Abstract
Background:
This study aimed to investigate the superiority of nab-paclitaxel plus S-1 (AS) over oxaliplatin plus S-1 (SOX) in patients with advanced gastric cancer (AGC).
Methods:
In this multicenter, randomized, phase III superiority trial, eligible patients with unresectable, locally advanced gastric adenocarcinoma were recruited and randomly assigned (1:1) to receive AS (nab-paclitaxel 260 mg/m2 on day 1 or 130 mg/m2 on days 1 and 8; oral S-1 40–60 mg twice daily for 14 days) or SOX (130 mg/m2 oxaliplatin on day 1; oral S-1 40–60 mg twice daily for 14 days) every 3 weeks for up to six cycles. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were overall survival, objective response rate, and safety.
Results:
Owing to slow enrolment, an unplanned interim analysis was performed, resulting in the early termination of the study on 31 December 2021 (data cutoff). Between March 2019 and March 2021, 97 patients (AS, n = 48; SOX, n = 49) were treated and evaluated for efficacy and safety of AS and SOX. As of the data cutoff, the median follow-up was 23.13 months [95% confidence interval (CI), 13.39–32.87]. The median PFS was 9.03 months (95% CI, 6.50–11.56) in the AS group and 5.07 months (95% CI, 4.33–5.81) in the SOX group, demonstrating a better PFS tendency following AS treatment than SOX treatment (hazard ratio = 0.59; 95% CI, 0.37–0.94; p = 0.03). The most common grade 3 or worse adverse events were anemia, neutropenia, and leukopenia in both groups, with a higher incidence of thrombocytopenia in the SOX group.
Conclusion:
Although this study was terminated early, the results demonstrated a better PFS tendency in patients with AGC who were treated with AS than in those treated with SOX, with controllable toxicities.
Trial registration:
Clinical Trials.gov identifiers: NCT03801668. Registered January 11, 2019.
Keywords: advanced gastric cancer, clinical trial, nab-paclitaxel, oxaliplatin, S-1
Introduction
Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide, especially in East Asian countries. 1 Epidemiological investigations have shown that GC deaths in China account for approximately 50% of the deaths worldwide annually. 2 Unfortunately, most patients are diagnosed at an advanced stage and have a poor prognosis. 3 Despite considerable improvements in surgical and comprehensive therapies,4–6 the 5-year survival rate remains dismally low, estimated at 20% for advanced GC (AGC).7,8
Combined chemotherapy with fluoropyrimidine [e.g. 5-fluorouracil (5-FU) and capecitabine] and platinum (e.g. cisplatin and oxaliplatin) has been recommended as the standard of care for AGC in the first-line setting.9–11 However, the platinum-based regimen has a high risk of toxic events. 12 Furthermore, the progression-free survival (PFS) remains poor (approximately 6 months) due to disease metastasis. 13 S-1, an oral 5-FU derivative, is designed to maintain high levels of 5-FU in the plasma and tumor cells with reduced gastrointestinal toxicity. 14 Thus, S-1 alone or in combination with paclitaxel was evaluated and showed promising benefits in patients with AGC in multiple clinical trials.15–19 Solvent-based paclitaxel is usually associated with hypersensitivity and anaphylactic reactions in some patients. 20 Thus, equivalent and low-toxicity taxane-based doublets warrant further exploration.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) was developed as a solvent-free paclitaxel formulation that eliminates the risk of hypersensitivity reactions. 21 More importantly, nab-paclitaxel exhibits distinct biodistribution properties and increased antitumor efficacy compared with solvent-based paclitaxel. 22 Owing to its promising activity, nab-paclitaxel has been approved as a therapeutic option for various cancers, including breast, pancreatic cancer, and GC. Notably, preclinical data have shown that the combination of nab-paclitaxel and S-1 has a synergistic effect on suppressing tumor progression in a mouse model. 23 In early stage trials, the efficacy and toxicity of nab-paclitaxel plus S-1 (AS) as first-line treatment for AGC have also been preliminarily established.24,25 However, the results of phase I or II studies cannot be considered conclusive. Therefore, we performed this study to compare the efficacy and safety of AS with that of oxaliplatin plus S-1 (SOX) in patients with chemotherapy-naïve AGC. Owing to the slow enrolment of patients and changes in the treatment landscape, an unplanned interim analysis was performed to decide the subsequent action regarding study discontinuation.
Patients and methods
Study design and participants
The GAPSO study is a multicenter, open-label, randomized, phase III superiority trial, comparing the efficacy and safety of AS with that of SOX in patients with chemotherapy-naïve AGC. Patients from three cancer centers across China were enrolled in this study.
Patients aged 18–75 years were eligible for enrolment if they had histologically or cytologically confirmed unresectable, locally advanced, recurrent, or metastatic gastric adenocarcinoma, measurable, or non-measurable disease (massive malignant ascites or peritoneal dissemination) according to RECIST version 1.1. Eligible patients were also required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 and a life expectancy of at least 3 months. In addition, eligible patients had to have a left ventricular ejection fraction of at least 50% and adequate organ function (including bone marrow, heart, hepatic, and renal functions). Patients were also eligible if at least 6 months had passed since the final administration of neoadjuvant or adjuvant chemotherapy. Patients were excluded if they met any of the following criteria: received prior chemotherapy for locally advanced or metastatic diseases; human epidermal growth factor receptor (HER2) positive; symptomatic central nervous system metastases; grade 2 or higher peripheral neuropathy as per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03; active viral hepatitis or positive test for human immunodeficiency virus (HIV); serious uncontrolled systemic concomitant illness; active gastrointestinal bleeding and digestive tract obstruction; other active synchronous cancer within 5 years, except for carcinoma in situ of the cervix or basal cell carcinoma; pregnancy, or breastfeeding.
Procedures
Eligible patients were randomly assigned (1:1) to the AS or SOX group using a randomization sequence created by a computer program. The investigators and patients were not blinded to the treatment allocation. Patients assigned to the AS group received intravenous nab-paclitaxel (keaili®, CSPC Ouyi Pharmaceutical Co. Ltd., Shijiazhuang, Hebei, China; 260 mg/m2 on day 1 or 130 mg/m2 on days 1 and 8) and oral S-1 twice daily on days 1 through 14 at a dose calculated according to the patient’s body surface area (<1.25 m2, 40 mg; 1.25–1.5 m2, 50 mg; ⩾1.50 m2, 60 mg). The dosing regimen of nab-paclitaxel was determined by the investigators based on the physical condition of the patients. If patients could not tolerate a 260 mg/m2 dose, 130 mg/m2 was administered on days 1 and 8. Patients assigned to the SOX group received intravenous oxaliplatin (130 mg/m2 on day 1) and oral S-1 at the same doses as those in the AS group. The treatment regimens were administered every 21 days for up to six cycles until disease progression, death, unacceptable toxicity, or withdrawal of consent. Patients who had stable disease (SD) or a response following six cycles of combined chemotherapy could continue maintenance chemotherapy with S-1, based on the choice of the patients and their physicians.
Toxicity was graded according to NCI-CTCAE version 4.03 and managed with dosing interruptions, dose reduction, or supportive care. Dosing interruptions were performed according to protocol guidelines. The treatment cycle was delayed until the non-hematological (⩽grade 1) and hematological toxicities resolved [absolute neutrophil count (ANC): ⩾1.5 × 109/L and platelet count: ⩾90 × 109/L). Dose reduction was allowed if one of the following events occurred: (1) ANC: <0.5 × 109/L; (2) febrile neutropenia; (3) ANC: 1 × 109/L–0.5 × 109/L and platelet count: 50 × 109/L–25 × 109/L; (4) platelet count: <25 × 109/L, bleeding, or transfusion requirement; (5) grade 3 or worse anemia; or (6) grade 2 or worse neuropathy. Up to two steps of dose reduction were permitted (⩽25% of the initial dose) if the dose reduction criterion was fulfilled. Patients requiring >3 weeks of dose interruption or >2 dose reductions were required to withdraw from the study. Dose re-escalation was not allowed between the cycles. Prophylaxis with colony-stimulating factor and erythropoietin was permitted during treatment. Supportive care was recommended to manage significant bone marrow suppression and febrile neutropenia.
Assessments
Physical examination and routine laboratory tests (including hematology, blood chemistry, and liver function tests) were performed prior to each treatment cycle. Radiological evaluation was performed using computed tomography or magnetic resonance imaging every two cycles following treatment initiation until disease progression. Tumor response was assessed by the investigator according to RECIST version 1.1. Adverse events (AEs) were monitored throughout the study period and classified according to NCI-CTCAE version 4.03. To assess the quality of life (QOL), patients were required to fill out the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) every two cycles. In addition, neurotoxicity was determined every cycle as per the neurotoxicity scale (16-item Taxane score). 26
Endpoints
The primary endpoint was PFS, defined as the time from the date of randomization to the first documentation of disease progression, or date of death from any cause. The secondary endpoints were objective response rate [ORR; defined as the proportion of patients who achieved a complete response (CR) or partial response], overall survival (OS; defined as the time from randomization to death from any cause), and safety (defined as AEs associated with chemotherapy).
Statistical analysis
This study was designed to assess the superiority of AS over SOX in terms of PFS. Based on a previous SOX phase III study, 27 we presumed that the median PFS was 5.5 and 8.0 months in the SOX and AS groups, respectively. Assuming an enrolment period of 18 months and a follow-up period of 12 months, 240 events were required to show the superiority of AS over SOX using a log-rank test with a significance level of 5% (two-sided) and 80% power. After adjusting for a dropout rate of 10%, we enrolled 294 patients (147 per group).
Interim analysis was planned after a defined number of events (120 PFS events) were observed. Using the O’Brien-Fleming error spending function, the two-sided nominal significance level for the interim analysis was found to be 0.003051. The independent data and safety-monitoring committee reviewed the interim analysis report and decided to stop the study early with the consensus of the study group and sponsor. If survival for AS was found to be higher than that for SOX, with a p value less than the nominal significance level (0.003051), termination of the study due to efficacy was considered.
Efficacy analysis was performed in the modified intent-to-treat (mITT) population, which consisted of all randomly assigned patients who had received at least one dose of the study treatment. PFS and OS were estimated using the Kaplan–Meier analysis and expressed as median values with corresponding two-sided 95% confidence intervals (CIs). Comparisons between the treatment groups were performed using the log-rank test. The proportion of patients who achieved an overall response was compared between the treatment groups using the chi-squared test. For post-hoc analyses of the interactions between the treatment and subgroup, the hazard ratio (HR) with two-sided 95% CIs was estimated using the Cox proportional hazards regression model in the pre-planned subgroups. Safety was evaluated in the safety analysis set, which consisted of all the patients who received at least one study treatment. All reported p values were two-sided. SPSS 22.0 software was used for the statistical analyses.
Results
Patient characteristics
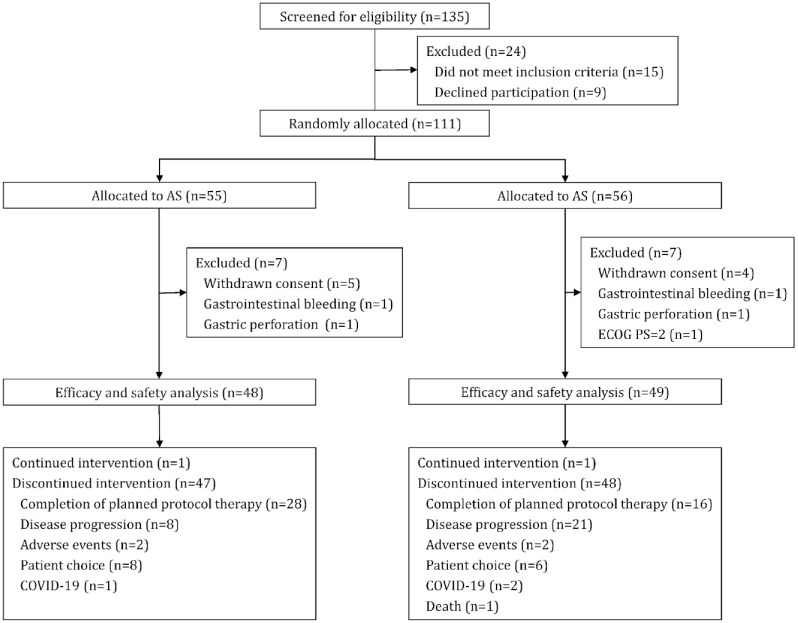
Between March 2019 and March 2021, 135 patients were screened from three cancer centers, and 111 patients were randomly assigned to either the AS (n = 55) or SOX (n = 56) group. Seven patients each from the two groups were excluded from the study prior to treatment initiation owing to withdrawal of consent (AS, n = 5; SOX, n = 4) and inclusion ineligibility (AS, n = 2; SOX, n = 3). The remaining 97 patients (AS, 48 versus SOX, 49) were treated and included in the mITT population for efficacy and safety analyses (Figure 1). At the data cutoff, two patients (one in each group) were still undergoing treatment, and 44 patients [AS, n = 28 (58.33%); SOX, n = 16 (32.65%)] completed the planned therapeutic protocol. The remaining 51 patients (AS, n = 19; SOX, n = 32) did not complete the planned six cycles of treatment due to disease progression (8 versus 21), AEs (2 versus 2), patient choice (8 versus 6), coronavirus disease 19 (COVID-19; 1 versus 2), and death (0 versus 1). The median durations of AS and SOX therapy were six and four cycles, respectively.
Figure 1.
Trial profile.
AS, nab-paclitaxel plus S-1; COVID-19, coronavirus disease 19; ECOG PS, Eastern Cooperative Oncology Group performance status; SOX, oxaliplatin plus S-1.
The patient characteristics are listed in Table 1. Of the 97 patients included in the study, 71 had measurable target lesions, whereas 26 had no measurable target lesions, but had massive malignant ascites or peritoneal dissemination. The demographic characteristics of all randomly assigned patients were well balanced between the two treatment groups (Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| AS group (n = 48) | SOX group (n = 49) | |
|---|---|---|
| Sex | ||
| Male | 25 (52.08%) | 29 (59.18%) |
| Female | 23 (47.92%) | 20 (40.82%) |
| Age, years | ||
| Median | 54.00 (49.00–63.50) | 56.00 (47.00–63.00) |
| <65 | 39 (81.25%) | 39 (79.59%) |
| ⩾65 | 9 (18.75%) | 10 (20.41%) |
| ECOG PS | ||
| 0 | 24 (50.00%) | 22 (44.90%) |
| 1 | 24 (50.00%) | 27 (55.10%) |
| Primary tumor location | ||
| Proximal | 10 (20.83%) | 8 (16.33%) |
| Body | 17 (35.42%) | 17 (34.69%) |
| Distal | 13 (27.08%) | 20 (40.82%) |
| Multiple/diffuse | 6 (12.50%) | 2 (4.08%) |
| Gastric remnant | 2 (4.17%) | 2 (4.08%) |
| Histology | ||
| Well differentiated | 1 (2.08%) | 0 |
| Moderately differentiated | 2 (4.17%) | 2 (4.08%) |
| Poorly differentiated/signet-ring cell | 35 (72.92%) | 35 (71.43%) |
| Unknown | 10 (20.83%) | 12 (24.49%) |
| Metastatic site | ||
| Liver | 12 (25.00%) | 13 (26.53%) |
| Lung | 2 (4.17%) | 2 (4.08%) |
| Peritoneum | 25 (52.08%) | 25 (51.02%) |
| Lymph node | 32 (66.67%) | 37 (75.51%) |
| Ovary | 9 (18.75%) | 8 (16.33%) |
| Others | 22 (45.83%) | 17 (34.69%) |
| Number of organs with metastases | ||
| <2 | 11 (22.92%) | 13 (26.53%) |
| ⩾2 | 37 (77.08%) | 36 (73.47%) |
| Massive ascites | ||
| Yes | 12 (25.00%) | 11 (22.45%) |
| No | 36 (75.00%) | 38 (77.55%) |
| Prior treatment | ||
| Curative gastrectomy | 8 (16.67%) | 4 (8.16%) |
| Palliative gastrectomy/metastasectomy | 13 (27.08%) | 14 (28.57%) |
| Prior adjuvant chemotherapy | 6 (12.50%) | 3 (6.12%) |
Data are median (IQR) or n (%).
AS, nab-paclitaxel plus S-1; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; SOX, oxaliplatin plus S-1.
Efficacy
Survival analysis and tumor response
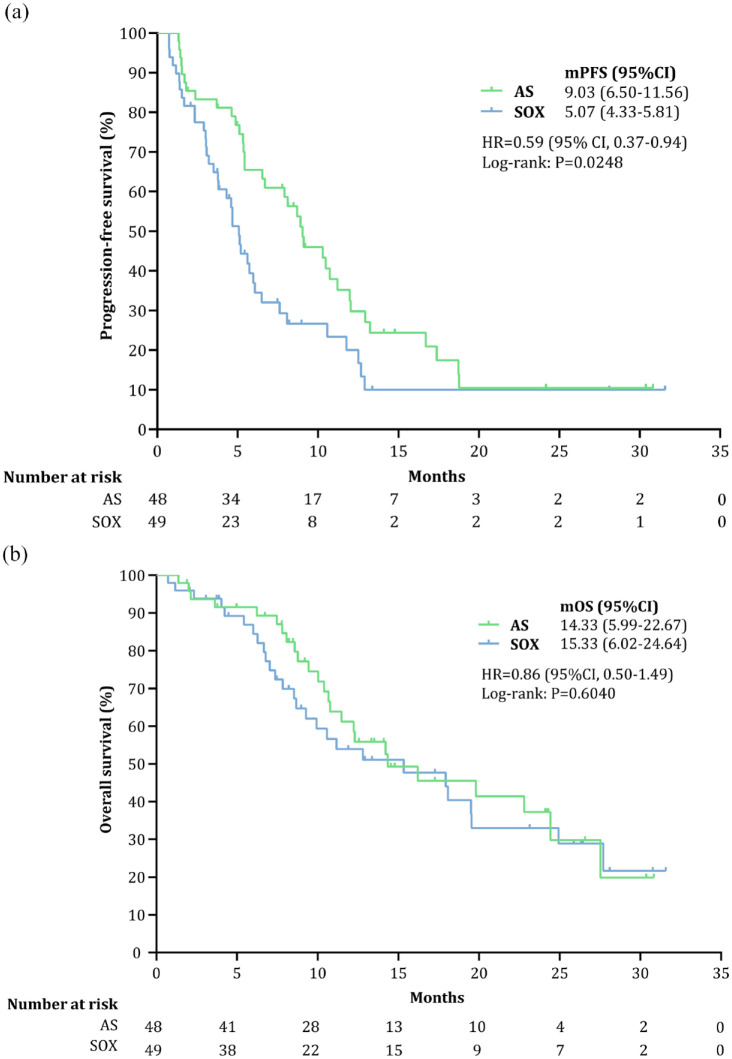
At the data cutoff (31 December 2021), 73 patients had PFS events [AS, n = 35 (72.92%); SOX, n = 38 (77.55%)]. At a median follow-up of 23.13 months (95% CI, 13.39–32.87), the median PFS was 9.03 months (95% CI, 6.50–11.56) in the AS group and 5.07 months (95% CI, 4.33–5.81) in the SOX group (HR = 0.59; 95% CI, 0.37–0.94; p = 0.03), with 6-month PFS rates of 65.47% and 36.94%, respectively (Figure 2(a)), demonstrating that patients treated with AS tended to have a better PFS than those treated with SOX. As of the cutoff date, 25 (52.08%) and 27 (55.10%) deaths occurred in the AS and SOX groups, respectively, with similar 1-year OS rates (61.20% versus 53.93%). The median OS was 14.33 months (95% CI, 5.99–22.67) in the AS group and 15.33 months (95% CI, 6.02–24.64) in the SOX group, with an HR of 0.87 (95% CI, 0.50–1.49; p = 0.60; Figure 2(b)).
Figure 2.
Survival endpoints in the full analysis set population: (a) PFS (primary endpoint) and (b) OS (secondary endpoint).
AS, nab-paclitaxel plus S-1; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; SOX, oxaliplatin plus S-1.
According to RECIST 1.1, 19 (39.58%; 95% CI, 25.77–54.73%) of the 48 patients in the AS group and 16 (32.65%; 95% CI, 19.95–47.54%) of the 49 patients in the SOX group achieved an objective response (p = 0.53; Table 2). Among them, only one patient (2.04%) in the SOX group had CR; besides, 10 patients (20.83%) in the AS group and 11 patients (22.45%) in the SOX group achieved SD (p = 0.48). Thus, the disease control rate (DCR) also did not differ significantly between the two groups [81.25% (95% CI, 67.37–91.05%) versus 69.39% (95% CI, 54.58–81.75%); p = 0.24]. Supplemental Figure S1 shows the tumor response during treatment.
Table 2.
Tumor response according to RECIST 1.1.
| AS (n = 48) | SOX (n = 49) | p Value * | |
|---|---|---|---|
| Complete response | 0 (0.00) | 1 (2.04%) | 0.478 |
| Partial response | 19 (39.58%) | 15 (30.61%) | |
| Stable disease | 10 (20.83%) | 11 (22.45%) | |
| Progressive disease | 9 (18.75%) | 15 (30.61%) | |
| Non-CR/non-PD | 10 (20.83%) | 7 (14.29%) | |
| Objective response (95% CI) | 19 (39.58%; 25.77–54.73) | 16 (32.65%;19.95–47.54) | 0.530 |
| Disease control (95% CI) | 39 (81.25%; 67.37–91.05) | 34 (69.39%; 54.58–81.75) | 0.240 |
Data are n (%).
p value for χ2 test.
AS, nab-paclitaxel plus S-1; CI, confidence interval; CR, complete response; PD, progressive disease; SOX, oxaliplatin plus S-1.
Owing to slow patient enrolment, an unplanned interim analysis was performed. The data monitoring committee recommended early termination of the study on 31, December 2021 (data cutoff) based on the results of the interim analysis.
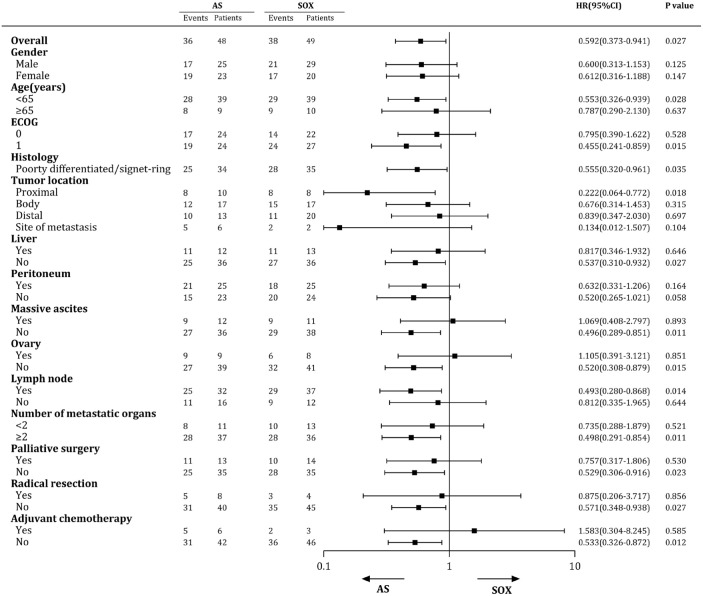
Subgroup analysis
A post-hoc subgroup analysis of PFS based on patient characteristics revealed that patients aged <65 years who had an ECOG PS of 1, primary tumor of the proximal stomach, lymph node metastasis, ⩾2 metastatic sites, no liver metastases, no massive ascites, no ovarian metastasis, or had not received prior treatment were more likely to benefit from first-line AS than SOX (Figure 3). Multivariate analysis showed that liver metastasis, ECOG PS 1, and SOX as initial treatment were associated with decreased PFS (Supplemental Table S1). However, subgroup analysis of OS showed no correlation between the assigned regimen and patient characteristics (Supplemental Figure S2).
Figure 3.
Subgroup analyses of PFS based on baseline characteristics.
AS, nab-paclitaxel plus S-1; CI, confidence interval; HR, hazard ratio; PFA, progression-free survival; SOX, oxaliplatin plus S-1.
In a cohort of patients with measurable disease (n = 71), the 6-month PFS rates were 58.69% and 36.39% in the AS and SOX groups, respectively. PFS was better in AS-treated patients (8.10 months; 95% CI, 5.15–11.05) compared to SOX-treated patients (5.07 months; 95% CI, 3.86–6.29), with an HR of 0.66 (95% CI, 0.38–1.13; p = 0.13; Supplemental Figure S3A). AS treatment reduced the risk of progression by 34.4% in this cohort (Supplemental Figure S3A). No differences in median OS [14.33 months versus 15.33 months; HR = 0.93 (95% CI, 0.50–1.73); p = 0.81; Supplemental Figure S3B], ORR (54.29% versus 44.44%; p = 0.48; Supplemental Table S2), and DCR (82.86% versus 75.00%; p = 0.56; Supplemental Table S2) were found between the two groups in this cohort. Similar results were observed in a cohort of patients with non-measurable disease (n = 26). Patients treated with AS (11.20 months; 95% CI, 8.86–13.54) tended to have a better PFS than those treated with SOX (4.67 months; 95% CI, 2.72–6.63), with an HR of 0.40 (95% CI, 0.16–1.01; p = 0.05; Supplemental Figure S4A). Thus, treatment with AS reduced the risk of disease progression by 59.7% in patients with non-measurable disease (Figure S4A). Furthermore, we did not observe any differences in the OS (Supplemental Figure S4B), ORR (Supplemental Table S2), or DCR (Supplemental Table S2) between the two groups in this cohort. Overall, the analysis of these two subgroups showed efficacy results that were consistent with that of the overall population.
Expanded follow-up analysis
Patients who progressed following first-line therapy were included in the expanded follow-up analysis. In total, 52.08% (25/48) of patients in the AS group and 44.90% (22/49) of patients in the SOX group received subsequent therapies, including nab-paclitaxel (7 versus 16), oxaliplatin (10 versus 0), irinotecan (3 versus 4), immune checkpoint inhibitors (8 versus 6), antiangiogenic therapy (5 versus 5), and others (3 versus 7). Patients who received subsequent therapies tended to have longer survival times than those who did not receive these therapies (17.93 months versus 10.77 months, p = 0.29; Supplemental Figure S5A). In addition, when these patients were stratified based on whether or not underwent nab-paclitaxel-based subsequent therapies, the OS tended to be better in the nab-paclitaxel-treated patients, although the difference was not significant (19.50 months versus 12.30 months, p = 0.15; Supplemental Figure S5B).
Assessment of QOL
The EORTC QLQ-C30 was administered to 27 patients in each group. According to the QOL assessment, a large proportion (7.41–44.44%) of patients in the two groups achieved improvement on 15 scales, and a better trend was observed in the AS group (Supplemental Figure S6).
Safety
The median relative dose intensity was 85.30% [interquartile range (IQR), 77.18–94.41%] for nab-paclitaxel, 84.00% (IQR, 66.67–95.45%) for S-1 in the AS group, 91.35% (IQR, 79.01–97.89%) for oxaliplatin, and 87.50% (IQR, 70.00–100.00%) for S-1 in the SOX group. In all, 11 patients [AS, n = 4 (8.33%); SOX, n = 7 (14.29%)] required dose reduction. Treatment was delayed by one or more times in 43 (89.58%) of the 48 patients in the AS group and in 34 (69.39%) of the 49 patients in the SOX group. The results of the neurotoxicity assessment of 54 patients (27 per group) demonstrated similar neurotoxicity in the two groups (Supplemental Figure S7). The main treatment-emergent AEs are summarized in Table 3. Treatment-emergent AEs of any grade occurred in 45 (93.75%) of the 48 patients undergoing AS therapy and in 45 (91.84%) of the 49 patients undergoing SOX therapy. Most treatment-related AEs were grade 1–2 and manageable. Only two patients (4.17%; one with acute renal failure and one with thrombocytopenia) in the AS group and three patients (6.12%; two with gastrointestinal bleeding and one death) in the SOX group experienced serious AEs, and only thrombocytopenia was considered drug related. In the two groups, the most common non-hematological AEs were fatigue, nausea, and anorexia, whereas anemia, red blood cell decrease, neutropenia, and leukopenia were the most frequent hematological toxicities. The proportion of patients with thrombocytopenia was lower in the AS group than that in the SOX group (18.75% versus 40.82%, p = 0.02). Conversely, alopecia occurred more frequently in the AS group than in the SOX group (52.08% versus 12.24%, p < 0.001). Rash was observed only in the AS group [5 of 48 patients (10.42%), p = 0.03). No treatment-related deaths occurred in either of the groups.
Table 3.
Summary of treatment-related AEs.
| AS group | SOX group | p Value | ||||
|---|---|---|---|---|---|---|
| Any | ⩾3 Grade | Any | ⩾3 Grade | Any | ⩾3 Grade | |
| Hematological | ||||||
| Anemia | 43 (89.58) | 7 (14.58) | 41 (83.67) | 6 (12.24) | 0.393 | 0.735 |
| Red blood cell decreased | 40 (83.33) | 6 (12.50) | 37 (75.51) | 6 (12.24) | 0.341 | >0.999 |
| Neutropenia | 24 (50.00) | 10 (20.83) | 26 (53.06) | 12 (24.49) | 0.763 | 0.667 |
| Leucopenia | 24 (50.00) | 4 (8.33) | 24 (48.98) | 8 (16.33) | 0.920 | 0.232 |
| Thrombocytopenia | 9 (18.75) | 1 (2.08) | 20 (40.82) | 4 (8.16) | 0.018 | 0.362 |
| Non-hematological | ||||||
| Fatigue | 25 (52.08) | 0 (0.00) | 20 (40.82) | 0 (0.00) | 0.266 | – |
| Nausea | 21 (43.75) | 0 (0.00) | 17 (34.69) | 0 (0.00) | 0.409 | – |
| Anorexia | 22 (45.83) | 0 (0.00) | 16 (32.65) | 1 (2.04) | 0.184 | >0.999 |
| AST increased | 11 (22.92) | 0 (0.00) | 20 (40.82) | 1 (2.04) | 0.059 | >0.999 |
| Alopecia | 25 (52.08) | 0 (0.00) | 6 (12.24) | 0 (0.00) | <0.001 | – |
| Vomiting | 16 (33.33) | 0 (0.00) | 14 (28.57) | 0 (0.00) | 0.612 | – |
| Constipate | 17 (35.42) | 0 (0.00) | 11 (22.45) | 0 (0.00) | 0.159 | – |
| Sensory neuropathy | 12 (25.00) | 0 (0.00) | 8 (16.33) | 1 (2.04) | 0.291 | >0.999 |
| Diarrhea | 12 (25.00) | 0 (0.00) | 7 (14.29) | 0 (0.00) | 0.210 | – |
| Creatine | 3 (6.25) | 0 (0.00) | 5 (10.20) | 0 (0.00) | 0.715 | – |
| Total bilirubin | 3 (6.25) | 0 (0.00) | 3 (6.12) | 0 (0.00) | >0.999 | – |
| Rash | 5 (10.42) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.027 | – |
Data are n (%).
AE, adverse events; AS, nab-paclitaxel plus S-1; AST, aspartate aminotransferase; SOX, oxaliplatin plus S-1.
Discussion
In this study, we compared the efficacy and safety of AS and SOX in patients with chemotherapy-naïve AGC. The unplanned interim analysis of the GAPSO study did not demonstrate the superiority of AS over SOX; however, it showed a better PFS tendency in AS-treated patients with chemotherapy-naïve AGC. Moreover, multivariate analyses indicated that the AS regimen might be broadly effective in improving PFS. However, the study was terminated early because of the slow enrolment of patients and changes in the treatment landscape.
The present study showed an improvement in PFS in favor of AS versus SOX (9.03 months versus 5.07 months). The progression risk in AS-treated patients was 40.8% lower than that in SOX-treated patients. The median PFS of patients treated with AS was 9.03 months, which was consistent with the results of a previous phase II trial (9.63 months). 25 The improvement in PFS observed in the AS group may be partly attributed to the pharmacological advantages of nab-paclitaxel, which can deliver higher doses of paclitaxel over a shorter infusion time, enhance the transport of paclitaxel across endothelial cells, and allow better delivery of the drug to the tumor microenvironment. Thus, it is associated with more linear pharmacokinetics.28,29 Although the present study did not demonstrate the advantages of AS in OS and ORR compared to SOX, the OS (14.33 months) and ORR (39.58%) following AS treatment were generally comparable with the results of other studies on S-1 plus paclitaxel, such as the OGSG0402 trial in Japan (OS, 11.9 months; ORR, 31.4%) 18 and a randomized phase II study in China (OS, 14.0 months; ORR, 46.3%). 30 In addition, the use of subsequent therapies in the two arms may explain the lack of OS benefits from AS therapy. A significant proportion of patients who progressed after the first-line therapy received subsequent therapies, including nab-paclitaxel, immune checkpoint inhibitors, or antiangiogenic therapy. Thus, we cannot completely exclude the possibility of their effects on long-term outcomes. However, in our published abstract, the ORRs of the two groups were relatively higher than the present results because we only analyzed patients with measurable lesions. 31 Based on the above results, the AS combination regimen may be more favorable and satisfactory for improving PFS than the SOX regimen in first-line treatment for patients with AGC.
Multivariate analyses of PFS further indicated that the AS regimen may be broadly effective in improving PFS. Through the analyses of clinicopathological subgroups, the present study further revealed that several subpopulations of patients, such as patients aged <65 years who had an ECOG PS of 1 and lymph node metastasis, benefit from the AS regimen. Nonetheless, the results of subgroup analysis should be interpreted with caution because of the small sample size studied.
At the end of the follow-up period, approximately half of the patients in the two groups were still alive, which may have affected the estimation of median OS. Therefore, we evaluated the survival of patients who showed disease progression following first-line chemotherapy. Among these patients, whether the subsequent therapy contained nab-paclitaxel or not tended to induce a longer OS. Notably, four of the six patients in the AS group who were rechallenged with nab-paclitaxel as second-line chemotherapy survived for more than 20 months and were still alive at the last follow-up. Thus, for patients with tumor progression following first-line therapy, nab-paclitaxel-based chemotherapy is likely to produce a long-term benefit. In addition, we investigated the effects of AS and SOX on QOL and found that AS may improve QOL in more patients. However, we recognize that these results are preliminary and require further analysis.
Another advantage of the AS regimen is its favorable safety profile owing to the low toxicity of both nab-paclitaxel and S-1. The present study showed an acceptable and manageable safety profile for the AS combination regimen in Chinese patients with AGC. The AEs observed in this study were consistent with known profiles reported in previous clinical studies. 25 No new safety concerns were noted in this population. Regarding hematological toxicities, the occurrence of leukopenia, neutropenia, red blood cell decrease, and anemia was generally similar between the two groups. However, the SOX regimen was associated with a higher incidence of thrombocytopenia. We speculate that this result may be partly due to oxaliplatin-related hepatic sinusoidal obstruction syndrome, in addition to bone marrow suppression.32,33 Similarly, we found a higher incidence of elevated ALT levels in the SOX group, although no significant difference was observed between the groups. Conversely, the incidence of alopecia and rash was higher in the AS group than that in the SOX group. These results are consistent with the mechanism of action of each therapy. Although neurotoxicity is a major safety concern following taxane treatment, 34 peripheral sensory neuropathy and neurotoxicity assessment results were similar for the two groups in our study. Notably, a previous study indicated that paclitaxel-induced neuropathy improved immediately after chemotherapy cessation, whereas oxaliplatin-induced neuropathy worsened after treatment and improved until 3-month post-treatment, 35 which was consistent with our findings. Although the neurotoxicity assessment data were only available in 54 patients owing to the COVID-19 epidemic, we found that nab-paclitaxel-induced neuropathy was obvious in the first few cycles, but showed no obvious aggravation. Conversely, oxaliplatin-induced neuropathy showed sustained aggravation, particularly in the last few cycles. Based on this evidence, AS therapy appears to have a safety advantage over SOX therapy in this population. Overall, most of the AEs were grade 1–2 and resolved with supportive care and dose modification. No treatment-related deaths occurred in either of the groups. The overall safety profile was acceptable and manageable.
This study had several limitations. First, the expected number of events was not observed at the data cutoff point, which may have affected the strength of the results. In fact, owing to the successful use of immune checkpoint therapies in the treatment of AGC, chemotherapy combined with immunotherapy has been recommended in select patients,36–38 which has led to slow patient enrolment since March 2021. Furthermore, the slow enrolment was further aggravated by the COVID-19 epidemic. During the 9-month period, only nine patients were recruited and it was not possible to recruit the required number of patients. Thus, based on the decision of the data and safety-monitoring committee of the trial, the study was terminated early in December 2021 with a statistical power of 75% in the unplanned interim analysis. Because immunotherapy has been the dominant paradigm in first-line therapy for unresectable GC, clinical trials to determine which regimen would be the better partner for immunotherapy are being planned. A second limitation is the use of the mITT population for efficacy analysis, which is not the most frequent analytical policy in randomized clinical trials (RCTs). In fact, there are three reasons for using mITT instead of ITT: (1) according to ICH E9, there are several circumstances that might lead to exclusion of randomized subjects from the ITT, including eligibility violations, failure to take at least one dose of trial medication, and lack of any data post-randomization. Thus, such exclusions in mITT should always be justified39,40; (2) it was reported that deviation from an ITT analysis in RCTs is a potential source of biased estimates of treatment effects. 41 Thus, an increasing number of RCTs have also analyzed the efficacy of mITT.42–46 (3) Patients who did not receive any study treatment withdrew their consent; thus, they were not followed up based on ethical principles, leading to the lack of efficacy assessment. Third, patients with non-measurable diseases were included, which may have affected the ORR. However, subgroup analyses according to measurable disease showed efficacy results that were consistent with those of the overall population. In fact, as per RECIST 1.1, ascites (as a non-target lesion) can be used for tumor response assessment. Therefore, disease progression must be assessed qualitatively and independently by two radiologists to evaluate the response more accurately. Fourth, due to the COVID-19 epidemic, biomarker tests, assessment of QOL, and neurotoxicity were available from only a limited number of patients, resulting in a lack of biomarker analyses used to predict the response to treatment as well as a lack of complete analysis of the safety profile. Another limitation of our study was the open-label design, which had the potential to introduce a subconscious bias in favor of the experimental group. Nevertheless, the radiologists and safety-monitoring staff were blinded to the treatment groups, thereby reducing the risk of ascertainment bias.
Conclusions
This study showed a better PFS tendency in patients with chemotherapy-naïve HER2-negative AGC who were treated with AS compared to those treated with SOX. Furthermore, AS showed manageable toxicity in this population. However, owing to slow patient enrolment, the monitoring board of the study decided on an early termination of the study. The results of this study will support the completion of the ongoing trials investigating the efficacy of nab-paclitaxel-based regimens.
Supplemental Material
Supplemental material, sj-docx-8-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-9-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-4-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-1-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-5-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-6-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-7-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yu-Hong Dai, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Xiong-Jie Yu, Department of Oncology, Shiyan Renmin Hospital, Shiyan, Hubei, China.
Hui-Ting Xu, Department of Oncology, Hubei Cancer Hospital, Wuhan, Hubei, China.
Liang Zhuang, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Ming-Sheng Zhang, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Yan-Mei Zou, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Qiang Fu, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Hong Qiu, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, No.1095 Jie Fang Avenue, Wuhan, Hubei 430030, China.
Xiang-Lin Yuan, Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, No.1095 Jie Fang Avenue, Wuhan, Hubei 430030, China.
Declarations
Ethics approval and consent to participate: The ethical, medical, and scientific aspects of the study were reviewed and approved by the ethics committee of Tongji Hospital of Huazhong University of Science and Technology (Ethical approval: 2018S510-4) and the Institutional Review Board of each participating center prior to initiation of the study. All patients provided written informed consent before enrolment in the study. This study was performed in accordance with the international ethical recommendations of the Declaration of Helsinki and Good Clinical Practice guidelines. This study was registered at ClinicalTrials.gov (NCT03801668).
Consent for publication: Not applicable.
Author contribution(s): Yu-Hong Dai: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Xiong-Jie Yu: Investigation; Resources; Writing – review & editing.
Hui-Ting Xu: Investigation; Resources; Writing – review & editing.
Liang Zhuang: Data curation; Investigation; Resources; Writing – review & editing.
Ming-Sheng Zhang: Investigation; Resources; Writing – review & editing.
Yan-Mei Zou: Data curation; Investigation; Writing – review & editing.
Qiang Fu: Data curation; Investigation; Writing – review & editing.
Hong Qiu: Conceptualization; Project administration; Supervision; Writing – review & editing.
Xiang-Lin Yuan: Conceptualization; Project administration; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing Interests: The authors declare that there is no conflict of interest.
Availability of data and materials: All data analyzed in this study are included in the article. Further inquiries can be directed to the corresponding author.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Z, Gong YB, Xu HM. Neoadjuvant therapy strategies for advanced gastric cancer: current innovations and future challenges. Chronic Dis Transl Med 2020; 6: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Necula L, Matei L, Dragu D, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol 2019; 25: 2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018. 5th ed. Gastric Cancer 2021; 24: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019; 30: 19–33. [DOI] [PubMed] [Google Scholar]
- 6. Gao K, Wu J. National trend of gastric cancer mortality in China (2003-2015): a population-based study. Cancer Commun (Lond) 2019; 39: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marano L, Polom K, Patriti A, et al. Surgical management of advanced gastric cancer: an evolving issue. Eur J Surg Oncol 2016; 42: 18–27. [DOI] [PubMed] [Google Scholar]
- 8. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019; 14: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee KW, Chung IJ, Ryu MH, et al. Multicenter phase III trial of S-1 and cisplatin versus S-1 and oxaliplatin combination chemotherapy for first-line treatment of advanced gastric cancer (SOPP trial). Gastric Cancer 2021; 24: 156–167. [DOI] [PubMed] [Google Scholar]
- 10. Kang Y-K, Chin K, Chung HC, et al. S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): a randomised, open-label, phase 3 trial. Lancet Oncol 2020; 21: 1045–1056. [DOI] [PubMed] [Google Scholar]
- 11. Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016; 14: 1286–1312. [DOI] [PubMed] [Google Scholar]
- 12. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9: 215–221. [DOI] [PubMed] [Google Scholar]
- 13. Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017; 39: 1010428317714626. [DOI] [PubMed] [Google Scholar]
- 14. Shirasaka T, Shimamato Y, Ohshimo H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 1996; 7: 548–557. [DOI] [PubMed] [Google Scholar]
- 15. Tsushima T, Hironaka S, Boku N, et al. Safety and efficacy of S-1 monotherapy in elderly patients with advanced gastric cancer. Gastric Cancer 2010; 13: 245–250. [DOI] [PubMed] [Google Scholar]
- 16. Huang D, Ba Y, Xiong J, et al. A multicentre randomised trial comparing weekly paclitaxel + S-1 with weekly paclitaxel + 5-fluorouracil for patients with advanced gastric cancer. Eur J Cancer 2013; 49: 2995–3002. [DOI] [PubMed] [Google Scholar]
- 17. Mochiki E, Ogata K, Ohno T, et al. Phase II multi-institutional prospective randomised trial comparing S-1+paclitaxel with S-1+cisplatin in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer 2012; 107: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugimoto N, Fujitani K, Imamura H, et al. Randomized phase II trial of S-1 plus irinotecan versus S-1 plus paclitaxel as first-line treatment for advanced gastric cancer (OGSG0402). Anticancer Res 2014; 34: 851–857. [PubMed] [Google Scholar]
- 19. Bian NN, Wang YH, Min GT. S-1 combined with paclitaxel may benefit advanced gastric cancer: evidence from a systematic review and meta-analysis. Int J Surg 2019; 62: 34–43. [DOI] [PubMed] [Google Scholar]
- 20. Naganuma M, Tahara K, Hasegawa S, et al. Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration adverse event reporting system. SAGE Open Med 2019; 7: 2050312119836011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy V, LaPlant BR, Gross GG, et al. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann Oncol 2009; 20: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desai N. Nanoparticle Albumin-Bound Paclitaxel (Abraxane®). In: Otagiri M, Chuang VTG. (eds) Albumin in medicine. Singapore: Springer Singapore, 2016, pp.101–119. [Google Scholar]
- 23. Li J-A, Xu X-F, Han X, et al. Nab-paclitaxel plus s-1 shows increased antitumor activity in patient-derived pancreatic cancer xenograft mouse models. Pancreas 2016; 45: 425–433. [DOI] [PubMed] [Google Scholar]
- 24. Nakayama N, Ishido K, Chin K, et al. A phase I study of S-1 in combination with nab-paclitaxel in patients with unresectable or recurrent gastric cancer. Gastric Cancer 2017; 20: 350–357. [DOI] [PubMed] [Google Scholar]
- 25. He MM, Wang F, Jin Y, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci 2018; 109: 3575–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology. Cancer 2003; 98(4): 822–831. [DOI] [PubMed] [Google Scholar]
- 27. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol 2015; 26: 141–148. [DOI] [PubMed] [Google Scholar]
- 28. Palumbo R, Sottotetti F, Bernardo A. Targeted chemotherapy with nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in metastatic breast cancer: which benefit for which patients? Ther Adv Med Oncol 2016; 8: 209–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Design Dev Ther 2015; 9: 3767–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Wang ML, Zhou LY, et al. Randomized phase II study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol 2013; 15: 836–842. [DOI] [PubMed] [Google Scholar]
- 31. Dai Y, Yu X, Xu H, et al. A multicenter randomized phase III study of albumin-bound paclitaxel combined with S-1 (AS) versus oxaliplatin combined with S-1 (SOX) for first-line treatment of advanced gastric cancer (GAPSO study). J Clin Oncol 2022; 40(4_Suppl): 282–282.34874182 [Google Scholar]
- 32. Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010; 28: 2549–2555. [DOI] [PubMed] [Google Scholar]
- 33. Jardim DL, Rodrigues CA, Novis YAS, et al. Oxaliplatin-related thrombocytopenia. Ann Oncol 2012; 23: 1937–1942. [DOI] [PubMed] [Google Scholar]
- 34. da Costa R, Passos GF, Quintao NLM, et al. Taxane-induced neurotoxicity: Pathophysiology and therapeutic perspectives. Br J Pharmacol 2020; 177: 3127–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pachman DR, Qin R, Seisler D, et al. Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 2016; 24: 5059–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng Z, Wei J, Wang F, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res 2021; 27: 3069–3078. [DOI] [PubMed] [Google Scholar]
- 38. Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019; 30: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. In International conference on harmonisation E9 Expert Working Group. Stat Med 1999; 18: 1905–1942. [PubMed] [Google Scholar]
- 40. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abraha I, Cherubini A, Cozzolino F, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ 2015; 350: h2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016; 388: 1281–1290. [DOI] [PubMed] [Google Scholar]
- 43. Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 2014; 384: 309–318. [DOI] [PubMed] [Google Scholar]
- 44. Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol 2017; 35: 3449–3457. [DOI] [PubMed] [Google Scholar]
- 45. Kim GM, Jeung H-C, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur J Cancer 2012; 48: 518–526. [DOI] [PubMed] [Google Scholar]
- 46. Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ 2010; 340: c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-8-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-9-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-4-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-1-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-5-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-6-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-7-tam-10.1177_17588359221118020 for Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) by Yu-Hong Dai, Xiong-Jie Yu, Hui-Ting Xu, Liang Zhuang, Ming-Sheng Zhang, Yan-Mei Zou, Qiang Fu, Hong Qiu and Xiang-Lin Yuan in Therapeutic Advances in Medical Oncology