Abstract
Severe coronavirus (SARS-COV-2) infection often leads to systemic inflammation accompanied by cardiovascular complications including venous thromboembolism (VTE). However, it is largely undefined if inflammatory markers such as lipocalin-2 (LNC2), calprotectin (S100A8/A9), and cystatin C (CST3), previously linked with VTE, play roles in cardiovascular complications and advancement of COVID-19 severity. To investigate the same, hospitalized moderate and severe (presented pneumonia and required intensive care) COVID-19 patients were recruited. The levels of plasma LNC2, S100A8/A9, CST3, myoglobin, and cardiac Troponin I (cTnI) were assessed through enzyme-linked immunosorbent assay (ELISA). The investigation revealed a significantly upregulated level of plasma LNC2 at the moderate stage of SARS-CoV-2 infection. In contrast, the levels of S100A8/A9 and CST3 in moderate patients were comparable to healthy controls; however, a profound induction was observed only in severe COVID-19 patients. The tissue injury marker myoglobin was unchanged in moderate patients; however, a significantly elevated level was observed in the critically ill COVID-19 patients. In contrast, cTnI level was unchanged both in moderate and severe patients. Analysis revealed a positive correlation between the levels of S100A8/A9 and CST3 with myoglobin in COVID-19. In silico analysis predicted interactions of S100A8/A9 with toll-like receptor 4 (TLR-4), MyD88 LY96, and LCN2 with several other inflammatory mediators including MMP2, MMP9, TIMP1, and interleukins (IL-6, IL-17A, and IL-10). In summary, early induction of LCN2 likely plays a role in advancing the COVID-19 severity. A positive correlation of S100A8/A9 and CST3 with myoglobin suggests that these proteins may serve as predictive biomarkers for thromboembolism and tissue injury in COVID-19.
Keywords: COVID-19, calprotectin, lipocalin-2, cystatin C, cardiovascular, thromboembolism
Impact Statement
Cardiovascular complications including venous thromboembolism and cardiac injury emerge as strong comorbidity factors in COVID-19. Therefore, biomarkers are immensely required to predict cardiovascular complications in COVID-19. The inflammatory markers such as lipocalin-2, S100A8/A9, and cystatin C have recently been linked to COVID-19 severity; however, it is largely undefined when particularly these markers are induced during the COVID-19 pathogenesis and whether they are associated with cardiovascular complications in COVID-19. Here we report, for the first time, that lipocalin-2 induced during the early, and S100A8/A9 and cystatin C during the late severe, stage of COVID-19. Consistently, S100A8/A9 and cystatin C, but not lipocalin-2, were found to be positively correlated with the tissue injury marker myoglobin in the COVID-19 patients. Hence, these markers could serve as predictive markers for severity, thromboembolism, and tissue injury in COVID-19.
Introduction
Severe coronavirus (SARS-CoV-2) infection is frequently linked to coagulopathy and venous thromboembolism (VTE) which promotes tissue injury and multiorgan dysfunction.1,2 SARS-CoV-2 invades alveolar epithelial and endothelial cells through the angiotensin-converting enzyme-2 (ACE-2) receptor resulting in angiotensin-signaling dysregulation. 3 The infection leads to endotheliitis and injury to different soft organs including the kidney and heart.4,5 The viral myocarditis and kidney dysfunction contribute to elevated myoglobin levels and often lead to fatal complications in severe COVID-19.6,7 Studies reported that the SARS-CoV-2 infection of endothelial cells in the kidney, cardiovascular system, and brain leads to myoglobin induction. 8 Moreover, prolonged hypoxia condition in COVID-19 causes non-specific damage to multiple soft organs and further contributes to the level of myoglobin which serves as a good prognostic marker of COVID-19 pneumonia.
Venous thrombosis followed by pulmonary embolism (PE) is commonly seen in severe COVID-19 and found to be associated with increased severity and mortality rate.9,10 The precise mechanism of VTE is largely undefined though the induction of platelet activation markers at early moderate stage of infection, inflammatory mediator (cytokine storm), and acquired FXIII deficiency seem to play critical roles in VTE.2,11 Vascular inflammation induces endothelial activation, which promotes the prothrombotic state of the endothelial layer. 12 The endothelial dysfunction occurs not only due to SARS-CoV-2 invasion but also through inflammatory markers secreted from activated endothelial cells.13,14 Hence, inflammatory mediators linked to thromboembolism may play driving roles in tissue injury and multiorgan failure in severe COVID-19. A previous study suggested myoglobin as a powerful predictor of increased risk of fatal outcome in major PE 15 which is commonly observed in severe COVID-19.
The dysregulation of markers such as calprotectin (S100A8/A9),16,17 cystatin C (CST3),18,19 and lipocalin-2 (LCN2)20,21 is linked with cardiovascular complications including vascular inflammation and VTE and tissue injury. S100A8/A9 is secreted from myeloid cells in response to inflammatory reactions and regulates the pathogenesis of cardiovascular disease. 22 It has also been used as a prognostic marker in different inflammatory diseases including pulmonary inflammations, gastrointestinal inflammations, and inflammatory bowel disease.23,24 LCN2, also known as neutrophil gelatinase–associated lipocalin (NGAL), is a neutrophil-associated protein that plays a role in innate immunity and is found to be associated with cardiorenal diseases. 25 Similarly, CST3, a non-glycosylated cysteine protease inhibitor, has been linked to cardiovascular risk and renal dysfunction.26,27
Increased levels of plasma S100A8/A9, 28 LCN2, 29 and CST3 30 have recently been reported in COVID-19. SARS-CoV-2-mediated induction of these markers may contribute to the severity of the disease partly due to the effects on the cardiorenal system. The COVID-19 patients may develop cardiac complications even in the absence of pre-existing cardiac diseases likely due to injuries caused by myocarditis. 31 Cardiac injury is reported in hospitalized severe COVID-19 patients and is strongly associated with poor clinical outcomes.32,33 Hence, assessment of the level of LCN2, S100A8/A9, and CST3 in moderate and severe COVID-19 patients is important. Moreover, the correlation between the levels of said inflammatory markers and cardiac injury is the utmost requirement to establish their potential roles in the cardiorenal complication observed in severe COVID-19.
Therefore, here we sought to assess the role of LCN2, S100A8/A9, and CST3 in cardiovascular complications and in the progression of COVID-19 severity by recruiting moderate and severe COVID-19 patients. Our findings show that the levels of LCN2, S100A8/A9, and CST3 are strongly associated with the pathogenesis of COVID-19. The levels of myoglobin in COVID-19 patients positively correlate with the levels of S100A8/A9 and CST3. In silico analysis predicts the interaction of LCN2 and S100A8/A9 with many other key inflammatory markers previously linked with thrombosis or VTE.
Materials and methods
Patients and sample processing
A total of 30 hospitalized COVID-19 patients including 15 moderate (without pneumonia or intensive care requirement) and 15 severe (presented pneumonia and/or acute respiratory distress syndrome and required intensive care) patients were recruited from Rashid Hospital, Dubai, between May and June 2020. The SARS-CoV-2 infection in these patients was confirmed with the reverse transcription–polymerase chain reaction (RT-PCR). Moreover, a total of 10 healthy controls were recruited for comparison. A written informed consent and medical and family history were collected from patients and healthy controls before collecting the blood samples. Patients with a history of inflammatory diseases and/or pre-existing cardiovascular diseases including those with myocardial infarction, stroke, or deep vein thrombosis (DVT) were excluded from the study. The study protocol was approved by the ethical review boards of the University of Sharjah and Dubai Health Authority. The blood samples from the patients and healthy controls were collected in an appropriate anticoagulant vial. The whole blood samples were centrifuged at 3000 rpm for 20 min, and platelet-poor plasma (PPP) was transferred to a fresh tube and stored at −80°C.
Assessment of specific inflammatory and cardiac injury markers
The vascular inflammation markers S100A8/A9 (Abcam #ab267628), CST3 (ThermoFisher #EHCST3), and LCN2 (ThermoFisher #EHLCN2) were assessed in the plasma using enzyme-linked immunosorbent assay (ELISA) kits following the instruction manual. The cardiac injury markers myoglobin (ThermoFisher #BMS2259) and cTnI (Abcam #ab200016) were also assessed employing ELISA kits following manufacturer instructions. All the samples were assessed in duplicate, and the average was taken as a final reading.
Protein-protein interaction network analysis
The protein-protein interaction (PPI) was performed to predict the interacting partners of S100A8/A9, LCN2, and CST3 through an online tool, STRING database (https://string-db.org/). A PPI networking map was prepared for the network, co-occurrence, database, co-expression, neighborhood, fusion, and co-localization of the gene of interest and its interacting gene as described previously. 34 The name of the target gene (Homo sapiens) was entered in the STRING database. Predicted functional partners were represented based on scores (indicators of confidence where STRING judges PPI based on evidence). Scores rank from 0 to 1, where 1 being the highest possible confidence. 35
Statistics
Data were analyzed employing one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis for multiple comparisons. For correlation, a linear regression test was performed (Graph Pad Prism Software Inc., San Diego, CA). Data were presented as mean ± SEM (standard error of mean). A P value less than 0.05 was considered statistically significant.
Results
Demography and clinical manifestations
The median age of the moderate patients was 48 years and 57 years of the severe group. There was no fatality reported in the moderate group; however, a total of five (33.3%) of 15 patients died in the severe group. Most of the patients were on chloroquine or hydroxychloroquine with or without antiviral and anti-inflammatory medication like Kalerta (lopinavir/ritonavir), Favipiravir, Tocilizumab, Azithromycin, and Corticosteroids (Supplementary Table 1). A detailed laboratory investigation results of these patients were reported recently. 36 The patients in both moderate and severe groups presented normal mean serum ferritin, creatinine, and platelet counts, and a trend of increased activated partial thromboplastin time (APTT) and prothrombin time (PT) were observed. Moreover, the D-dimer level, white blood cells (WBCs), and absolute lymphocyte count (ALC) were significantly increased in the severe versus moderate group. These findings attest the induction of inflammation and coagulation activation in the severe COVID-19 patients.
SARS-CoV-2 induces the levels of LCN2 at moderate and S100A8/S100A9 and CST3 at the severe stage of infection
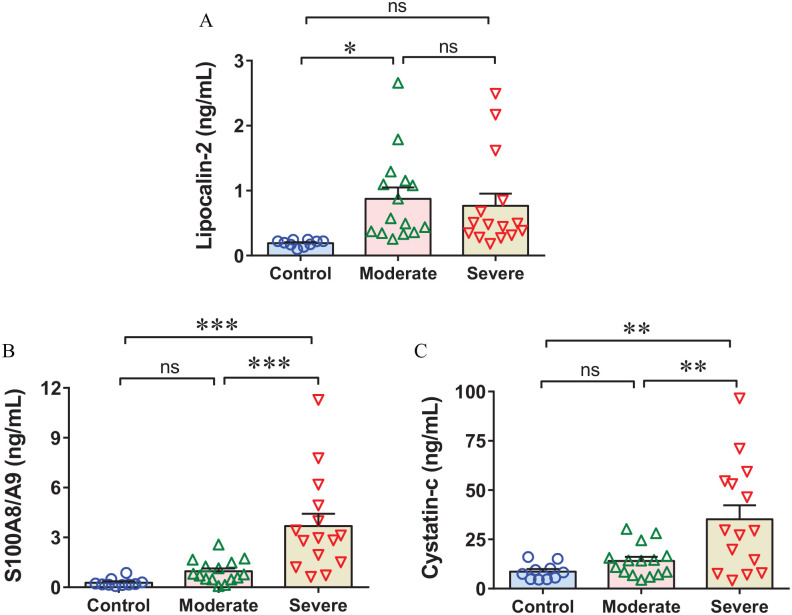
The SARS-CoV-2-induced inflammatory reaction accompanied by pro-coagulant changes in the vascular endothelium facilitates thrombosis and thromboembolism which determines the clinical presentation of COVID-19. Hence, we hypothesized that inflammatory markers, previously linked with thromboembolism, could potentially have associations with COVID-19 severity. Indeed, an increased level of LCN2 was identified in the moderate and only a trend of increase level was observed in the critically ill COVID-19 patients (Figure 1(A)). However, conversely, the levels of S100A8/S100A9 and CST3 were profoundly upregulated in the severe cohort and were unchanged in the moderate group (Figure 1(B) and (C)). These results suggest that LCN2 induction in moderate patients aggravates the COVID-19 severity and most likely plays an important role in the induction of cytokine storms. The elevated levels of S100A8/S100A9 and CST3 in severe patients are potentially caused due to inflammation induction.
Figure 1.
SARS-CoV-2 infection induces lipocalin-2 at moderate and calprotectin and cystatin C at severe stage of COVID-19 pathogenesis. The scatter dot plots show the levels of (A) plasma lipocalin-2 (LCN2), (B) calprotectin (S100A8/A9), and (C) cystatin C (CST3) in moderate and severe COVID-19 patients versus healthy controls. (A color version of this figure is available in the online journal.)
*P < 0.05; **P < 0.005; ***P < 0.0005.
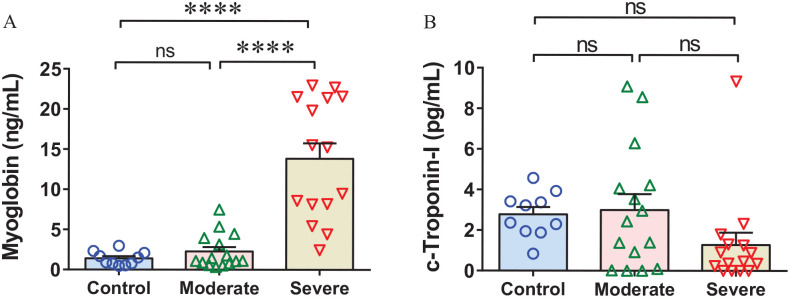
Severe SARS-CoV-2 infection promotes myoglobin induction
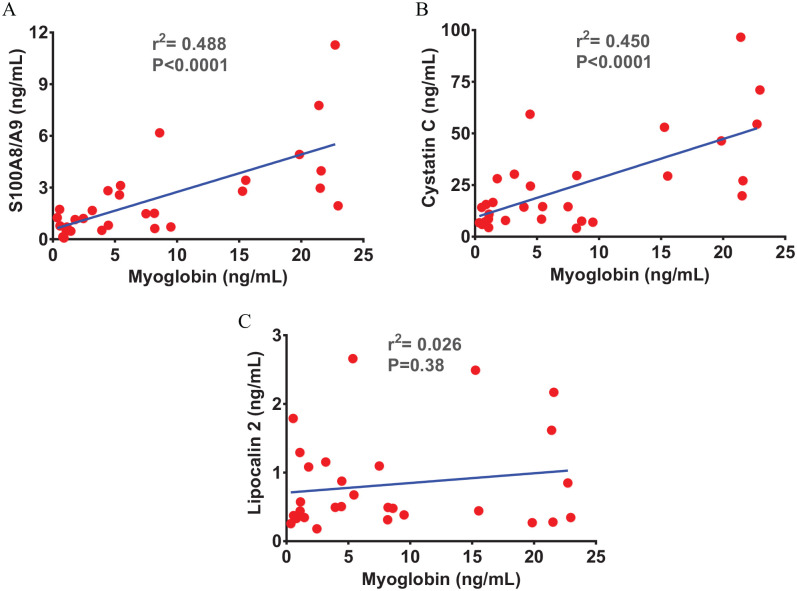
The association between SARS-CoV-2 infection-induced systemic inflammation and myocarditis-induced cardiac injury has been reported in the severe COVID-19.6,37 Therefore, we assessed the level of cardiac injury markers, myoglobin and cTnI, to know if the recruited COVID-19 patients potentially developed cardiac injury. The myoglobin level was found comparable between moderate patients and healthy controls; however, a profoundly elevated level was identified in the severe versus moderate COVID-19 patients and healthy controls (Figure 2(A)). Surprisingly, cTnI level was found unchanged both in moderate and severe COVID-19 groups (Figure 2(B)). The plasma S100A8/S100A9 and CST3 were positively correlated with the levels of myoglobin (Figure 3(A) and (B)); however, no correlation was observed between the levels of LCN2 and myoglobin in the recruited patients (Figure 3(C)). These observations suggest that SARS-CoV-2 infection leads to induction of plasma LCN2 at a relatively early stage. The positive correlations of S100A8/S100A9 and CST3 with cardiac injury marker myoglobin in severe patients suggest their potential roles in cardiovascular complications in COVID-19.
Figure 2.
Severe SARS-CoV-2 infection exaggerates cardiac injury in COVID-19. (A) The bar diagram shows an unchanged myoglobin level in the moderate and significantly increased levels in the severe COVID-19 patients. (B) The scatter bar diagram shows comparable levels of cardiac troponin I (cTnI) in moderate and severe COVID-19 patients. (A color version of this figure is available in the online journal.)
****P < 0.0001.
Figure 3.
Calprotectin and cystatin C show a positive correlation with cardiac injury markers. The scatter plots from linear regression analysis show a positive correlation between (A) calprotectin (S100A8/A9) and myoglobin and (B) cystatin C (CST3) and myoglobin. (C) Graph shows no correlation between lipocalin-2 (LCN2) and myoglobin. (A color version of this figure is available in the online journal.)
PPI analysis reveals the potential interacting partners of S100A8/S100A9, LCN2, and CST3
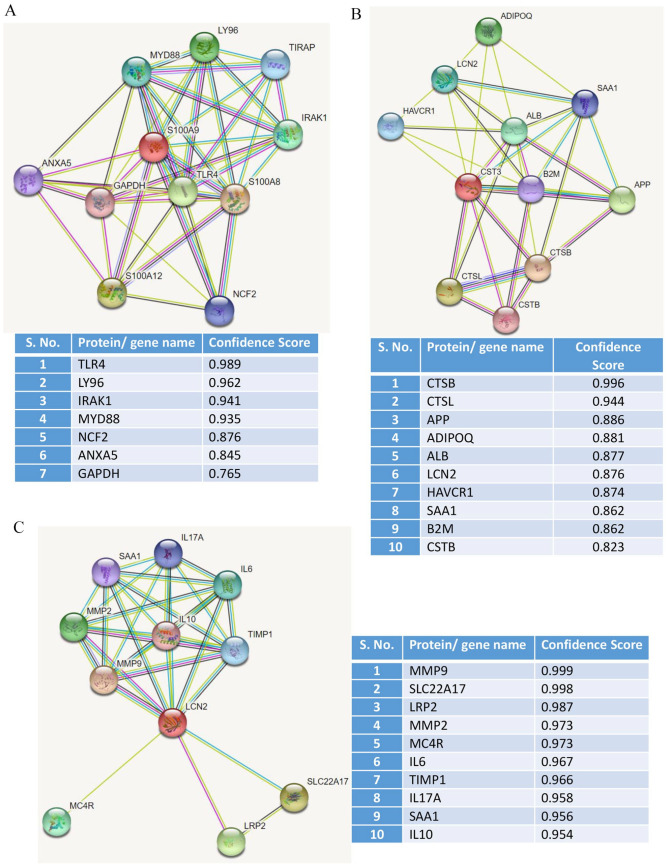
Next, we investigated how potentially S100A8/A9, CST3, and LCN2 regulate inflammation and cardiovascular complications. In silico PPI revealed interaction of S100A8/A9 with several key inflammatory mediators including toll-like receptor 4 (TLR4), lymphocyte antigen 6 (LY6), interleukin-1 receptor-associated kinase 1 (IRAK1), myeloid differentiation primary response 88 (MYD88/TIRAP), and neutrophil cytosolic factor 2 (NCF2) (Figure 4(A)). Moreover, PPI analysis for CST3 revealed its several interacting partners including cathepsin B (CTS-B, CTS-L), amyloid precursor protein (APP), adiponectin (ADIPOQ), albumin (ALB), LCN2, beta-2 microglobulin (B2M), and cystatin B (CSTB) (Figure 4(B)). Similar to S100A8/A9, LCN2 has also shown its interaction with inflammatory mediators including matrix metallopeptidases (MMP2 and MMP9), interleukins (IL-6, IL-10, and IL-17A), SLC22A17, melanocortin 4 receptor (MC4R), and tissue inhibitor matrix metalloproteinase 1 (TIMP1) (Figure 4(C)). Taken together, most of the indicated interacting inflammatory mediators are reported to be induced in severe COVID-19 and previously linked with either thrombosis or VTE. Therefore, these interacting inflammatory markers likely mediate cardiovascular complications in the COVID-19.
Figure 4.
STRING database analysis to identify the potential interacting partners of calprotectin cystatin C and lipocalin-2. The protein-protein interaction (PPI) analysis shows potential interaction between (A) calprotectin (S100A8/A9), (B) cystatin C (CST3), and (C) lipocalin-2 (LCN2) with other proteins. Here, PPI interaction is depicted in the form of nodes (proteins) and edges (functional interactions) where red-colored nodes represent the query protein. Predicted functional partners are represented based on scores. Scores are the indicators of confidence where STRING judges PPI based on evidence. Scores rank from 0 to 1, where 1 being the highest possible confidence. (A color version of this figure is available in the online journal.)
Discussion
Here, we investigated the potential roles of S100A8/A9, CST3, and LCN2 in the pathogenesis of COVID-19 and show induction of S100A8/A9 and CST3, particularly in severe COVID-19 patients who required intensive care. Most importantly, the level of LCN2 was found significantly upregulated at the early moderate stage of COVID-19. The plasma myoglobin was significantly elevated only in the severe patients and was found positively correlated with the levels of S100A8/A9 and CST3. In silico analysis show the potential interaction of S100A8/A9 and LCN2 with several other inflammatory mediators.
The observed elevated levels of LCN2 at the early moderate stage indicate its potential role in promoting the COVID-19 pathogenesis. The predicted interactions of LCN2 with MMPs and other inflammatory mediators further suggest the role of LCN2 in vascular inflammation induction. LCN2 forms a complex with and protects MMP9 which increases the deleterious effects of MMP9 on blood vessels. 38 Consistent with the early induction of LCN2 in our study, an early increase of plasma MMP9 was also previously reported in COVID-19. 39 LCN2 is an independent thrombotic risk factor, 40 and increased levels have been linked to thromboembolic complications. 41 Therefore, LCN2 may promote vascular inflammation and induce thromboembolism in COVID-19 largely through regulating the levels of MMP9 and other inflammatory mediators (Figure 5). The interactions between LCN2 and other inflammatory mediators need validation to define the specific roles of LCN2 in the regulation of thromboembolism.
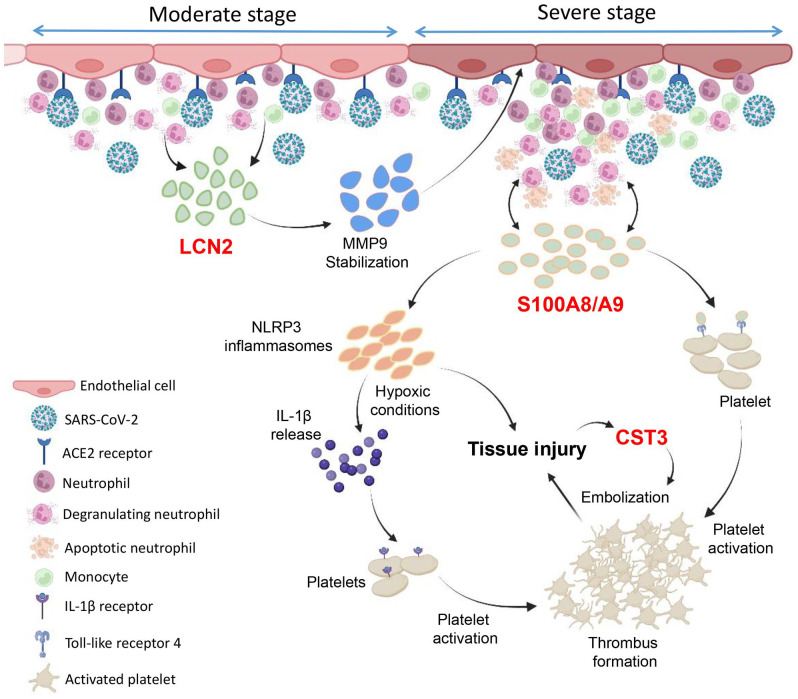
Figure 5.
Role of LCN2, S100A8/A9, and CST3 in COVID-19 pathogenesis. The schematic figure is drawn based on our findings and previous reports highlighting the key roles of lipocalin-2 (LCN2), calprotectin (S100A8/A9), and cystatin C (CST3) in the pathogenesis of COVID-19. SARS-CoV-2 infection at the moderate stage promotes neutrophil infiltration and activation which potentially induces the profound release of LCN2. The elevated level of plasma LCN2 increases MMP9 stabilization and in turn increases the deleterious effect of MMP9 on vasculatures which, partly, induces vascular inflammation. This further induces the myeloid cells infiltration and S100A8/A9 release. S100A8/A9 binds with toll-like receptor 4 (TLR4) receptors on neutrophils which further activates the immune cell population. S100A8/A9 also couple with TLR4 present on platelets which induces platelet activation and aggregation. Moreover, S100A8/A9 prime with NLRP3 inflammasomes ultimately leads to tissue injury and IL-1β secretion, particularly under hypoxic conditions, which activates platelets through binding with IL-1β receptor. Cystatin C (CST3) likely facilitates thrombus embolization and ultimately tissue injury which further elevates the levels of CST3. (A color version of this figure is available in the online journal.)
The unchanged S100A8/A9 at the moderate stage of SARS-CoV-2 infection indicates that the S100A8/A9 may play minimal roles during the early stage. S100A8/A9 expresses in neutrophils and monocytes and is rapidly released postinfection. 42 The induction of S100A8/A9 helps recruit leukocytes which further induces the release of cytokines and may contribute to the cytokine storms observed in the severe COVID-19. Consistent with our results, an elevated level of S100A8/A9 was recently reported in severe COVID-19 patients who required ventilators. 43 S100A8/A9, a TLR4 ligand that abundantly expresses on the platelets, has been linked to platelet activation, VTE, and organ damage in systemic inflammatory diseases such as systemic lupus erythematosus.44,45 S100A8/A9 also binds with TLR4 present on neutrophils and prime with leucine-rich-containing family, pyrin domain containing 3 (NLRP3) inflammasomes that induce IL-1β secretion and tissue injury. 46 Consistently, we observed that S100A8/A9 levels were correlated with the tissue injury marker myoglobin in COVID-19 cases. NLRP3 emerges as a key regulator of VTE, particularly under hypoxic conditions. 47 NLRP3 inflammasome-induced IL-1β binds with IL-1β receptor on platelets which leads to platelet aggregation and VTE under hypoxia. The critically ill COVID-19 patients suffer from severe hypoxia; therefore, S100A8/A9-induced NLRP3 inflammasomes potentially play a major role in VTE observed in severe COVID-19. Moreover, S100A8/A9 is predicted to interact with other inflammatory markers such as LY96, IRAK1, and MYD88 which are the key components of TLR4 receptor signaling. 48 Taken together, the S100A8/A9–TLR4 axis likely plays an important role in thrombo-inflammation and facilitates organ damage that ultimately leads to worse clinical outcomes in COVID-19 (Figure 5).
Similar to S100A8/A9, CST3 has also been associated with an increased risk of VTE 18 and is reported to be an indicator of cardiorenal injury.49–51 Induction of CST3 levels in severe COVID-19 may play a role in advancing the COVID-19 severity through inducing inflammation, VTE followed by organ injury. Consistent with our findings, elevated levels of CST3 have been associated with poor prognosis and increased mortality rate in COVID-19. 52 A study has shown that myoglobin level could be contributed by cardiac as well as kidney injury. 53 Consistently, the elevated levels of myoglobin, however, unchanged cTnI levels in our study indicate that increased myoglobin level in severe COVID-19 may be contributed due to kidney injury and injury to other soft organs. An unchanged cTnI level, particularly in severe COVID-19 patients, is surprising, but the cardiac injury cannot be ruled out based on this finding. The timing of sampling is a possible factor to be considered for this unusual outcome as the level of cTnI rapidly decreases and returns to a normal level within a few days after myocardial injury. Including cTnT marker would have provided a better picture of cardiac injury in these patients. Moreover, if the cardiac injury was not induced in these patients, then severe inflammatory reaction alone may not be entirely responsible, but other factors also potentially play roles in the development of cardiac injury in severe COVID-19.
In conclusion, we report that the LCN2 induction occurs at the early moderate stage of SARS-CoV-2 infection when patients do not need critical care. In contrast, the levels of S100A8/A9 and CST3 were unchanged at the early stage; however, a profound induction was observed in the critically ill COVID-19 patients. The positive correlations of S100A8/A9 and CST3 with myoglobin indicate their potential role in tissue injury. Therefore, LCN2 likely plays a role in the induction of systemic inflammation, and S100A8/A9 and CST3 facilitate more severe complications including tissue injury potentially through inducing the VTE.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221091990 for Lipocalin-2, S100A8/A9, and cystatin C: Potential predictive biomarkers of cardiovascular complications in COVID-19 by Anamika Gupta, Abaher O Al-Tamimi, Rabih Halwani, Hend Alsaidi, Meganathan Kannan and Firdos Ahmad in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: AG and AOA performed experiments and collected data, RH helped with data interpretation and manuscript writing, AJ helped with sample and clinical data collection, MK performed data interpretation and helped with manuscript writing, and FA designed the study, acquired funding, supervised the project, performed data analysis and interpretation, and wrote and revised the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol was approved by the ethical review boards of the University of Sharjah and Dubai Health Authority.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by COVID-19 (CoV-0302), Targeted (1801090144P), and Competitive (1901090162P) research grants from the University of Sharjah to Firdos Ahmad. This work was also supported by the Cardiovascular Research Group at the University of Sharjah.
ORCID iD: Firdos Ahmad  https://orcid.org/0000-0001-6530-6068
https://orcid.org/0000-0001-6530-6068
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad F, Kannan M, Ansari AW. Role of SARS-CoV-2-induced cytokines and growth factors in coagulopathy and thromboembolism. Cytokine Growth Factor Rev 2022;63:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318:H1084–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet 2020;395:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu F, Li W, Lin Q, Xu M, Du J, Li H. Myoglobin and troponin as prognostic factors in patients with COVID-19 pneumonia. Med Clin (Barc) 2021;157:164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Tamimi AO, Yusuf AM, Jayakumar MN, Ansari AW, Elhassan M, AbdulKarim F, Kannan M, Halwani R, Ahmad F. Induction of soluble P-selectin and CD40 ligand and, FXIII deficiency promote aberrant coagulation and thromboembolism in severe COVID-19. Circ Res 2021;129:AP357 [Google Scholar]
- 12. Kannan M, Ahmad F, Saxena R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev 2019;37:100583. [DOI] [PubMed] [Google Scholar]
- 13. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pruszczyk P, Bochowicz A, Kostrubiec M, Torbicki A, Szulc M, Gurba H, Kuczynska K, Berent H. Myoglobin stratifies short-term risk in acute major pulmonary embolism. Clin Chim Acta 2003;338:53–6 [DOI] [PubMed] [Google Scholar]
- 16. Martos L, Oto J, Fernandez-Pardo A, Plana E, Solmoirago MJ, Cana F, Hervas D, Bonanad S, Ferrando F, Espana F, Navarro S, Medina P. Increase of neutrophil activation markers in venous thrombosis-contribution of circulating activated protein C. Int J Mol Sci 2020; 21:5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res 2021;128:2017–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brodin EE, Braekkan SK, Vik A, Brox J, Hansen JB. Cystatin C is associated with risk of venous thromboembolism in subjects with normal kidney function —the Tromso study. Haematologica 2012;97:1008–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tutar N, Kemik NA, Yilmaz I, Buyukoglan H, Kanbay A, Dogan A, Oyak FS, Gülmez I, Demir R. Is serum cystatin C a predictor of acute pulmonary thromboembolism in patients with normal renal function? Clin Appl Thromb Hemost 2015;21:533–8 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y. Small lipid-binding proteins in regulating endothelial and vascular functions: focusing on adipocyte fatty acid binding protein and lipocalin-2. Br J Pharmacol 2012;165:603–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu H, Liu Z, Lu J, Yang X, Yan XX, Mi Y, Hua L, Li Y, Jing ZC, Du J. Lipocalin-2 predicts long-term outcome of normotensive patients with acute pulmonary embolism. Cardiovasc Toxicol 2020;20:101–10 [DOI] [PubMed] [Google Scholar]
- 22. Cai Z, Xie Q, Hu T, Yao Q, Zhao J, Wu Q, Tang Q. S100A8/A9 in myocardial infarction: a promising biomarker and therapeutic target. Front Cell Dev Biol 2020;8:603902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ricciuto A, Griffiths AM. Clinical value of fecal calprotectin. Crit Rev Clin Lab Sci 2019;56:307–20 [DOI] [PubMed] [Google Scholar]
- 24. Azramezani Kopi T, Shahrokh S, Mirzaei S, Asadzadeh Aghdaei H, Amini Kadijani A. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: a review study. Gastroenterol Hepatol Bed Bench 2019;12:183–9 [PMC free article] [PubMed] [Google Scholar]
- 25. Li D, Li H, Bauer C, Hu Y, Lewis JR, Xu A, Levinger I, Wang Y. Lipocalin-2 variants and their relationship with cardio-renal risk factors. Front Endocrinol (Lausanne) 2021;12:781763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serwin N, Cecerska-Heryc E, Pius-Sadowska E, Serwin K, Niedzwiedz A, Wisniewska M, Roszak M, Grygorcewicz B, Skwirczynska E, Machalinski B, Dolegowska B. Renal and inflammation markers-renalase, cystatin c, and NGAL levels in asymptomatic and symptomatic SARS-CoV-2 infection in a one-month follow-up study. Diagnostics (Basel) 2022;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem 2009;55:1932–43 [DOI] [PubMed] [Google Scholar]
- 28. Mellett L, Khader SA. S100A8/A9 in COVID-19 pathogenesis: impact on clinical outcomes. Cytokine Growth Factor Rev 2022;63:90–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu K, Shang N, Levitman A, Corker A, Kudose S, Yaeh A, Neupane U, Stevens J, Sampogna R, Mills AM, D’Agati V, Mohan S, Kiryluk K, Barasch J. Elevated neutrophil gelatinase-associated lipocalin is associated with the severity of kidney injury and poor prognosis of patients with COVID-19. Kidney Int Rep 2021;6:2979–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin L, Chen X, Chen J, Pan X, Xia P, Lin H, Du H. The predictive value of serum level of cystatin C for COVID-19 severity. Sci Rep 2021;11:21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, Channon KM, Mills NL, Sheikh A, Hippisley-Cox J. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chapman AR, Bularga A, Mills NL. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation 2020;141:1733–5 [DOI] [PubMed] [Google Scholar]
- 33. Jaiswal V, Sarfraz Z, Sarfraz A, Mukherjee D, Batra N, Hitawala G, Yaqoob S, Patel A, Agarwala P, Ruchika, Sarfraz M, Bano S, Azeem N, Naz S, Jaiswal A, Sharma P, Chaudhary G. COVID-19 infection and myocarditis: a state-of-the-art systematic review. J Prim Care Community Health 2021;12:21501327211056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin T, Wu L, Hua Q, Song Z, Pan Y, Liu T. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: a network pharmacology study and experimental validation. J Ethnopharmacol 2020;246:112128. [DOI] [PubMed] [Google Scholar]
- 35. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Tamimi AO, Yusuf AM, Jayakumar MN, Ansari AW, Elhassan M, AbdulKarim F, Kannan M, Halwani R, Ahmad F. SARS-CoV-2 infection induces soluble platelet activation markers and PAI-1 in the early moderate stage of COVID-19. Int J Lab Hematol. Epub ahead of print 9 March 2022 DOI: 10.1111/ijlh.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 2001;276:37258–65 [DOI] [PubMed] [Google Scholar]
- 39. Ueland T, Holter JC, Holten AR, Muller KE, Lind A, Bekken GK, Dudman S, Aukrust P, Dyrhol-Riise AM, Heggelund L. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect 2020;81:e41–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu G, Li H, Fang Q, Jiang S, Zhang L, Zhang J, Hou X, Lu J, Bao Y, Xu A, Jia W. Elevated circulating lipocalin-2 levels independently predict incident cardiovascular events in men in a population-based cohort. Arterioscler Thromb Vasc Biol 2014;34:2457–64 [DOI] [PubMed] [Google Scholar]
- 41. Rajnics P, Kellner A, Karadi E, Moizs M, Bodor C, Kiraly PA, Marosvari D, Andrikovics H, Egyed M. Increased Lipocalin 2 level may have important role in thrombotic events in patients with polycythemia vera and essential thrombocythemia. Leuk Res 2016;48:101–6 [DOI] [PubMed] [Google Scholar]
- 42. Wang S, Song R, Wang Z, Jing Z, Ma J. S100A8/A9 in inflammation. Front Immunol 2018;9:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi H, Zuo Y, Yalavarthi S, Gockman K, Zuo M, Madison JA, Blair C, Woodward W, Lezak SP, Lugogo NL, Woods RJ, Lood C, Knight JS, Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J Leukoc Biol 2021;109:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tyden H, Lood C, Gullstrand B, Jonsen A, Nived O, Sturfelt G, Truedsson L, Ivars F, Leanderson T, Bengtsson AA. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2048–55 [DOI] [PubMed] [Google Scholar]
- 45. Ding N, Chen G, Hoffman R, Loughran PA, Sodhi CP, Hackam DJ, Billiar TR, Neal MD. Toll-like receptor 4 regulates platelet function and contributes to coagulation abnormality and organ injury in hemorrhagic shock and resuscitation. Circ Cardiovasc Genet 2014;7:615–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan X, Zheng X, Huang Z, Lin J, Xie C, Lin Y. Involvement of S100A8/A9-TLR4-NLRP3 inflammasome pathway in contrast-induced acute kidney injury. Cell Physiol Biochem 2017;43:209–22 [DOI] [PubMed] [Google Scholar]
- 47. Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A 2017;114:4763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie H, Sheng L, Zhou H, Yan J. The role of TLR4 in pathophysiology of antiphospholipid syndrome-associated thrombosis and pregnancy morbidity. Br J Haematol 2014;164:165–76 [DOI] [PubMed] [Google Scholar]
- 49. Zivlas C, Triposkiadis F, Psarras S, Giamouzis G, Skoularigis I, Chryssanthopoulos S, Kapelouzou A, Ramcharitar S, Barnes E, Papasteriadis E, Cokkinos D. Left atrial volume index in patients with heart failure and severely impaired left ventricular systolic function: the role of established echocardiographic parameters, circulating cystatin C and galectin-3. Ther Adv Cardiovasc Dis 2017;11:283–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 2007;115:173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem 2005;38:1–8 [DOI] [PubMed] [Google Scholar]
- 52. Zinellu A, Mangoni AA. Cystatin C, COVID-19 severity and mortality: a systematic review and meta-analysis. J Nephrol 2022;35:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992;90:267–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221091990 for Lipocalin-2, S100A8/A9, and cystatin C: Potential predictive biomarkers of cardiovascular complications in COVID-19 by Anamika Gupta, Abaher O Al-Tamimi, Rabih Halwani, Hend Alsaidi, Meganathan Kannan and Firdos Ahmad in Experimental Biology and Medicine