Abstract
Escherichia coli fhuF mutants, a sufS::MudI mutant, and a sufD::MudI mutant were found to have the same phenotype: the inability to use ferrioxamine B as an iron source in a plate assay. In addition, the sufS and sufD genes were shown to be regulated by the iron-dependent Fur repressor. Sequence analysis revealed that the sufS open reading frame corresponds to orf f406. The protein SufS belongs to the family of NifS-like proteins, which supply sulfur for [Fe-S] centers. The protein FhuF contains a [2Fe-2S] center. A mutation in the upstream sufD gene (orf f423) caused the same phenotype. The T7 expression system and a His tag allow the isolation in good yield of the FhuF protein from a wild-type strain. In contrast, overproduction of the protein in a ΔsufD strain failed. Radioactive labeling of N-His-FhuF with [35S]methionine showed that the protein was unstable in the ΔsufD mutant.
Under low-iron growth conditions, many bacteria secrete specific chelators called siderophores. Production of siderophores and transport systems for Fe3+-loaded siderophores is regulated in gram-negative bacteria by the repressor protein Fur with Fe2+ as a corepressor (5). In Escherichia coli, a fhuF-lacZ operon fusion has been used as a reporter to study iron regulation (15) since this fusion reacts very sensitively to slight changes in the iron concentration of the medium. The only phenotype observed for fhuF mutations is a diminished ability of the cells to use ferrioxamine B as an iron source. Ferrioxamine B is a poor iron source for E. coli K-12 (7, 12) because this strain lacks a specific receptor for it in the outer membrane; this receptor is found in yersiniae (2) and other enterobacteria (10). The FhuF protein has been purified and found to be an unusual [2Fe-2S] protein (12).
Isolation of sufS mutants and their phenotype.
Mutants with a FhuF-like phenotype were selected with the aim of further defining the function of FhuF in ferrioxamine B uptake. Iron-regulated lacZ operon fusions were isolated by using the transposing phage MudI (Ap lac) as previously described (6, 12). Under low-iron growth conditions, transcription of the fusion was derepressed. The mutants were tested for the ability to utilize various siderophores as iron sources in a plate assay. Enterochelin, citrate, ferrichrome, and coprogen stimulated normal growth under iron-limiting conditions. Only ferrioxamine B and rhodotorulic acid caused a reduced growth zone on an iron-limited medium in a filter paper disc assay (Table 1). This phenotype was identical to that of fhuF mutants (12). Mapping experiments using P1 (data not shown) indicated that the phage was not located in or near fhuF at 99 min on the genetic map of E. coli (3).
TABLE 1.
Growth response of parent strain H1443 and suf mutants to iron-loaded siderophores on iron-limiting nutrient broth dipyridyl platesa
| Strain | Growth zone diam (mm)b
|
|
|---|---|---|
| Ferrioxamine B | Rhodotorulic acid | |
| H1443 | 23 | 35 |
| H1489 sufS::MudI | 11 | 26 |
| H1490 sufD::MudI | 13 | 26 |
Described in reference 12.
Filter paper discs containing 15 μl of 2 mM ferrioxamine B or 0.5 mM rhodotorulic acid were applied, and growth zones were measured after 16 h at 27°C.
Sequencing of the sufS::lacZ fusion site and localization on the E. coli genetic map.
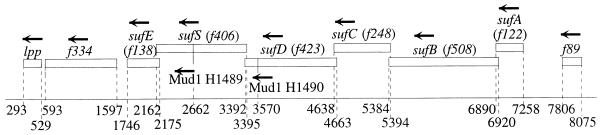
Two independently isolated lacZ operon fusions in E. coli H1489 and H1490 were studied. To identify the fusion site, chromosomal DNA of the mutants was digested with HincII and cloned into the SmaI site of the vector pSU18 (1). By PCR with a MudI-derived primer (CACGTACATGCCGCCAAACTCACCA) and the UNI primer (CGACGTTGTAAAACGACGGCCAGT), 0.95- and 0.9-kb fragments were obtained from strains H1489 and H1490, respectively. Cloning and DNA sequencing revealed that the MudI phage in strain H1489 was inserted in open reading frame orf f406 upstream of bp 2662 (Fig. 1) in E. coli K-12 MG1655 section 153 (of 400) of the complete genome (4). Since f406 is most likely involved in the mobilization of sulfur (see below), it will be called sufS. It is located at 38 min near lpp (structural gene for the murein lipoprotein) on the genetic map of E. coli (3). The MudI phage of strain H1490 was inserted into orf f423 (sufD) upstream of sufS and upstream of bp 3750 in section 153 of the E. coli K-12 genome (4). sufS and sufD seem to be part of an operon with six open reading frames, where each gene following the first open reading frame, f122 (sufA), has a start codon overlapping the stop codon of the previous gene (Fig. 1).
FIG. 1.
Map of the suf gene cluster at 37.8 min on the linkage map of E. coli (3). Stop and start positions and the insertion sites of the two MudI phages are given according to the base pair numbering of section 153 of the E. coli genome sequence (4). The designations of the open reading frames in the GenBank database are given in parentheses. The arrows indicate the direction of transcription.
To confirm the determined positions of the insertions, the mutants were transduced with P1 carrying the marker lpp::Tn10 by using the donor strain WR19-1 (14). A cotransduction frequency of 95% was observed with each inserted phage, as expected from the short distance between sufS and lpp (Fig. 1); these results support the sequencing data.
Regulation of sufD and sufS by iron.
Derepression of lacZ by low iron had been the selection criterion for the sufD and sufS mutants. This indicated that the putative operon may be regulated by Fur, and indeed, inspection of the promoter region revealed a possible Fur binding site at positions 7305 to 7323 in section 153 of the E. coli K-12 genome (4) (GATAATGATtATCAgTtca; the lowercase letters differ from the Fur box consensus sequence GATAATGATAATCATTATC).
To test whether the observed regulation is Fur dependent, fur-28 zbf::Tn10 was transduced by P1 into strains H1489 and H1490, which resulted in strains SIP593 and SIP596, respectively. The phenotype of the transductants was tested on MacConkey lactose iron plates; indeed, the fur mutants formed red colonies, while their parent strains formed white colonies. Quantitative determination of β-galactosidase activities confirmed these observations. Strains H1489 and H1490 had β-galactosidase activities of 13 to 16 U during exponential growth on TY (12) medium, and after 1 h of growth with 50 μM EDDA (7) added, activities of 38 to 46 U were observed. The Fur mutants grown on TY medium contained β-galactosidase activities of 46 to 48 U; addition of 50 μM EDDA slightly enhanced the activities to 59 to 62 U.
Synthesis of N-His-FhuF in a sufD mutant.
Strain H1490 sufD::MudI was grown at 42°C. Of 10 temperature-resistant colonies tested, two were ampicillin sensitive, which indicated deletion of the integrated MudI phage. One of these strains, H5385 ΔsufD, was transformed with plasmids pGP1-2, which encodes the T7 RNA polymerase (16), and pKF191, which carries the gene encoding a FhuF protein with a His tag cloned downstream of the T7 Φ10 promoter region (12).
The His tag allows one-step isolation of the N-His-FhuF protein with Ni2+-nitrilotriacetate resin (12). The membranes of the ruptured cells of strain WM1576/pKF191 fhuF+ were sedimented, and the clear brown supernatant was applied to an Ni2+-nitrilotriacetate-agarose column. At the top of the gel matrix, a deep brown band appeared, which migrated through the column during elution with 0.5 M imidazole. About 40 mg of protein/g (wet weight) of cells was isolated. The same procedure was used with cells of strain H5385 ΔsufD (pGP1-2 pKF191 fhuF+). However, no brown band appeared, and only very small amounts of N-His-FhuF protein could be demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the imidazole eluate. The amounts were so small that we were unable to determine by UV–visible-light spectroscopy whether the eluted FhuF protein contained the [2Fe-2S] center.
To determine whether the N-His-FhuF protein was produced in the ΔsufD strain, the protein was selectively labeled with [35S]methionine (12, 16). After 10 min, [35S]methionine was chased with 50 mM unlabeled methionine. After SDS-PAGE, a strongly labeled N-His-FhuF protein band was observed, and after a 30-min chase with unlabeled methionine, most of the labeled protein band disappeared (Fig. 2); in contrast, the same protein was stable in a suf+ background (Fig. 2).
FIG. 2.
Section of an autoradiograph after SDS-PAGE of [35S]methionine-labeled N-His-FhuF protein. Strains H5385 pGP1-2 (lanes 1 and 2) and WM1576 (lanes 3 to 5) were transformed with plasmid pKF191, which allowed the exclusive expression of the gene encoding N-His-FhuF by the T7 RNA polymerase system (12, 16). Ten minutes after addition of [35S]methionine, whole cells were pelleted by centrifugation and an aliquot was separated by SDS-PAGE (lanes 1 and 3). For the chase, the remaining cells were suspended in fresh medium containing 50 mM unlabeled methionine and incubated for 15 min (lane 4) and 30 min (lanes 2 and 5) before loading on the polyacrylamide gel.
Conclusions.
The NifS protein is required for the construction of the [Fe-S] cluster of the nitrogenase in Azotobacter vinelandii. Three open reading frames coding for proteins with similarities to NifS have been identified in the E. coli K-12 genome (4). One is the NifS-like protein, now called IscS (17), which has been purified and described as a pyridoxal phosphate-dependent cysteine desulfurase by Flint (8) and is encoded at 57 min on the genetic map of E. coli. A second E. coli protein of this family has been characterized as a selenocysteine lyase (called Csd) with cysteine sulfinate desulfinase activity (11). The corresponding gene has been cloned and mapped at 63 min on the E. coli map. The similarity of NifS-like proteins has been discussed extensively by Mihara et al. (11), who pointed out that Csd and NifS are members of two subfamilies. The SufS protein and Csd show 43% amino acid identity, and SufS and IscS have 22% identity, which reflects the fact that SufS also belongs to the Csd group of NifS-like proteins. In addition, it is interesting that SPL1 from yeast, a protein involved in RNA splicing, also belongs to the group of NifS-like proteins (similarity to NifS, 40%; similarity to SufS, 21%).
Examination of the genes in the neighborhood of sufS revealed an operon structure (Fig. 1). The product of the first gene, sufA, is similar to IscA from E. coli and A. vinelandii (Fig. 3), which is encoded by the iscSUA gene cluster (17). Genes encoding counterparts of IscSUA have been found near nifS in A. vinelandii and also in different bacteria and eukaryotes. The function of IscA and IscU is not known, but it is assumed that the proteins from these operons synthesize iron-sulfur centers of various proteins (17). An obvious counterpart for IscU or NifU is missing in the SufABCDSE cluster. However, SufE and YgdK from E. coli have 35% amino acid identity. ygdK encodes an open reading frame directly downstream of csd, which again points to a certain relationship between Csd and SufS.
FIG. 3.
Comparison of SufA-like proteins IscA from E. coli (8, 17), IscA from A. vinelandii (17), and YadR from E. coli (4) with SufA. A star indicates identity, and a caret indicates similarity. Percent similarity to SufA is given.
The derived gene product of open reading frame sufC has clear-cut similarities to the ATPase subunits of ABC transporters. The gene products of open reading frames sufB and sufD are predicted in the databases to be the membrane components of a family of ABC transporters. However, using various protein structure prediction programs, no membrane-spanning helices were detected for these proteins and their homologues in chloroplasts. No published experimental data which demonstrate the membrane localization of any protein of the SufB-related family were found.
Since the insertion of MudI into sufS and sufD only had an effect on ferrioxamine B utilization, no other vital functions seem to depend solely on these genes. In this early stage of the investigation, it is not known if the sufD product is directly involved in ferrioxamine utilization or if the Mu insertion has a polar effect on sufSE. However, it is interesting that these genes are regulated by Fur and iron, as observed for fhuF.
The instability of FhuF could have various causes. One may be that FhuF is part of the Suf complex and is unstable as single protein. Another interpretation, which we prefer, is that the Suf complex is necessary for the building of the “distorted” [2Fe-2S] center in FhuF and that the apo-FhuF protein is unstable in the cell. However, this instability must be very peculiar since the various FhuF Cys→Ser mutant proteins which did not contain a [2Fe-2S] center were isolated in relatively large amounts (12).
Acknowledgments
We thank Christian Bayertz for technical assistance, Volkmar Braun (Tübingen) and Uwe Stroeher (Tübingen) for reading the manuscript and discussion, and Karen A. Brune (Allensbach) for editing the manuscript.
This research was supported by the Deutsche Forschungsgemeinschaft, Schwerpunktprogramm “Molekulare Analyse von Regulationsnetzwerken in Bakterien” and Sonderforschungsbereich 323.
REFERENCES
- 1.Bartolome B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBr322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 2.Bäumler A J, Hantke K. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol Microbiol. 1992;6:1309–1321. doi: 10.1111/j.1365-2958.1992.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 3.Berlyn M K B. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Iron transport and storage in microorganisms, plants and animals. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 67–145. [PubMed] [Google Scholar]
- 6.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mulac bacteriophage: in vitro probe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chart H, Buck M, Stevenson P, Griffiths E. Iron regulated outer membrane proteins of Escherichia coli: variations in expression due to the chelator used to restrict the availability of iron. J Gen Microbiol. 1986;132:1373–1378. doi: 10.1099/00221287-132-5-1373. [DOI] [PubMed] [Google Scholar]
- 8.Flint D H. Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J Biol Chem. 1996;271:16068–16074. [PubMed] [Google Scholar]
- 9.Garg R P, Vargo C J, Cui X, Kurtz D M., Jr A [2Fe-2S] protein encoded by an open reading frame upstream of the Escherichia coli bacterioferritin gene. Biochemistry. 1996;35:6297–6301. doi: 10.1021/bi9600862. [DOI] [PubMed] [Google Scholar]
- 10.Matzanke B F, Berner I, Bill E, Trautwein A X, Winkelmann G. Transport and utilization of ferrioxamine-E-bound iron in Erwinia herbicola (Pantoea agglomerans) Biol Metals. 1991;4:181–185. doi: 10.1007/BF01141312. [DOI] [PubMed] [Google Scholar]
- 11.Mihara H, Kurihara T, Yoshimura T, Soda K, Esaki N. Cysteine sulfinate desulfinase, a NIFS-like protein of Escherichia coli with selenocysteine lyase and cysteine desulfurase activities. Gene cloning, purification, and characterization of a novel pyridoxal enzyme. J Biol Chem. 1997;272:22417–22424. doi: 10.1074/jbc.272.36.22417. [DOI] [PubMed] [Google Scholar]
- 12.Müller K, Matzanke B F, Schünemann V, Trautwein A X, Hantke K. FhuF, an iron-regulated protein of Escherichia coli with a new type of [2Fe-2S] center. Eur J Biochem. 1998;258:1001–1008. doi: 10.1046/j.1432-1327.1998.2581001.x. [DOI] [PubMed] [Google Scholar]
- 13.Quail M A, Jordan P, Grogan J M, Butt J N, Lutz M, Thomson A J, Andrews S C, Guest J R. Spectroscopic and voltammetric characterisation of the bacterioferritin-associated ferredoxin of Escherichia coli. Biochem Biophys Res Commun. 1996;229:635–642. doi: 10.1006/bbrc.1996.1856. [DOI] [PubMed] [Google Scholar]
- 14.Rotering H, Fiedler W, Rollinger W, Braun V. Procedure for the identification of Escherichia coli mutants affected in components containing glycerol derived from phospholipid turnover: isolation of mutants lacking glycerol in membrane-derived oligosaccharides (MDO) FEMS Microbiol Lett. 1984;22:61–68. [Google Scholar]
- 15.Stojiljkovic I, Bäumler A J, Hantke K. Fur regulon in Gram-negative bacteria: identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 16.Tabor S. Unit 16.2: expression using the T7 RNA polymerase/promoter system. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 16.2.1–16.2.11. [Google Scholar]
- 17.Zheng L, Cash V L, Flint D H, Dean D R. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]