Abstract
Background:
Antiangiogenic therapy combined with chemotherapy could improve pathological complete response (pCR) for breast cancer. Apatinib is an oral tyrosine kinase inhibitor that selectively inhibits vascular endothelial growth factor receptor 2. We assessed the efficacy and safety of apatinib combined with standard neoadjuvant chemotherapy in patients with triple-negative breast cancer (TNBC).
Materials and methods:
This single-arm, phase II study enrolled patients aged 18–70 years with previously untreated stage IIA-IIIB TNBC. Patients received oral apatinib at a dose of 250 mg once daily and intravenously docetaxel every 3 weeks for four cycles, followed by epirubicin plus cyclophosphamide every 3 weeks for four cycles. The primary endpoint was the pCR rate in the breast and lymph nodes. Secondary endpoints included objective response rate, event-free survival (EFS), overall survival (OS), and safety.
Results:
In all, 31 patients were enrolled, and the median follow-up time was 22.9 months (range: 10.1–41.6 months). The pCRs in both breast and lymph nodes were achieved in 17 [54.8%; 95% confidence interval (CI): 36.0–72.7] of 31 patients. Objective responses were achieved in 29 patients (93.5%; 95% CI: 78.6–99.2), and disease control was achieved in 31 patients (100%; 95% CI: 88.8–100.0). The 2-year EFS and 2-year OS were 90.9% and 94.4%, respectively. The five most common treatment-related adverse events were fatigue (51%), hypertension (41%), anorexia (39%), hand–foot syndrome (35%), and diarrhea (32%). Few grade 3 or more adverse events were observed.
Conclusion:
The combination of apatinib with docetaxel followed by epirubicin plus cyclophosphamide showed excellent efficacy and manageable toxicities; and further randomized controlled phase III trials are warranted.
Trial registration:
This trial was registered with ClinicalTrials.gov (NCT03243838) on 5 August 2017.
Keywords: apatinib, neoadjuvant chemotherapy, pathological complete response, survival, triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) accounts for 10–15% of invasive breast cancers and is highly proliferative, with high risk for recurrence, relatively poorer prognosis, and rapid disease progression.1–4 Since currently no targeted agents are approved for treatment of TNBC, chemotherapy remains a standard treatment. Neoadjuvant chemotherapy (NACT) has become a standard treatment for stage II-III TNBC, since it can improve the rates of breast-conserving surgery,5–7 increase the rates of sentinel lymph node biopsy,8–10 and prolong the patients’ outcomes. 11 Approximately 28–34% of patients with stage II to III TNBC treated with anthracycline- and taxane-based NACT can achieve a pathological complete response (pCR), which is associated with low rates of relapse and death.12–14 Nowadays, combination of immune checkpoint inhibitor (ICI) with NACT has improved pCR and survival outcomes. In Keynote 522, the 18-month event-free survival (EFS) was 91.3% and 85.3% with and without ICI, respectively, and the 3-year overall survival (OS) was 89.7% and 86.9% with and without ICI, respectively.
Several findings suggested that angiogenesis, a validated target in the treatment of TNBC, plays an important role in tumor formation, growth, invasion, and metastasis.15–17 Vascular endothelial growth factor (VEGF) is one of the most potent promoters of angiogenesis; overexpression of VEGF has been observed in breast cancer, and is associated with worse relapse-free and OS. 18 Inhibiting angiogenesis has been a potential strategy for treatment of TNBC because genes involved in angiogenesis are frequently activated in basal-like tumors. 13 In recent years, antiangiogenic treatment has been one of the important strategies for TNBC, and antiangiogenic drugs combined with chemotherapy has significantly increased the rate of pCR and improved the survival of breast cancer patients, especially for TNBC patients.12,13,19,20 In SWOG S0800 study, 12 the addition of bevacizumab to nab-paclitaxel followed by doxorubicin and cyclophosphamide significantly increased the pCR rate in TNBC (59% versus 29%; p = 0.014); while in GeparQuinto study, 13 the rates of pCR were 27.9% with epirubicin and cyclophosphamide followed by docetaxel (EC-D) and 39.3% with EC-D plus bevacizumab among TNBC patients (p = 0.003).
Apatinib, an oral small-molecule tyrosine kinase inhibitor that selectively targets VEGF receptor 2 (VEGFR-2) to inhibit tumor angiogenesis, has been approved for the treatment of advanced gastric cancer in China 21 and has demonstrated efficacy to inhibit proliferation, encouraging antitumor activities and tolerable toxicities across a wide range of solid tumors. 22 Moreover, apatinib monotherapy has exhibited objective efficacy and acceptable toxicity in metastatic breast cancer, especially in metastatic TNBC.23,24 However, there is no study regarding apatinib as a NACT drug for treatment of early-stage breast cancer. Therefore, we conducted a single-arm phase II trial (LANCET) to determine whether adding apatinib to docetaxel followed by epirubicin plus cyclophosphamide would improve the pCR in NACT of operable TNBC.
Materials and methods
Patient population
The following criteria were used to enroll patients: Eastern Cooperative Oncology Group performance status of 0–1; aged 18–70 years; histologically or pathologically confirmed noninflammatory invasive TNBC; and a clinical stage of IIA–IIIB and previously untreated. The diagnosis of TNBC was defined below: the estrogen receptor and progesterone receptor negativity rates were less than 10%,13,19 and the human epidermal growth factor receptor type 2 (HER2) staining was 0, 1+, or 2+, and fluorescence in situ hybridization detected no HER2 gene amplification. Other inclusion criteria were negative pregnancy tests if female subjects were at childbearing age, adequate bone marrow function (hemoglobin concentration of ⩾8.0 g/dL, white blood cell count of ⩾3000 cells per μL, absolute neutrophil count of ⩾1500 cells per μL, platelet count of ⩾70,000 cells per μL), adequate renal function (creatinine was the upper limit of normal or lower), and adequate liver function (total bilirubin was the upper limit of normal or lower, and aspartate aminotransferase or alanine aminotransferase was twice the upper limit of normal or lower).
Staging evaluation composed of computed tomography scanning for chest and abdomen and/or abdominal sonography and radionuclide bone imaging. Patients with a history of malignancy at another site (except for cervical carcinoma in situ and basal cell or squamous cell carcinoma of the skin) that had been fully treated were qualified if no recurrent disease occurred for more than 5 years. Exclusion criteria included uncontrolled blood pressure, previous exposure to apatinib, known allergies to any of the excipients, and a history of unstable angina, myocardial infarction, or class III/IV congestive heart failure (defined by the New York Heart Association) within the past 6 months before day 1 of this trial.
Study procedures
The LANCET study was designed as a single-arm, open-label, phase II trial (clinicaltrials.gov identifier: NCT03243838). All enrolled patients were administered oral apatinib at a dose of 250 mg once daily (days 1–21) and intravenously docetaxel (100 mg/m2) every 3 weeks for four cycles, followed by epirubicin (90 mg/m2) plus cyclophosphamide (600 mg/m2) every 3 weeks for four cycles. All the treatments were continued until disease progression, patient withdrawal, or unacceptable toxic effects. Granulocyte colony-stimulating factor used for prophylaxis of febrile neutropenia was permitted according to the American Society of Clinical Oncology guidelines. 8 The biochemical and hematological indexes were evaluated every cycle. According to the International Ki67 in Breast Cancer Working Group consensus and St. Gallen International Consensus Guidelines, the threshold for Ki67 was defined as 30%.25,26 The treatment was continued until disease progression, unacceptable toxicity, or patient withdrawal. Dose modifications, including dose interruptions and dose reductions, were allowed in the event of toxicity. Sequential dose reduction for apatinib (250 mg on days 1–17 and days 1–14), or treatment interruption was permitted for any grade 3 or worse adverse events or grade 2 adverse events deemed intolerable by the patient. Dose re-escalation was not allowed in the protocol. Treatment interruption due to toxicity was permitted a maximum of two times or for a maximum of 14 days (continuously or cumulatively) within a month.
At 3–8 weeks after completing all cycles of the NACT, definitive surgery was performed. The types of breast surgery (mastectomy or breast-conserving surgery) and axillary treatment (sentinel lymph node biopsy or axillary lymph node dissection) were determined by the treating surgeon. After surgery, patients who had residual disease were offered adjuvant chemotherapy with capecitabine (initial dose 1250 mg/m2, reduced to 1000 mg/m2 when intolerable to toxic effects) administered twice a day for 1–14 days and cycled every 21 days for 6–8 cycles. 9 Long-term follow-up for disease condition and survival status was scheduled every 3 months for the first 2 years after initiation of treatment, then every 6 months for the third to fifth years, and then annually after 5 years.
The primary endpoint was the pCR rate in the breast and lymph nodes (ypTis/0ypN0), which was defined as the absence of invasive tumor cells in the breast and lymph nodes and was determined by a local pathologist. Secondary endpoints included objective response rate (ORR), EFS, OS, and safety. ORR was assessed according to RECIST version 1.1, which included patients with measurable disease who achieved a complete or partial response of target lesions. EFS was defined as the interval from the start of treatment to progression prior to surgery, post-surgery recurrence, or death due to any cause. 27 OS was defined as the time from registration to death from any cause. 27 The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE 4.0) was employed to assess the treatment-related safety.
Data collection and analysis
We used Simon’s two-stage design with a one-sided α error of 5% and a power of 80%. 28 The previously reported data indicated that the pCR rate of EC-D regimen for regional or local advanced breast cancer was 28%. 13 We expected that the pCR rate for apatinib combined with T-EC regimen would be 50%. Under these assumptions, 15 patients were to be treated in the first stage of the study. At least five responses were required to continue to the second stage, and 15 more patients would be enrolled in the second stage for a total sample size of 30. Overall, if totally 13 responses or more were observed, the treatment regimen would be considered a success.29,30
Treatment responses according to RECIST 1.1 criteria, EFS, and OS were assessed in the intention-to-treat (ITT) population, which included all enrolled patients who were compliant with the protocol. The target lesion and regional lymph nodes were examined by palpation at baseline and every cycle of chemotherapy. Ultrasound examination of breast and axilla was repeated at baseline and after every two cycles; ultrasound examination and mammography were performed before breast surgery. Safety was assessed in the safety population, which included all patients with complete safety data. The median follow-up time was calculated the using Kaplan–Meier curve. We calculated the proportion of patients who achieved an objective response and the corresponding 95% confidence interval (CIs) using the Clopper–Pearson method. EFS and OS analyses and associated 95% CIs were estimated using the Kaplan–Meier method. Data were analyzed with SPSS statistics version 26 (IBM Corporation, Armonk, NY, USA). The final date of data acquisition for this study was 31 December 2021.
Results
Patient characteristics
From 1 August 2018 to 10 March 2021, 34 patients were screened, of which 31 patients were enrolled in this study and included in the primary endpoint analysis as well as safety analysis (Figure 1). Among the enrolled patients, the mean age was 48 years (range: 31–63 years). Most of the patients were in T2 stage (90.3%), N0-2 stage (93.5%), and high Ki67 expression (96.8%). And approximately two-thirds of the patients were in American Joint Committee on Cancer stage II (67.7%) and histological grade III (67.7%). After NACT, the proportions of patients who underwent mastectomy and those who underwent breast-conserving surgery were comparable (48.4% and 45.2%, respectively), and most of the enrolled patients underwent sentinel lymph node biopsy only (61.3%) for axillary surgery (Table 1).
Figure 1.
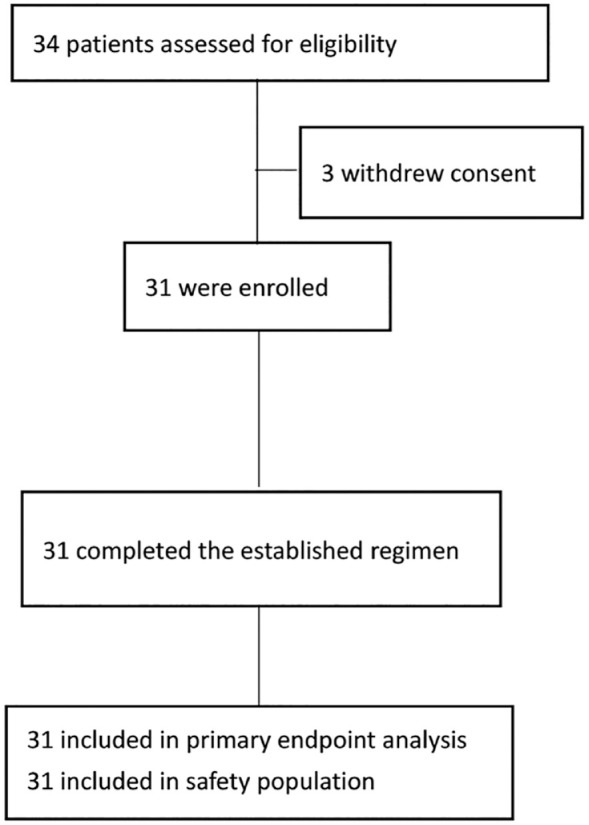
Consort diagram.
Table 1.
Baseline characteristics.
| Parameters | Percentage |
|---|---|
| Overall | 31 (100) |
| Age, mean (range) | 48 (31–63) |
| ECOG performance status | |
| 0 | 31 (100) |
| 1 | 0 (0) |
| T stage | |
| T1 | 0 (0) |
| T2 | 28 (90.3) |
| T3 | 1 (3.2) |
| T4 | 2 (6.5) |
| N stage | |
| N0 | 16 (51.6) |
| N1 | 6 (19.4) |
| N2 | 7 (22.6) |
| N3 | 2 (6.5) |
| AJCC stage | |
| I | 0 (0) |
| II | 21 (67.7) |
| III | 10 (32.3) |
| Histological grade | |
| I | 0 (0) |
| II | 10 (32.3) |
| III | 21 (67.7) |
| Ki67 expression | |
| <30% | 1 (3.2) |
| ⩾30% | 30 (96.8) |
| Androgen receptor | |
| Negative | 19 (61.3) |
| Positive | 5 (16.1) |
| Unknown | 7 (22.6) |
| Breast surgery | |
| Mastectomy | 15 (48.4) |
| BCS | 14 (45.2) |
| NSM | 2 (6.5) |
| Axillary surgery | |
| SNB | 19 (61.3) |
| ALND | 4 (12.9) |
| SNB + ALND | 8 (25.8) |
AJCC, American Joint Committee on Cancer; ALND, axillary lymph node dissection; BCS, breast-conserving surgery; ECOG, Eastern Cooperative Oncology Group; NSM, nipple-sparing mastectomy; SNB, sentinel lymph node biopsy.
Clinical efficacy
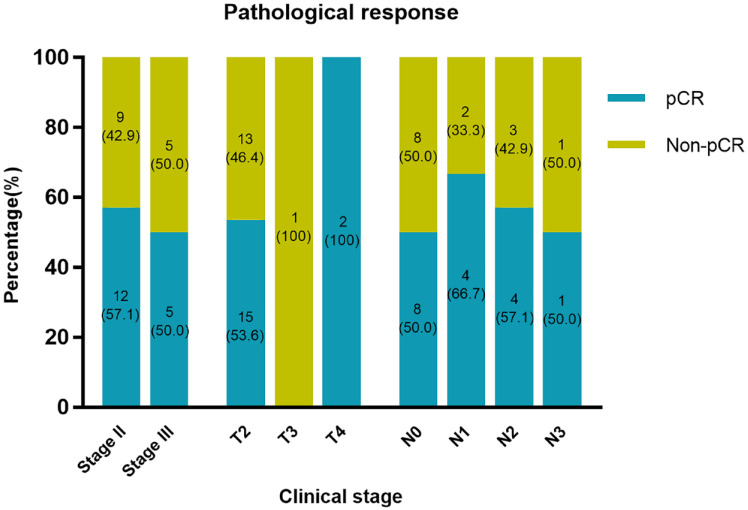
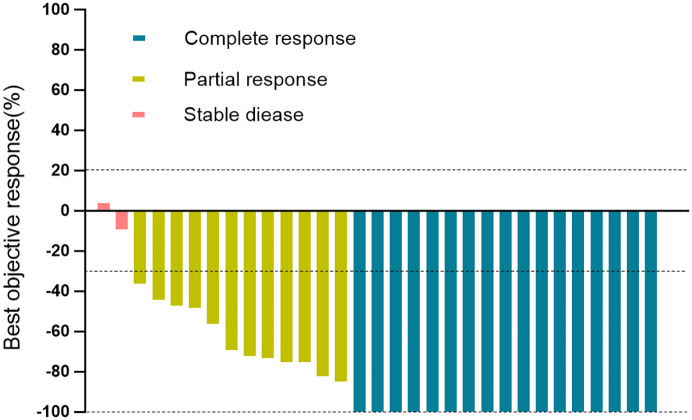
In the first assessable 15 patients included in the study, pCRs in both breast and lymph nodes were noted in 10 (67.7%) patients. The pCR threshold for the first stage of Simon’s two-stage design was reached, and the trial continued to the second stage. pCRs were achieved in 17 (54.8%; 95% CI: 36.0–72.7) of 31 patients in the ITT population (Table 2). Objective responses were achieved in 29 patients (93.5%; 95% CI: 78.6–99.2), and disease control was achieved in 31 patients (100%; 95% CI: 88.8–100.0) (Table 2). The pCRs in both breast and lymph nodes at different clinical stages are shown in Figure 2. The best percentage change from baseline in target lesions is shown in Figure 3, and tumor shrinkage was noted in 30 (96.8%) of 31 patients.
Table 2.
Treatment responses.
| Parameters | ITT population (n = 31) |
|---|---|
| Complete response | 17 (54.8%; 36.0–72.7) |
| Partial response | 12 (38.7%) |
| Stable disease | 2 (6.5%) |
| Progressive disease | 0 (0) |
| Objective response | 29 (93.5%; 78.6–99.2) |
| Disease control | 31 (100%; 88.8–100.0) |
Data are n (%) and n (%; 95% CI).
CI, confidence interval; ITT, intention-to-treat.
Figure 2.
Percentages of pCR according to clinical stage of breast cancer.
pCR, pathological complete response.
Figure 3.
Waterfall plot for the best percentage change in target lesion size.
Waterfall plot for the best percentage change in target lesion size is shown for 31 patients who had at least one post-baseline efficacy assessment. The color indicates the type of response. The dashed line at 20% represents the boundary for determination of progressive disease, and the dashed line at −30% represents the boundary for determination of partial response.
Survival analysis
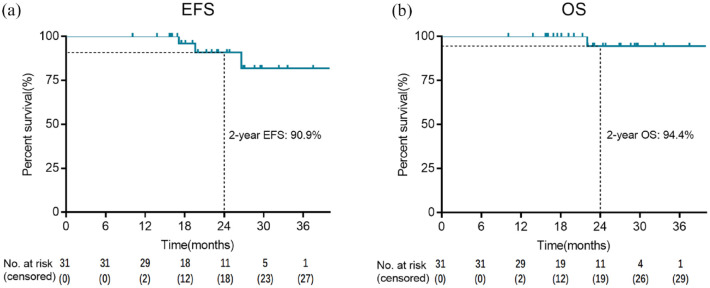
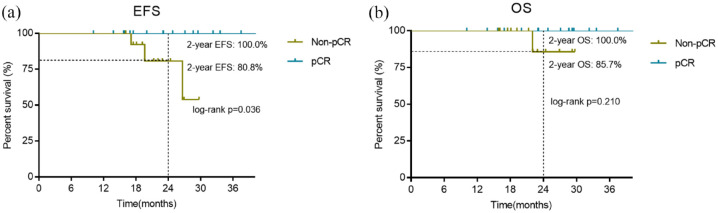
At the date of cutoff on 31 December 2021, the median follow-up time was 22.9 months (range: 10.1–41.6 months). Of the 29 patients with an objective response, one (3.4%) had local recurrence at data cutoff. Another two patients with stable disease had recurrence at 17.1 months and 26.6 months, respectively; and the former had distant metastasis and died at 22 months. The 2-year EFS and 2-year OS were 90.9% and 94.4%, respectively (Figure 4). Subgroup analysis according to pCR status showed that the 2-year EFS of patients who had pCR and those non-pCR were 100.0% and 80.8%, respectively (p = 0.036), while the 2-year OS of patients who had pCR and those non-pCR were 100.0% and 85.7%, respectively (p = 0.210) (Figure 5).
Figure 4.
Kaplan–Meier survival curves: EFS (a) and OS (b) in patients with at least one post-baseline efficacy assessment (n = 31).
EFS, event-free survival; OS, overall survival.
Figure 5.
Kaplan–Meier survival curves: EFS (a) and OS (b) in patients with at least one post-baseline efficacy assessment (n = 31) according to pCR status.
EFS, event-free survival; OS, overall survival; pCR, pathological complete response.
Treatment-related toxicity
In all, 31 patients were included in the safety analysis (Table 3). The incidence of adverse events (any grade) was 100% regardless of causality. The five most common treatment-related adverse events were fatigue (51%), hypertension (41%), anorexia (39%), hand–foot syndrome (35%), and diarrhea (32%). Two patients (6%) reported to have grade 3 of hypertension, and other grade 3 adverse events were reported in fatigue, hand–foot syndrome, oral mucositis, diarrhea, and vomiting [1 (3%) of 31 patients, respectively]. No treatment-related grade 4 or more adverse events were observed. Dose reductions occurred in 7 (23%) of 31 patients for apatinib, all of whom required only one dose reduction, and none of them had two dose reductions. No patient was discontinued to use apatinib. One patient died during the follow-up period due to disease progression; however, her death was not deemed to be treatment related.
Table 3.
Treatment-related adverse events in the safety population (n = 31).
| Adverse event* | Grade 1–2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Hypertension | 11 (35%) | 2 (6%) | 0 |
| Fatigue | 15 (48%) | 1 (3%) | 0 |
| Hand–foot syndrome | 10 (32%) | 1 (3%) | 0 |
| Anorexia | 12 (39%) | 0 | 0 |
| Oral mucositis | 6 (19%) | 1 (3%) | 0 |
| Diarrhea | 9 (29%) | 1 (3%) | 0 |
| Nausea | 8 (26%) | 0 | 0 |
| Vomiting | 2 (6%) | 1 (3%) | 0 |
| Headache | 5 (16%) | 0 | |
| Peripheral edema | 6 (19%) | 0 | 0 |
| Proteinuria | 4 (13%) | 0 | 0 |
| Increased AST | 3 (10%) | 0 | 0 |
| Increased ALT | 2 (6%) | 0 | 0 |
| Anemia | 3 (10%) | 0 | 0 |
| Thrombocytopenia | 1 (3%) | 0 | 0 |
| Neutropenia | 2 (6%) | 0 | 0 |
Adverse events related to treatment-related toxicity.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
TNBC displays highest risk of distant recurrence and poorest prognosis among all breast cancer subtypes. 31 However, systemic treatment of TNBC is still mainly limited to chemotherapy. In recent years, a large number of studies have shown that breast cancer patients who obtained pCR after NACT had significantly improved survival compared with those with residual tumor at the time of surgery.14,32,33 The CTNeoBC study, 14 which performed a pooled analysis of 12 trials, demonstrated that breast cancer patients who achieved pCR had improved disease-free survival and OS, especially in the TNBC subgroup. The pCR rate of TNBC after anthracycline and taxane-based NACT was 33.6% in CTNeoBC study. 14 While in GeparTrio study, the pCR rate of TNBC cohort after eight cycles of docetaxel, doxorubicin, and cyclophosphamide NACT was 37%. 34 Therefore, how to improve the pCR rate of TNBC becomes more and more important.
Tumor angiogenesis is considered a critical prognostic factor in breast carcinoma 35 and plays a significant role in promoting tumor development and metastasis, which makes antiangiogenic drugs improve the efficacy of traditional chemotherapy in treatment of breast cancer. 36 Antiangiogenic treatment such as bevacizumab combined with chemotherapy has been proven to increase pCR in early and locally advanced HER2-negative breast cancer in previous studies. In GeparQuinto study, 13 the rates of pCR were 14.9% with EC-D and 18.4% with EC-D plus bevacizumab (p = 0.04); the corresponding rates of pCR were 27.9% and 39.3% among TNBC patients (p = 0.003). In NSABP-B40 study, 37 the addition of bevacizumab to chemotherapy, as compared with chemotherapy alone, significantly increased the rate of pCR (34.5% versus 28.2%, p = 0.02), but no significant difference was found among TNBC patients (52% versus 47%, p = 0.34). Therefore, it is still unclear whether using antiangiogenic drug will improve the rate of pCR for TNBC patients or not.
Aberrant tumor-associated neovasculature has been proven to induce various immunosuppressive features, and antiangiogenic therapy can ameliorate antitumor immunity.38–40 The IMpassion 130 TME exploratory analysis identified angiogenesis as associated with reduced progression-free survival (PFS). And the FUTURE-C-Plus study 40 found that patients with advanced immunomodulatory TNBC benefited more from immune checkpoint blockade plus angiogenesis inhibition, supported by a respectable efficacy (ORR = 81.3%; median PFS = 13.6 months) and good tolerability in 48 patients enrolled to receive famitinib, camrelizumab, and nab-paclitaxel. However, whether TNBC patients benefit more from neoadjuvant immune checkpoint blockade plus angiogenesis inhibition remains unclear and needs further studies in the future.
Apatinib, an oral selective inhibitor of VEGFR-2, has been widely used in the treatment of various tumors, including gastric cancer, liver cancer, colorectal cancer, osteogenic sarcoma, and lung cancer.41–44 Metastatic breast cancer often tends toward a poor prognosis and has a median PFS of less than 4 months with traditional chemotherapy after second-line therapy or more.45,46 Two phase II clinical trials had been conducted to evaluate the efficacy of apatinib in metastatic breast cancer patients who were previously treated with anthracycline and taxanes.23,24 Hu et al. 23 had conducted a multicenter, single-arm, phase II study, which enrolled 38 metastatic non-TNBC patients who had received prior chemotherapy, and found that using apatinib alone achieved a median PFS and OS of 4.0 months and 10.3 months, respectively; the ORR and disease control rate (DCR) were 16.7% and 66.7%, respectively. Another phase II study, which evaluated the efficacy of apatinib monotherapy in heavily pretreated metastatic TNBC patients in China, demonstrated that the median PFS and OS were 3.3 months and 10.6 months, respectively; the ORR was 10.7% and the clinical benefit rate was 25% in heavily pretreated patients. 24 In addition, results from a retrospective study, which investigated the antitumor activity and safety of apatinib combined with chemotherapy in 66 pretreated advanced breast cancer patients, revealed that the median PFS and OS were 6.0 months and 10.0 months, respectively. 47 Another observational study analyzed the efficacy and safety of apatinib combined with chemotherapy in 85 patients with previously treated advanced breast cancer, and concluded that the median PFS and OS in the TNBC group were 5.2 months and 11.4 months, respectively, while the median PFS and OS in the non-TNBC group were 4.3 months and 11.3 months, respectively. 48 In consideration of the efficacy of apatinib in advanced TNBC, our study aimed to evaluate the efficacy and safety of neoadjuvant apatinib combined with standard chemotherapy for early-stage TNBC patients. It demonstrated that the pCR rate of the 31 evaluable patients was 54.8%, and the ORR and DCR were 90.6% and 96.9%, respectively. Moreover, the 2-year EFS was 90.9%, similar to the Keynote-522 study in which the Kaplan–Meier estimate of EFS at 18 months and 36 months were 91.3% (95% CI: 88.8–93.3) and 84.5% (95% CI: 81.7–86.9) at the pembrolizumab chemotherapy group, respectively.49,50 However, the follow-up period at this early time point was not long enough to assess mature survival data, which is an important consideration in patients receiving potentially curative treatment. Therefore, subsequent analyses are ongoing to further assess the survival of the enrolled patients.
To the best of our knowledge, this study was the first to assess the efficacy and safety of adding apatinib to docetaxel followed by the epirubicin plus cyclophosphamide in early-stage TNBC patients. The results of our study indicated promising antitumor activity of the combination of apatinib and standard chemotherapy. NeoCART study, 51 a multicenter, randomized controlled, phase II trial conducted by our institution to assess the efficacy and safety of docetaxel plus carboplatin versus EC-D in untreated stage II-III TNBC, showed that the rate of pCR in the EC-D group was 38.6%. Since the rate of pCR (54.8%) achieved in combination of apatinib and standard chemotherapy was dramatically higher than that of chemotherapy alone (38.6%), there may exist promising synergistic effect between apatinib and standard chemotherapy. Randomized controlled studies will be designed to further determine whether adding apatinib to standard chemotherapy could improve the pCR in NACT of early-stage TNBC in the future.
Hypertension, proteinuria, and hand–foot syndrome are the most common adverse events of antiangiogenic drugs. Some previous studies have suggested 250–850 mg dose of apatinib in patients with gastric cancer or breast cancer.21,23,41,52 Concerning the side effect of apatinib combined with standard chemotherapy, we used 250 mg once daily as the dose in our clinical trial and expected all the enrolled patients could be tolerant to apatinib of 250 mg dose for 21 consecutive days combined with chemotherapy in 3-week cycle. In this trial, the most frequent all-grade adverse events were fatigue (47%), anorexia (38%), hypertension (34.0%), and hand–foot syndrome (31%), which was similar to those reported in previous studies.23,24,48,50 Most of the reported adverse events were grade 1 to 2; only a few of the enrolled patients reported grade 3 adverse events, and no grade 4 adverse events were observed. No treatment-associated mortality occurred during the combined treatment, and no patient needed dose reduction due to toxicity and intolerance.
There are some limitations in our study. First, this is a single-arm, single-center clinical trial with no control group for comparison; thus, the selection bias was inevitable. Second, the small sample size enrolled in this trial reduces certainty of the effectiveness observed. Third, since the reported pCR rate of neoadjuvant anthracycline–taxane chemotherapy for TNBC patients varied from 27.9% to 36.6%,12–14,20 the assumption that pCR rate of neoadjuvant anthracycline–taxane chemotherapy was 28% was relatively lower among available data, and might represent an underestimation to some extents. Fourth, biomarkers including Tau protein, Bcl-2, EGFR, AR, PD-1, PD-L1, and CDK5/6 were only detected in some of the enrolled patients, and no biomarker was found to be a significant prognostic factor for the effect of the scheduled chemotherapy regimen. In the future, a randomized controlled study in larger sample size may be warranted to further validate the efficacy of apatinib as well as the prognostic effect of biomarkers in NACT among TNBC patients.
Conclusion
In summary, our results showed excellent efficacy and manageable toxicities in adding apatinib to docetaxel followed by epirubicin plus cyclophosphamide, with significantly improved pCR in NACT of early-stage TNBC. However, prospective studies with larger sample sizes as well as longer follow-up time are expected to investigate the long-term effect of apatinib on prognosis in patients with early-stage TNBC.
Acknowledgments
The authors thank all of the patients analyzed in this study.
Footnotes
ORCID iD: Kun Wang  https://orcid.org/0000-0001-9851-7080
https://orcid.org/0000-0001-9851-7080
Contributor Information
Ciqiu Yang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Junsheng Zhang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Yi Zhang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Fei Ji, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Yitian Chen, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Teng Zhu, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Liulu Zhang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Hongfei Gao, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Mei Yang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Jieqing Li, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Minyi Cheng, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Kun Wang, Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No. 123 Huifu West Road, Guangzhou, 510080, China; Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2017195H(R1)). The study conformed to the Declaration of Helsinki, and all participants provided written informed consent. It was registered at ClinicalTrials.gov (NCT03243838).
Consent for publication: This manuscript does not include any individual person’s details, so patient consent for publication is not applicable in this case. All authors listed approved the present manuscript and consented for its publication.
Author contribution(s): Ciqiu Yang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Junsheng Zhang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft; Writing – review & editing.
Yi Zhang: Data curation; Formal analysis; Methodology; Project administration; Validation; Writing – review & editing.
Fei Ji: Data curation; Investigation; Software; Writing – review & editing.
Yitian Chen: Formal analysis; Project administration; Resources; Software; Validation; Visualization; Writing – review & editing.
Teng Zhu: Data curation; Investigation; Methodology; Writing – review & editing.
Liulu Zhang: Data curation; Methodology; Writing – review & editing.
Hongfei Gao: Data curation; Investigation; Writing – review & editing.
Mei Yang: Data curation; Investigation; Writing – review & editing.
Jieqing Li: Data curation; Formal analysis; Investigation; Writing – review & editing.
Minyi Cheng: Data curation; Investigation; Writing – review & editing.
Kun Wang: Conceptualization; Funding acquisition; Resources; Supervision; Validation; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by grants from National Natural Science Foundation of China (82171898), High-level Hospital Construction Project (DFJH202109), Science and Technology Planning Project of Guangzhou City (202002030236), Beijing Medical Award Foundation (YXJL-2020-0941-0758), and Guangdong Basic and Applied Basic Research Foundation (2022A1515012277). Funding sources were not involved in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the article for publication.
Competing Interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Due to Informed Consent Form, data privacy, and Intellectual Property Rights-related restrictions, the clinical data cannot be made public, that is, accessible for anyone, for any purpose without a review process and without putting an agreement in place. Nevertheless, raw data are available upon request and any requests can be directed to the study team.
References
- 1. Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan HG, Malmgren JA, Atwood M. T1N0 triple negative breast cancer: risk of recurrence and adjuvant chemotherapy. Breast J 2009; 15: 454–460. [DOI] [PubMed] [Google Scholar]
- 3. Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003; 100(14): 8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009; 9: 29–33. [DOI] [PubMed] [Google Scholar]
- 5. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 6. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 7. Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol 2009; 27: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 8. Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2005; 23: 2694–2702. [DOI] [PubMed] [Google Scholar]
- 9. Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003; 21: 4165–4174. [DOI] [PubMed] [Google Scholar]
- 10. Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg 2009; 250: 558–566. [DOI] [PubMed] [Google Scholar]
- 11. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 12. Nahleh ZA, Barlow WE, Hayes DF, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat 2016; 158: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. New Engl J Med 2012; 366: 299–309. [DOI] [PubMed] [Google Scholar]
- 14. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 15. Li C, Li R, Song H, et al. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. J Surg Oncol 2011; 103: 184–192. [DOI] [PubMed] [Google Scholar]
- 16. Tolaney SM, Boucher Y, Duda DG, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci USA 2015; 112: 14325–14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammed RA, Ellis IO, Mahmmod AM, et al. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Modern Pathol 2011; 24: 774–785. [DOI] [PubMed] [Google Scholar]
- 18. Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 2006; 24: 769–777. [DOI] [PubMed] [Google Scholar]
- 19. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Earl HM, Hiller L, Dunn JA, et al. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trial. Lancet Oncol 2015; 16: 656–66. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016; 34: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res 2019; 25: 515–523. [DOI] [PubMed] [Google Scholar]
- 23. Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014; 14: 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014; 135: 1961–1969. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst 2021; 113: 808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 2021; 32: 1216–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol 2015; 26(5): 873–879. [DOI] [PubMed] [Google Scholar]
- 28. Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials 1989; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 29. Lan CY, Wang Y, Xiong Y, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol 2018; 19: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 30. Liu C, Jia Q, Wei H, et al. Apatinib in patients with advanced chordoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2020; 21: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 31. Fitzpatrick A, Tutt A. Controversial issues in the neoadjuvant treatment of triple-negative breast cancer. Ther Adv Med Oncol 2019; 11: 1758835919882581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–804. [DOI] [PubMed] [Google Scholar]
- 33. von Minckwitz G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 2011; 125(1): 145–156. [DOI] [PubMed] [Google Scholar]
- 34. von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2013; 31(29): 3623-30. [DOI] [PubMed] [Google Scholar]
- 35. Hopson MB, Lee S, Accordino M, et al. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res Treat 2021; 188: 427–432. [DOI] [PubMed] [Google Scholar]
- 36. Magnussen AL, Mills IG. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Brit J Cancer 2021; 125: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. New Engl J Med 2012; 366: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019; 7: 387–401. [DOI] [PubMed] [Google Scholar]
- 39. Lan C, Shen J, Wang Y, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase ii trial. J Clin Oncol 2020; 38: 4095–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen L, Jiang YZ, Wu SY, et al. Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-Plus): an open-label, single-arm, phase ii trial. Clin Cancer Res 2022; 28: 2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013; 31: 3219–3225. [DOI] [PubMed] [Google Scholar]
- 42. Langer CJ, Mok T, Postmus PE. Targeted agents in the third-/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat Rev 2013; 39: 252–260. [DOI] [PubMed] [Google Scholar]
- 43. Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst 2014; 106: djt371. [DOI] [PubMed] [Google Scholar]
- 44. Liao Z, Li T, Zhang C, et al. Clinical study of apatinib in the treatment of stage IV osteogenic sarcoma after failure of chemotherapy. Cancer Biol Med 2020; 17: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: The PAKT trial. J Clin Oncol 2020; 38: 423–433. [DOI] [PubMed] [Google Scholar]
- 46. Kontani K, Hashimoto SI, Murazawa C, et al. Metronomic chemotherapy for metastatic breast cancer to prolong time to treatment failure to 12 months or more. Mol Clin Oncol 2013; 1: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Z, Shan J, Yu Q, et al. Real-world data on apatinib efficacy – results of a retrospective study in metastatic breast cancer patients pretreated with multiline treatment. Front Oncol 2021; 11: 643654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu A, Yuan P, Wang J, et al. Apatinib combined with chemotherapy in patients with previously treated advanced breast cancer: an observational study. Oncol Lett 2019; 17: 4768–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020; 382: 810–21. [DOI] [PubMed] [Google Scholar]
- 50. Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 2022; 386: 556–567. [DOI] [PubMed] [Google Scholar]
- 51. Zhang L, Wu ZY, Li J, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label phase II trial. Int J Cancer 2022; 150: 654–662. [DOI] [PubMed] [Google Scholar]
- 52. Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer 2020; 8: e000696. [DOI] [PMC free article] [PubMed] [Google Scholar]