mediating inflammation and immune responses via paracrine signaling and subsequent suppression of pro-inflammatory molecules, as well as expressing anti-inflammatory cytokines, accordingly. This effect is highly sought out by physicians in order to attenuate severe pain in patients who suffer from persistent inflammatory diseases; namely, OA.
Cell Proliferation and Differentiation
In addition to modulating inflamed host tissues, SVF provides a rich population of stem cells, which are highly appealing due to their inherent capabilities of differentiating into specific lineages when provided with the appropriate biochemical signals [14]. Furthermore, SVF has been reported to trigger host cell proliferation when delivered into specific regions which include adipose tissue, nerves and injured areas such as diabetic foot ulcers and burn wounds [52,74–81]. This particular feature of SVF has picked interest among researchers.
To further elucidate, proliferation of fibroblasts appears to increase upon exposure to SVF in diabetic foot ulcers, for instance, which contributes to senescent cell signaling and the promotion of wound contraction [81,82]. Similarly, fibroblast activity and proliferation is improved in burn wounds treated with SVF intradermal injections [78]. Regarding OA, intra-articular infiltration of SVF has demonstrated positive results. A recent retrospective article [49] evaluated SVF clinical outcomes in 100 joints from 50 patients with moderate to severe knee OA. The authors observed that this approach not only decreased catabolic and pro-inflammatory molecules but it also induced a significant increase in anabolic and anti-inflammatory molecules, such as IGF-1 and IL-10. This is greatly attributed to the signaling properties of SVF cells, which can initiate a cascade of molecular and structural events [49]. MSC population found in SVF, for instance, can undergo proliferation and differentiate into chondrogenic lineages [44].
In addition, the immunomodulatory effects these cells exert can control exacerbated inflammation [83], which ultimately reestablishes regular joint homeostasis. However, a previous in vitro study [84] concluded that more cartilage is generated from chondrocytes cocultured with SVF rather than expanded adipose stem cells. The authors explain that under this specific circumstance, such occurrence may be in fact better attributed to the trophic role of SVF-derived MSCs in stimulating chondrocyte proliferation and matrix production rather than MSCs undergoing chondrogenic differentiation.
Enhancement of Extracellular Matrix Function
Another important aspect of regeneration which is specifically addressed by the application of SVF is extracellular matrix (ECM) regulation. ECM is a vital component of cellular structure, filling the role of a scaffold and therefore contributing to accelerated tissue regeneration [85]. It is made up of a mixture of proteins and molecules such as collagen, laminin, elastin, and fibronectin, which are often secreted in abundance by fibroblasts [14,86,87]. The ECM primary role is to establish and maintain dynamic interaction with integrins present on adhesive cell surfaces. This interaction dictates several biological responses due to the adequate binding of integrins to ECM receptors leading to signaling cascades and alterations in cell activity [88].
Additional effects mediated by this interaction include cell migration, since they rely on the proper attachment of integrins to cytoskeletons in order to pull themselves into motion [89,90]. For these reasons, SVF-secreted ECM proteins can further contribute to regenerative processes, especially via the growth of vascular networks, since they control morphogenesis and migration speed during angiogenic events [91].
In addition to these beneficial properties, the biological value of ECM is also partially attributed to its role as a reservoir for bioactive molecules. ECM has the capability of storing and confiscating cytokines and growth factors, shifting and regulating their concentrations and bioavailability [86]. The fibroblast growth factor family, for instance, can establish strong chemical bonds with heparin sulfate proteoglycan chains. These, in turn, are involved in the binding, transportation and activation of pivotal transcription factors, such as Wnt and hedgehog proteins [86]. In regular tissue homeostasis, controlled proteolysis releases the bioactive cytokines and growth factors, which can subsequently regulate other physiological and pathological responses on their own, such as angiogenesis or even ligand maturation [86,92].
ECM also exhibits biochemical and mechanical properties, which allow it to sense and interact with the extracellular microenvironment through means of signal transduction pathways. These chemical signals are produced by some ECM proteins such as integrins and fibronectin, as well as growth factors and other similar signaling molecules [93,94]. Interaction between these molecules and different matrices with specific sets of receptors is responsible for variations in the generated cellular responses [93,94]. Its mechanical features allow it to function as a physical barrier, an anchorage site or even a movement track for cell migration [92].
ECM offers rigidity, porosity, density, insolubility and topography, providing cells with physical cues. These are detected by cell surface proteins, primarily integrins, which then connect the ECM extracellular compartment to the actin cytoskeleton inside cells.
Stiff ECMs are known to promote integrin clustering, robust focal adhesions, and activation of Rho and MAP kinase pathways, which in conjunction may lead to increased contractile functions and cell proliferation [92]. Such trait also appears to affect differentiation, since on a soft ECM the MSCs are more inclined towards differentiation into neurogenic cells, whereas on a stiff matrix they seem to undergo osteogenic lineage differentiation pathways, for instance [86,95].
By now it seems quite evident that in cases of chronic inflammatory and degenerative disorders, injured tissues can greatly benefit from SVF administration, considering that it is a natural biological material that can sustain a regenerative microenvironment.
Conclusion
Stromal vascular fraction obtained from processed adipose tissue may be presented as an excellent orthobiologic tool for the treatment of many complicated and impactful musculoskeletal disorders, such as osteoarthritis. The diverse cell populations found in this biologic material elicit multiple effects such as the enhancement of angiogenesis, immunomodulation, and cell differentiation and proliferation. Mesenchymal stem cells, in particular, secrete a variety of cytokines and growth factors. These bioactive molecules can promote anti-inflammatory effects and additional biologic responses that can redirect the excessive inflammation towards more regenerative pathways in the joint microenvironment. Since osteoarthritis is mainly characterized by progressive degeneration and persistent inflammation, the application of SVF as an orthobiologic tool for the management of this orthopedic condition appears to be a viable solution, considering its relatively easy processing methods and high cell yield.
Glossary
List of Abbreviations
- SVF:
Stromal vascular fraction
- OA:
Osteoarthritis
- NSAID:
Non-Steroidal Anti-Inflammatory Drug
- PRP:
Platelet Rich Plasma
- BMA:
Bone Marrow Aspirate
- BMAC:
Bone Marrow Aspirate Concentrate
- ADSC:
Adipose-Derived Stem Cell
- GMP:
Good Manufacturing Practice
- MSC:
Mesenchymal Stem Cell
- CAL:
Cell-Assisted Lipotransfer
- JKOM:
Japanese Knee Osteoarthritis Measure
- VAS:
Visual Analogue Scale
- WOMAC:
Western Ontario and McMaster Universities Osteoarthritis Index
- KOOS/HOOS:
modified Knee injury and Osteoarthritis Outcome Score/Hip disability, and Osteoarthritis Outcome Score
- EPC:
Endothelial Progenitor Cell
- VEGF:
Vascular Endothelial Growth Factor
- HGF:
Hepatocyte Growth Factor
- TGF-β:
Transforming Growth Factor Beta
- IL:
Interleukin
- TNF-α:
Tumor Necrosis Factor Alpha
- IGF-1:
Insulin-Like Growth Factor 1
- ECM:
Extracellular Matrix
- MAP:
Mitogen-Activated Protein
- BMI:
Body Mass Index
- Mφ2:
Macrophage Type 2
- GBD:
Global Burden of Disease
Conflict of Interest
None
References
- 1.Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020;396((10264)):1711–1712. doi: 10.1016/S0140-6736(20)32230-3. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Collaborative Network. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results.; 2019
- 3.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26((3)):355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzini GOM, Santos GS, Visoni SBC, Azzini VOM, Santos RGD, Huber SC, Lana JF. Metabolic syndrome and subchondral bone alterations: The rise of osteoarthritis - A review. J Clin Orthop Trauma. 2020;11((Suppl 5)):S849–S855. doi: 10.1016/j.jcot.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189–2196. doi: 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setti T, Arab MGL, Santos GS, Alkass N, Andrade MAP, Lana JFSD. The protective role of glutathione in osteoarthritis. J Clin Orthop Trauma. 2020;15:145–151. doi: 10.1016/j.jcot.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafsi K, McKay J, Li J, Lana JF, Macedo A, Santos GS, Murrell WD. Nutritional, metabolic and genetic considerations to optimise regenerative medicine outcome for knee osteoarthritis. J Clin Orthop Trauma. 2019;10((1)):2–8. doi: 10.1016/j.jcot.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcum ZA, Hanlon JT. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann Longterm Care. 2010;18((9)):24–27. [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon MS, Behera P, Patel S, Shetty V. Orthobiologics and platelet rich plasma. Indian J Orthop. 2014;48((1)):1–9. doi: 10.4103/0019-5413.125477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purita J, Lana JFSD, Kolber M, Rodrigues BL, Mosaner T, Santos GS, Caliari-Oliveira C, Huber SC. Bone marrow-derived products: A classification proposal - bone marrow aspirate, bone marrow aspirate concentrate or hybrid? World J Stem Cells. 2020;12((4)):241–250. doi: 10.4252/wjsc.v12.i4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huddleston HP, Maheshwer B, Wong SE, Chahla J, Cole BJ, Yanke AB. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Oper Tech Sports Med. 2020;28:150759. [Google Scholar]
- 13.Han S, Sun HM, Hwang KC, Kim SW. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit Rev Eukaryot Gene Expr. 2015;25((2)):145–52. doi: 10.1615/critreveukaryotgeneexpr.2015013057. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Nguyen A, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J Plast Reconstr Aesthet Surg. 2016;69((2)):180–8. doi: 10.1016/j.bjps.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Boquest AC, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2((4)):319–29. doi: 10.1007/BF02698059. [DOI] [PubMed] [Google Scholar]
- 16.Huang T, He D, Kleiner G, Kuluz J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med. 2007;30(Suppl 1(Suppl 1)):S35–40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24((5)):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 18.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7((1)):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochette L, Mazini L, Malka G, Zeller M, Cottin Y, Vergely C. The Crosstalk of Adipose-Derived Stem Cells (ADSC), Oxidative Stress, and Inflammation in Protective and Adaptive Responses. Int J Mol Sci. 2020;21((23)):9262. doi: 10.3390/ijms21239262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13((2)):305–12. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 21.Andersen E, Ingerslev LR, Fabre O, Donkin I, Altıntaş A, Versteyhe S, Bisgaard T, Kristiansen VB, Simar D, Barrès R. Preadipocytes from obese humans with type 2 diabetes are epigenetically reprogrammed at genes controlling adipose tissue function. Int J Obes (Lond). 2019;43((2)):306–318. doi: 10.1038/s41366-018-0031-3. [DOI] [PubMed] [Google Scholar]
- 22.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275((5302)):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 23.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196((3)):430–43. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 24.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7((10)):803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 25.Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36((2)):451–5. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar BV, Connors TJ, Farber DL. Human T Cell Development, Localization, and Function throughout Life. Immunity. 2018;48((2)):202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27((1-4)):201–6. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 29.Larosa DF, Orange JS. 1. Lymphocytes. J Allergy Clin Immunol. 2008;121((2 Suppl)):S364–9. doi: 10.1016/j.jaci.2007.06.016. quiz S412. [DOI] [PubMed] [Google Scholar]
- 30.Haack-Sørensen M, Follin B, Juhl M, Brorsen SK, Søndergaard RH, Kastrup J, Ekblond A. Culture expansion of adipose derived stromal cells. A closed automated Quantum Cell Expansion System compared with manual flask-based culture. J Transl Med. 2016;14((1)):319. doi: 10.1186/s12967-016-1080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thirumala S, Goebel WS, Woods EJ. Manufacturing and banking of mesenchymal stem cells. Expert Opin Biol Ther. 2013;13((5)):673–91. doi: 10.1517/14712598.2013.763925. [DOI] [PubMed] [Google Scholar]
- 32.Rodbell M. Metabolism of isolated fat cells. i. effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–80. [PubMed] [Google Scholar]
- 33.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8((1)):145. doi: 10.1186/s13287-017-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adães S, Mendonça M, Santos TN, Castro-Lopes JM, Ferreira-Gomes J, Neto FL. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res Ther. 2014;16((1)):R10. doi: 10.1186/ar4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12((12)):3375–82. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 36.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander RW. Understanding Mechanical Emulsification ( Nanofat ) Versus Enzymatic Isolation of Tissue Stromal Vascular Fraction ( tSVF ) Cells from Adipose Tissue: Potential Uses in Biocellular Regenerative Medicine. J Prolotherapy. 2016;8:e947–e960. [Google Scholar]
- 38.Xu T, Yu X, Yang Q, Liu X, Fang J, Dai X. Autologous Micro-Fragmented Adipose Tissue as Stem Cell-Based Natural Scaffold for Cartilage Defect Repair. Cell Transplant. 2019;28((12)):1709–1720. doi: 10.1177/0963689719880527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremolada C, Colombo V, Ventura C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr Stem Cell Rep. 2016;2((3)):304–312. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tremolada C, Ricordi C, Caplan AI, Ventura C. Mesenchymal Stem Cells in Lipogems, a Reverse Story: from Clinical Practice to Basic Science. Methods Mol Biol. 2016;1416:109–22. doi: 10.1007/978-1-4939-3584-0_6. [DOI] [PubMed] [Google Scholar]
- 41.Kaewsuwan S, Song SY, Kim JH, Sung JH. Mimicking the functional niche of adipose-derived stem cells for regenerative medicine. Expert Opin Biol Ther. 2012;12((12)):1575–88. doi: 10.1517/14712598.2012.721763. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22((11)):2063–77. doi: 10.3727/096368912X657855. [DOI] [PubMed] [Google Scholar]
- 43.Shah FS, Wu X, Dietrich M, Rood J, Gimble JM. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15((8)):979–85. doi: 10.1016/j.jcyt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7((6)):718–29. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krampera M, Franchini M, Pizzolo G, Aprili G. Mesenchymal stem cells: from biology to clinical use. Blood Transfus. 2007;5((3)):120–9. doi: 10.2450/2007.0029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalek J, Vrablikova A, Darinskas A, Lukac L, Prucha J, Skopalik J, Travnik J, Cibulka M, Dudasova Z. Stromal vascular fraction cell therapy for osteoarthritis in elderly: Multicenter case-control study. J Clin Orthop Trauma. 2019;10((1)):76–80. doi: 10.1016/j.jcot.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21((1)):207. doi: 10.1186/s12891-020-03231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lapuente JP, Dos-Anjos S, Blázquez-Martínez A. Intraarticular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res. 2020;15((1)):137. doi: 10.1186/s13018-020-01664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simunec D, Salari H, Meyer J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells. 2020;9((9)):2096. doi: 10.3390/cells9092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ude CC, Shah S, Ogueri KS, Nair LS, Laurencin CT. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen Eng Transl Med. 2021. [DOI] [PMC free article] [PubMed]
- 52.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32((1)):48–55. doi: 10.1007/s00266-007-9019-4. discussion 56-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokota N, Yamakawa M, Shirata T, Kimura T, Kaneshima H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther. 2017;6:108–112. doi: 10.1016/j.reth.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanke CW, Bernstein G, Bullock S. Safety of tumescent liposuction in 15,336 patients. National survey results. Dermatol Surg. 1995;21((5)):459–62. doi: 10.1111/j.1524-4725.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 55.Venkataram J. Tumescent liposuction: a review. J Cutan Aesthet Surg. 2008;1((2)):49–57. doi: 10.4103/0974-2077.44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, Rybar J, Chaloupka A, Darinskas A, Michalek J, Kristek J, Travnik J, Jabandziev P, Cibulka M, Skopalik J, Kristkova Z, Dudasova Z. Stromal Vascular Fraction Cells of Adipose and Connective Tissue in People with Osteoarthritis: A Case Control Prospective Multi-Centric Non-Randomized Study. Glob Surg, 2017;3((3)):1–9. [Google Scholar]
- 57.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8((41)):55–60. [PubMed] [Google Scholar]
- 58.Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019;47((1)):882–890. doi: 10.1080/21691401.2019.1576710. [DOI] [PubMed] [Google Scholar]
- 59.Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, Vesey G, Herbert BR. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6((3)):e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104((12)):1410–20. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 62.Koh YJ, Koh BI, Kim H, Joo HJ, Jin HK, Jeon J, Choi C, Lee DH, Chung JH, Cho CH, Park WS, Ryu JK, Suh JK, Koh GY. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31((5)):1141–50. doi: 10.1161/ATVBAHA.110.218206. [DOI] [PubMed] [Google Scholar]
- 63.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109((10)):1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 64.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97((6)):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 65.Kwon HM, Hur SM, Park KY, Kim CK, Kim YM, Kim HS, Shin HC, Won MH, Ha KS, Kwon YG, Lee DH, Kim YM. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63((1)):19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Zhu M, Dong Z, Gao J, Liao Y, Xue J, Yuan Y, Liu L, Chang Q, Lu F. Adipocyte regeneration after free fat transplantation: promotion by stromal vascular fraction cells. Cell Transplant. 2015;24((1)):49–62. doi: 10.3727/096368913X675133. [DOI] [PubMed] [Google Scholar]
- 67.Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials. 2014;35((19)):5065–78. doi: 10.1016/j.biomaterials.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 68.Fu S, Luan J, Xin M, Wang Q, Xiao R, Gao Y. Fate of adipose-derived stromal vascular fraction cells after coimplantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg. 2013;132((2)):363–373. doi: 10.1097/PRS.0b013e31829588b3. [DOI] [PubMed] [Google Scholar]
- 69.Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev. 2013;22((6)):985–97. doi: 10.1089/scd.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007;31((9)):1420–8. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 71.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104((49)):19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu L, Yang F, Zhang F, Guo D, Li L, Wang X, Liang T, Wang J, Cai Z, Jin H. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018;9((9)):905. doi: 10.1038/s41419-018-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, Gimble JM, Bunnell BA. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med. 2013;2((10)):789–96. doi: 10.5966/sctm.2013-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Premaratne GU, Ma LP, Fujita M, Lin X, Bollano E, Fu M. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg. 2011;6:43. doi: 10.1186/1749-8090-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Dijk A, Naaijkens BA, Jurgens WJ, Nalliah K, Sairras S, van der Pijl RJ, Vo K, Vonk AB, van Rossum AC, Paulus WJ, van Milligen FJ, Niessen HW. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011;7((3)):219–29. doi: 10.1016/j.scr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 76.Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21((4)):545–53. doi: 10.1111/wrr.12051. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Gao J, Cha P, Chang Q, Liao Y, Liu C, Li K, Lu F. Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatol Surg. 2013;39((3 Pt 1)):449–56. doi: 10.1111/dsu.12058. [DOI] [PubMed] [Google Scholar]
- 78.Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40((7)):1375–83. doi: 10.1016/j.burns.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Chung MT, Paik KJ, Atashroo DA, Hyun JS, McArdle A, Senarath-Yapa K, Zielins ER, Tevlin R, Duldulao C, Hu MS, Walmsley GG, Parisi-Amon A, Momeni A, Rimsa JR, Commons GW, Gurtner GC, Wan DC, Longaker MT. Studies in fat grafting: Part I. Effects of injection technique on in vitro fat viability and in vivo volume retention. Plast Reconstr Surg. 2014;134((1)):29–38. doi: 10.1097/PRS.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63((9)):1544–52. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 81.You HJ, Han SK. Cell therapy for wound healing. J Korean Med Sci. 2014;29((3)):311–9. doi: 10.3346/jkms.2014.29.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 2010;18((4)):342–8. doi: 10.1111/j.1524-475X.2010.00593.x. [DOI] [PubMed] [Google Scholar]
- 83.Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu L, Prins HJ, Leijten J, Helder MN, Evseenko D, Moroni L, van Blitterswijk CA, Lin Y, Karperien M. Chondrocytes Cocultured with Stromal Vascular Fraction of Adipose Tissue Present More Intense Chondrogenic Characteristics Than with Adipose Stem Cells. Tissue Eng Part A. 2016;22((3-4)):336–48. doi: 10.1089/ten.TEA.2015.0269. [DOI] [PubMed] [Google Scholar]
- 85.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13((5)):377–83. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 86.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23((8 Suppl 1)):S20–3. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4((1)):a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200((4)):423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 89.Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8((2)):51–4. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 90.Friedl P, Zänker KS, Bröcker EB. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43((5)):369–78. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 91.Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol. 2009;5((7)):e1000445. doi: 10.1371/journal.pcbi.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3((12)):a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3((5)):a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013;24((5)):447–58. doi: 10.1016/j.devcel.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun M, Chi G, Xu J, Tan Y, Xu J, Lv S, Xu Z, Xia Y, Li L, Li Y. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem Cell Res Ther. 2018;9((1)):52. doi: 10.1186/s13287-018-0798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


