Abstract
Liver fibrosis is the common pathological change of chronic liver diseases characterized by increased deposition of extracellular matrix and reduced matrix degradation. In response to liver injury caused by a variety of pathogenic agents, such as virus and alcohol, hepatic stellate cells (HSCs) are differentiated into myofibroblast-like cells and produce excessive collagens, thus resulting in fibrogenesis. Natural killer (NK) cells are the essential innate immune cells in the liver and generally control fibrosis by killing activated HSCs. This review briefly describes the fibrogenesis process and the phenotypic features of hepatic NK cells. Besides, it focuses on the antifibrotic mechanisms of NK cells and explores the potential of activating NK cells as a therapeutic strategy for the disease.
Keywords: Natural killer cells, fibrosis, hepatic stellate cells, apoptosis
Impact Statement
Liver fibrosis can progress to irreversible cirrhosis and cancer, so it remains as an unresolved health burden. Currently, many treatments have improved fibrosis at the early stage of this disease, but fail to halt fibrosis progression in the advanced stage. A new angle for therapy is required and activating natural killer is a target worth paying attention to as it is an essential component of innate immunity in the liver. This work details crucial insights on the antifibrotic mechanisms of NK cells for the development of novel therapeutic targets for liver fibrosis.
Introduction
Liver fibrosis is a common pathological feature of various hepatic diseases, such as viral hepatitis, alcoholic liver disease and nonalcoholic steatohepatitis, ultimately leading to irreversible cirrhosis and to liver cancer. 1 The pathogenic mechanisms of fibrosis are still not fully understood and consequently an effective treatment is yet to be developed. Generally, the fibrosis is initiated by intrahepatic hepatocyte death, which induces hepatic stellate cells (HSCs) proliferation, differentiation, collagen overproduction, and thus fibrogenesis. 2 As an important component of innate immunity, natural killer (NK) cells are enriched in the liver. 3 NK cells play critical roles in innate immune defense against bacterial, viral, and parasitic pathogens, as well as tumor suppression through the natural cytotoxicity and cytokine secretion. 4 Accumulating evidence indicates that NK cells are able to limit liver fibrosis via killing HSCs through inducing apoptosis and generating antifibrotic cytokines. 5 Several recent studies have shown that exosomes derived from NK cells also exert an inhibitory effect on HSCs proliferation and activation, thereby alleviating fibrosis.6,7 However, with the progress of the hepatic injury to the chronic or advanced stage, the function of NK cells is exhausted and they fail to halt fibrosis. Therefore, activating NK cells at the early stage of diseases or restoring them at the advanced stage of fibrosis might provide promising therapeutic strategies for this disease. This review briefly summarizes the fibrogenesis, the phenotypic characteristics of NK cells in the liver, as well as emphasizes the antifibrotic mechanisms of NK cells and discusses the potential of NK cells as therapeutic targets for liver fibrosis.
The formation of fibrosis
In the process of fibrogenesis, hepatocyte death is regarded as an initial event. The main causes of hepatic damage are lipid deposition and abnormality of cholesterol metabolism in hepatocytes. Lipid peroxidation induce oxidative stress and mitochondrial dysfunction, thus leading to hepatocyte necrosis and apoptosis. 8 Lipid overload in hepatocytes also drives the inflammatory response that further induces the inflammasome activation and hepatocyte pyroptosis. 9 In addition, free cholesterol mediates the apoptotic and necrotic hepatocyte death through the JNK1 signaling. 10 Dead hepatocytes releases a variety of intracellular contents, such as nucleic acids, intracellular proteins, and mitochondrial compounds, which trigger inflammatory responses and immune reactions, exacerbating hepatocyte damage. 11
Hepatocyte death provides sufficient signals for the hepatic fibrogenesis. The extracellular matrix is mainly produced by myofibroblasts, which are derived from the activated HSCs, portal fibroblasts, and bone marrow-derived progenitor cells. 12 HSCs are activated to induce fibrogenesis in response to toxic liver injury, whereas both activated HSCs and portal fibroblasts are responsible for cholestatic liver fibrosis. 13 Thus, this review will focus on the role of HSCs in liver fibrosis and the effects of NK cells on HSCs. Cellular debris and contents released from dead hepatocytes provoke the activation and transformation of HSCs. For instance, the phagocytosis of apoptotic bodies by HSCs triggers a profibrogenic response. 14 DNA fragments interacting with Toll-like receptor (TLR) 9, a major components of innate immunity, promotes HSCs activation. 15 Inflammasomes recruits and activates inflammatory cells which secrete cytokines and growth factors to establish the inflammatory microenvironment that induces HSCs activation and fibrogenesis. 16 For example, platelet-derived growth factor, interleukin (IL)-1, angiotensin II, tumor necrosis factor (TNF)-α and leptin are proved to be mediators of HSCs proliferation, while transforming growth factor β, and IL-17 evoke the differentiation of HSCs into myofibroblast-like cells, which express α-smooth muscle actin and generate numerous collagens, thus leading to liver fibrosis.17,18
With the overproduction and deposition of extracellular matrix proteins in the liver, fibrosis may progress to cirrhosis and even to cancer, due to normal liver tissue is replaced by hepatocytic nodules that cause hepatic dysfunction and failure. 19 Therefore, suppression of HSCs activation and reduction of collagen deposition in the liver might provide a promising strategy for the treatment of liver fibrosis.
NK cells in the liver
As a basic component of the innate immune system, NK cells elicit the innate immune reaction in response to various pathogens, such as tumors, viruses, and bacteria. They are abundant in the liver, comprising roughly 40% and 20% of total intrahepatic lymphocytes in human and mouse, respectively. 3 The liver-specific NK cells are originated from circulating NK cell precursors and form their peculiar morphology and function. Compared with peripheral or splenic NK cells, liver NK cells expresses higher levels of CD69, an activation marker, and cytotoxic mediators, such as perforin, granzyme B and TNF-related apoptosis-inducing ligand (TRAIL, a classical apoptotic inducing molecule), suggesting an increased activated potential and cytotoxicity.20,21 According to the expression level of CD56, human NK cells are classified to the more cytokine responsive CD56bright and the more cytotoxic CD56dim subsets. The CD56bright NK cells are predominant in the peripheral blood and spleen, but their frequency is equal to the CD56dim NK cells in the liver,4,22 indicating that NK cells harbor the equivalent sensitivity and cytotoxicity against abnormal cells in liver diseases. However, the meaning of this distinction needs to be investigated.
NK cells recognize their target cells through the receptor-ligand interaction. Receptors expressed on NK cells are categorized into the stimulatory and inhibitory receptors, by which the opposing signals determine the activation status of NK cells and their killing effect on target cells. 23 The stimulatory receptors, such as NK group 2D (NKG2D), NKp36, NKp44, NKp30, NKp46, NKp80, and CD94/NKG2 C, can induce NK cell activation; while the inhibitory receptors, including the killer cell immunoglobulin-like receptor (KIR), and Ly49, are involved in the suppression of NK cell activation. 24 Thus, activating NK cells by regulating the expression level of these receptors might increase their cytotoxicity and provide a potential therapeutic strategy for chronic liver diseases. A major question that remains is, what switches the expression of the stimulatory and inhibitory receptors on NK cells in various liver diseases and whether these two kinds of receptors have reciprocal effects.
Mechanisms of NK cell-mediated cytotoxicity to HSCs
Receptor-ligand interactions
The function of NK cells is regulated by various receptors, and their cytotoxicity against target cells mainly depends on the interactions between activating receptors or inhibitory receptors of NK cells and corresponding ligands on target cells. Under physiological condition, HSCs express major histocompatibility complex (MHC) I, which binds to inhibitory receptors of NK cells and suppresses their activation. 25 Once stimulated by hepatocyte damage signals, HSCs generate amounts of retinoic acid and increases the expression of retinoic acid early transcript (RAE)-1, a ligand for the activating NK cell receptors, thus leading to the NK cell killing of activated HSCs. However, NK cells fail to kill quiescent HSCs because of their low express level of RAE-1. 26
Increasing activating NK cell receptors have been discovered, among which NKG2D and NKp46 are considered to play a vital role in the killing effect of NK cells. NKG2D binding to its ligands such as MICA (MHC class I polypeptide-related sequence A), ULBP (UL16 binding protein), both of which are expressed on activated HSCs, effectively triggers NK cell-mediated cytotoxicity. 27 With the HBV-infected liver fibrosis progression, the expression level of NKG2D is increased, which regulates the anti-viral function of NK cells by killing the HBV-infected hepatocytes. 28 Similarly, NK cells from patients with non-alcoholic steatohepatitis (NASH) have higher levels of NKG2D than NK cells from healthy individuals, along with elevated gene and protein expression of its ligands MICA in HSCs,29,30 suggesting an increased cytotoxic activity of NK cells against HSCs. However, in patients with alcoholic liver fibrosis, the number and cytolytic activity of NK cells expressing NKG2D are significantly decreased compared to those of patients without liver disease. 31 In addition to stimulating NK cells to kill activated HSCs, NKp46 also participates in hepatic tissue repair and remodeling. The NKp46-mediated killing to HSCs is attributed to the interaction with its ligand NCR1. 32 In HBV-related advanced hepatic fibrosis, intrahepatic NK cells have a reduced expression of NKp46 and cytotoxic function, implying the inhibition of immune functions of NK cells. 33 The circulating NK cells from chronic HBV-infected patients present decreased NKp46 expression and cytolytic activity, which indicates that NKp46 could suppress HBV replication and alleviate liver damage and fibrosis. 34 Likewise, both NKp46 and NKp30 are diminished in chronic HCV-infected patients, and NK cells are incapable of controlling virus load. 35 One explanation might be that NK cells with low expression of NKp46 have a reduced interferon (IFN)-γ secretion, resulting in HCV replication and liver fibrosis. 36 Besides, hepatic NK cells expressing NKp46 produce numerous TNF compared to circulating NK cells, which might contribute to virus clearance and thus alleviating fibrosis process. 37 These results imply that activating NK cell receptors block hepatitis virus replication and mediate the killing of NK cells to activated HSCs, ameliorating hepatic fibrosis. However, the expression level of activating NK cell receptors decrease in advanced stage of liver diseases remains further solved. Furthermore, increasing studies have demonstrated that several NK cell receptors also mediate the killing of NK cells to activated HSCs, such as KLRG1 38 and TLR9. 39 Therefore, further clarifying activating NK cell receptors and their functions are expected to provide potential therapies for liver fibrosis.
Meanwhile, reducing the expression of inhibitory NK cell receptors also enhances the cytotoxic attack of NK cells against HSCs. Once activated, HSCs downregulate the expression of MHC I, which reduces the function of inhibitory NK cell receptors and increased cytotoxicity. 40 For instance, the NKG2A and KIR are the common inhibitory NK cell receptors for inhibiting NK cell activation. In HCV-infected patients, the increased expression of NKG2A leads to a reduced cytolytic activity against virus-stimulated hepatocytes and an enhanced virus load. 35 Experimentally, reducing the expression of KIR in NK cells by siRNA-mediated knockdown increases HSCs death and attenuates hepatic fibrosis. 40 Besides, silencing the inhibitory NK cell receptor Ly49 in mice stimulates NK cells and facilitates their antifibrogenic activity. 41 These findings indicate that decreasing the expression of inhibitory NK cell receptors suppresses hepatitis virus replication and augments the killing effect of NK cells, thereby alleviating liver fibrosis. However, whether the expression of inhibitory NK cell receptors alters with the progression of hepatic diseases remains to be determined. Investigating the functions and the regulatory mechanisms of these receptors will provide new insight in the etiology of fibrogenesis.
Granule exocytosis
Granule exocytosis is defined as a process involving the generation of perforin and granzyme (A, B) containing granules, which are secreted from NK cells after interaction with target cells. 42 For elimination of target cells, perforin perforates the cell membrane and subsequently granzyme release into target cells where both caspase-dependent and independent pathways are activated to induce cell apoptosis. 43 In liver fibrosis, activated NK cells tend to present potent degranulation activity, and produces a large amount of perforin and granzyme, which induce HSCs apoptosis and thus limit fibrosis. 44 At the initial stages of liver fibrosis, the invariant NK T cells accumulate in the liver and eliminate activated HSCs by granule exocytosis pathway. 45 Moreover, this pathway is necessary for the specific NK cell killing of senescent fibroblasts and the clearance of senescent activated HSCs. 46 This result suggests that NK-cell-mediated cytotoxicity to senescent HSCs exerts immune surveillance to ameliorate liver fibrosis. The chemokine CXCL10 exhibits potent chemotactic capacity for cells expressing its cognate receptor CXCR3. In CXCL10-deficient mice, the number of NK cells and granzyme B expression are increased to control HSC activity and fibrosis. 47 Intrahepatic NK cells, recruited through CXCL10-CXCR3 interaction, exhibit high levels of activation and play a protective role against the fibrosis progression in NASH. 48
Whether CXCL10 affecting the killing function of NK cells remains to be further explored. In addition, the degranulation activity of NK cells is influenced by hepatic fibrosis progression. In HCV-infected patients, the number of intrahepatic NK cells increases at early stage of inflammation whereas their degranulation activity is impaired in the advanced inflammation and fibrosis stage. 49 Therefore, uncovering the factor that responsible for suppression of degranulation activity in advanced hepatic diseases, will be conducive to develop promising strategies to limit liver fibrosis progression.
Death receptor-mediated apoptosis
The ligands on the surface of NK cells, including TRAIL and Fas ligand (FasL, a member of the TNF family of death-inducing ligands), bind to corresponding receptors on target cells, contributing to caspase activation and cell apoptosis. The quiescent HSCs can escape from the killing by NK cells since a lacking of ligands for NK cell activation. 1 Activation of HSCs results in increased expression of the TRAIL receptors, leading to enhanced cytotoxicity of NK cells against HSCs. In the preclinical setting, recombinant expression of human TRAIL on HSCs induces HSCs apoptosis, while blocking TRAIL by antibodies antagonizes this effect. 50 This finding suggests that NK cells effectively eliminate activated HSCs in a death receptor-dependent manner, thus mitigating fibrosis. This process is regulated by various signal pathways. For example, NK cells are activated to kill HSCs in response to IL-18 and TLR3 ligand stimulation via the p38/PI3 K/AKT signaling, a key pathway to preserve energy balance and coordinate metabolism in eukaryotic cells. 51 Activation of STAT1, an essential component of IFN-signaling, attenuates liver fibrosis through increasing TRAIL expression on NK cells and the killing attack against activated HSCs. 52 In addition, bone marrow-derived M1 macrophages increase the number of NK cells, which release numerous TRAIL and induce HSCs apoptosis, suggesting that recruitment of activated NK cells by modulating the hepatic microenvironment could elicit HSCs death. 53 A series of clinical investigations also confirm the death receptor-mediated NK cell killing of HSCs. In HCV-associated fibrogenesis, NK cells from HCV-infected patients induce apoptosis of activated HSCs in the TRAIL and FasL-dependent manner. 54 With the disease progression to chronic HCV infection, the TRAIL expression of intrahepatic NK cells decreases and their cytotoxicity is compromised, which results in viral persistence and fibrosis aggravation. 55 In addition, NK cells promote TRAIL-mediated death of HSCs at early stages of NASH, but excess lipid accumulation in hepatocytes contributes to the loss of cytotoxic activity in NK cells, which causes the progression of fibrosis at later stages of the disease. 56 Chronic alcohol consumption reduces the expression of TRAIL on NK cells and predisposes them to decreasing in function, which aggravates the development of fibrosis. 57 However, in HBV-related liver failure, highly activated NK cells kill hepatocytes through Fas/FasL pathway. 58 Besides, increased circulating inflammatory cytokines, such as IL-6 and IL-8, expedite the TRAIL-mediated NK cell cytotoxicity against hepatocytes. 59 Therefore, hyperactivated NK cells induce hepatocytes damage in the advanced stage of hepatic diseases, thereby exacerbating liver fibrosis. Experimentally, administration of PEGylated TRAIL induces apoptosis of activated HSCs and further blocks liver fibrotic progression. 60 Activation of death receptor-mediated HSCs death by NK cells might provide a promising therapy for liver fibrosis.
Cytokine-induced death
As an important antifibrotic cytokine, IFN-γ is released from NK cells to accelerate activated HSCs death.61,62 IFN-γ binds to its receptor expressed on the surface of HSCs and subsequently induces phosphorylation of JAK, leading to the activation of STAT1 signals, ultimately to cell cycle arrest and proliferation inhibition of HSCs. 52 In both HBV-infected patients and the liver fibrosis mouse model, IFN-γ inhibits the proliferation of liver progenitor cells that are associated with inflammation and fibrosis in chronic liver diseases, thus attenuating fibrosis. 63 Besides, NK cells regulate hepatic macrophages polarization via IFN-γ in a NASH mouse model, suppressing the expression of profibrogenic genes, including α-smooth muscle actin, type I collagen, and tissue inhibitor of metalloproteinase. 64 NK cells are activated to limit tissue damage and fibrosis during NASH, whereas NK cell deficiency exhibits more severe NASH disease phenotype. 64 Loss of the cytotoxic activity of NK cells during NASH could potentially cause the higher susceptibility of NASH patients to liver cancer at later stages of the disease. 65 However, in a steatohepatitis mouse model, the expression levels of these fibrosis-related genes are suppressed by IFN-γ knockdown, since the NK cell-secreted IFN-γ stimulates macrophages to produce TNF-α that further triggers liver inflammation and fibrosis. 66 These results suggest that NK cell-derived IFN-γ exerts dual effects through modulating macrophage-mediated inflammation under different pathological conditions, is associated with the development of fibrosis. The different effects of IFN-γ on fibrosis in different disease models might be associated with different cell types and coexistent cytokines in the microenvironment,67,68 but this needs confirmation. Moreover, other cytokines, such as IFN-α, has been found to increase expression of TRAIL on the surface of NK cells, 69 suggesting NK cell-secreted cytokines activate the death-receptor pathway to induce HSCs apoptosis. The release of IFN-γ from NK cells is regulated by various mediators. A recent study has revealed that activation of mGluR5 in NK cells increased the production of IFN-γ via the MEK/ERK pathway, contributing to the increased cytotoxicity against activated HSCs. 70 In HCV-related hepatic fibrosis, hepatocytes produce profibrotic factors such as macrophage colony-stimulating factor and IL-34, which suppress the IFN-γ generation of NK cells and thus impair the IFN-γ-induced killing of HSCs. 71 The retinol and its metabolites are implicated in the killing of HSCs by NK cells; inhibition of ADH3, one of retinol metabolizing enzyme, enhances IFN-γ production and cytotoxicity of NK cells against HSCs, thus hampering alcohol-induced fibrogenesis.72,73 However, direct IFN-γ treatment fail to activate NK cells or suppress activated HSCs in chronically alcohol-fed mice, showing no beneficial effects of IFN-γ in alcoholic liver fibrosis. 74 One explanation might be that alcohol-induced IL-10 and TGF-β release by monocytes and activated HSCs restrains the activation status of NK cells. 75 Consequently, theses regulators that are responsible for IFN-γ generation in NK cells might contribute to developing potential therapeutic targets for liver fibrosis. Important to note is that peripheral invariant NKT cells, expressing markers of both T and NK cells, produce large amounts of cytokines, including IL-4 and IL-13, which cause excessive activation HSCs and accelerate the progression of fibrosis to cirrhosis in HBV-related diseases.76,77 Therefore, future studies to clarify the role of NK cell-derived cytokines in different stages of hepatic diseases are needed.
The effects of NK cells on hepatocytes
Generally, healthy hepatocytes express MHC-I that interacts with inhibitory receptors on NK cells and prevents the activation of NK cells. 78 During acute viral infection, both HBV and HCV invade hepatocytes, which upregulates the expression of NKG2D ligands and further induces NK cell activation.79,80 Clinical observations found that NK cells from acute hepatitis virus-infected patients displayed the increased cytotoxicity and IFN-γ production, and were associated with viral clearance in these patients.80,81 Preclinical settings further unveiled that NK cells activation with elevated degranulation and IFN-γ is capable of controlling viral replication by recognizing and lysing virus-infected hepatocytes during acute viral hepatitis.82,83 However, in chronic viral hepatitis, frequencies and cytotoxic functions of NK cells are diminished, which contributes to fibrosis and malignant transformation. 84 Thus, improving NK cell cytotoxicity against virus-infected hepatocytes in acute viral hepatitis has been regarded as a potential therapeutic strategy to postpone diseases progression. 85
NK cells play a key role in mediating the cytolysis of infected hepatocytes in the FasL and TRAIL dependent manners.58,59 Previous study showed that TRAIL (+) NK cells were enriched in the liver of patients with chronic HBV infection, and were accompanied by hepatocytes that expressed increased levels of DR5, a TRAIL receptor. 86 Similarly, deletion of hepatic protective factors, such as NEMO and GNMT, caused the upregulation of DR5, which activated TRAIL (+) NK cells to induce hepatocytes death.87,88 These results imply that TRAIL (+) NK cells are the main inducer of hepatocytes death through the TRAIL/DR5 interaction. However, there is evidence that TRAIL (+) NK cells from patients with virus-related liver cirrhosis induce normal hepatocyte apoptosis in vitro. 89 The reason for this is that TRAIL (+) NK cells lack the Ly-49 inhibitory receptor that recognizes MHC I, which makes them more susceptible to hepatocytes. 90 By contrast, the remaining TRAIL (-) NK cells that account for 60%-70% of hepatic NK cells upregulate the expression of Ly-49 receptor in response to toxic stimulus and prevent normal hepatocyte killing. 90
In conclusion, the killing of NK cells on hepatocytes provides some insight into the NK cell-activating therapeutic strategies in liver fibrosis. In the acute stages of viral hepatitis, activated NK cells participate in elimination of virus-infected hepatocytes, thus reducing viral replication and HSCs activation. However, TRAIL (+) NK cells kill both injured and normal hepatocytes due to devoid of inhibitory receptors that recognize MHC I, whereas TRAIL (-) NK cells express inhibitory receptors and protect normal hepatocytes from killing. Thus, upregulating the expression of inhibitory receptors in TRAIL (+) NK cells, as well as increasing the expression of activating receptors in TRAIL (-) NK cells might exert the antifibrotic function of NK cells with safety and efficacy.
Activating NK cells for the anti-fibrotic therapy
With liver disease progression to fibrosis, the activity of NK cells increases at the early stage but reduces at the advanced stage. A previous study showed that intrahepatic NK cells, isolated from HCV-infected patients, produced amounts of IFN-γ at early stage of inflammation whereas the degranulation activity of these cells was compromised in advanced inflammation and fibrosis stage. 49 This defective function of NK cells also occurs in chronic HCV infection, where NK cells accumulate in the liver but display impaired degranulation and decreased IFN-γ secretion in response to HSCs. 91 Also, in HIV/HCV-coinfected patients, the loss of NK cell killing effects is related to advanced liver fibrosis. 92 When the HCV-related fibrosis progresses to cirrhosis, virus eradication partially restores the function of NK cells, 93 suggesting an irreversible damage to NK function at the advanced stage of liver fibrosis. Consistent with this result found in HCV-related fibrosis, the expression of activating NK cell receptors, the production of cytokines, and the cytotoxic function of NK cells are suppressed in chronic HBV 94 and Schistosoma japonicum infection. 95 The dysfunction of NK cells facilitates the development of liver fibrosis, and the diminished NK cell activity is related to the severity of liver damage. 96 Although the exact mechanism of abnormality of NK cells in the advanced stage of liver fibrosis remains an enigma, one possible explanation for that is the activated HSCs disrupt the antifibrotic capacity of NK cells through a TGF-β-dependent emperipolesis. 97 A recent study has found that increased insulin levels enhance NK cell cytotoxic activity to HSCs in the early stage of NASH-related fibrosis whereas insulin resistance impairs NK cell activity in the advanced stage of fibrosis, suggesting the aberrant function of NK cells is related to insulin levels. 98 Therefore, the maintenance of antifibrotic function of NK cells at the early stage of liver diseases halt the development of fibrosis.
Modulation of the expression of activating and inhibitory NK cell receptors is regarded as a promising strategy to activate NK cells. In carbon tetrachloride (CCl4)-induced liver fibrosis mouse model, cultured mycelium cordyceps sinensis increased the numbers of hepatic NK cells and the expression levels of NKG2D, which alleviates the fibrosis development. 99 Analogous to this effect, Salvia Miltiorrhiza, a Chinese herbal medicine, is also shown to promote the expressions of NKG2D and inhibit the HSCs activation. 100 However, the antifibrotic function of NK cells is abrogated by chronic alcohol consumption. 74 These findings indicate that increasing the expression of activating NK cell receptors could intensify the antifibrotic function. In addition, using siRNA to reduce the expression of KIR, 40 hepatic fibrosis is attenuated, suggesting that suppression of inhibitory NK cell receptors stimulates NK cells and promotes their antifibrogenic activity. Therefore, increasing the expression of activating NK cell receptors and/or reducing the expression of inhibitory NK cell receptors are expected to halt fibrosis progression, but of course this needs to be confirmed in clinical investigations.
In addition, an enhancement of NK cell-derived cytokines promotes the cytotoxicity of NK cells against HSCs. A study revealed that administration of herbal agents stimulate NK cells to produce IFN-γ, thus exerting an antifibrotic effect. 100 Another herbal agent, Yu Gan Long, is also shown to accelerate the extracellular matrix degradation by modulating the balance among IL-4, IL-17α, and IFN-γ in CCl4-induced hepatic fibrosis. 101 Furthermore, exogenous IFN-γ supplement specific delivering to HSCs increases its antifibrotic potency and safety for the treatment of liver fibrosis. 102 In HCV-infected patients, NK cells express high levels of TRAIL in response to PEG-IFN-α therapy, which is associated with control of HCV infection and improvement of liver fibrosis. 69 Therefore, increasing the level of antifibrotic cytokines alleviate liver fibrosis, but the efficacy and safety still need further verification.
Considering that NK cells eliminate virus-infected hepatocytes in the acute stages of viral hepatitis, activating NK cells is beneficial to improve virus-related fibrosis. However, it must be noted that TRAIL (+) NK cells also kill normal hepatocytes. Thus, activating NK cells by modulating the activating and inhibitory receptors should consider whether NK cells express TRAIL.
Conclusion and future direction
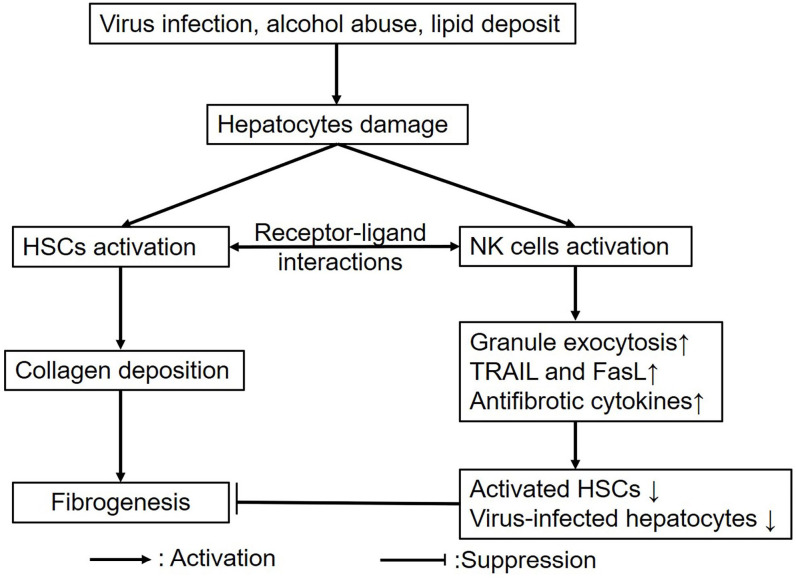
As an essential component of the innate immune system in the liver, NK cells exert the antifibrotic function in liver fibrosis. In response to toxic stimulus, hepatocytes damage activates both HSCs and NK cells. By receptor-ligand interactions, NK cells kill activated HSCs and virus-infected hepatocytes via several mechanism including granule exocytosis, TRAIL- and FasL-mediated apoptosis, and secretion of antifibrotic cytokines, thus alleviating fibrosis (Figure 1). In a series of preclinical settings, activating NK cells has been employed as a novel therapeutic strategy for liver fibrosis, including herbal-mediated NK cell activation, modulating the expression of activating and inhibitory receptors, and supplement with antifibrotic cytokines (Table 1). However, the efficacy and safety still need to be confirmed.
Figure 1.
The roles of NK cells in liver fibrosis. Both HSCs and NK cells are activated in response to various stimulus; moreover, NK cells target HSCs via receptor-ligand interactions, and further kill HSCs and virus-infected hepatocytes through granule exocytosis, TRAIL and FasL-mediated apoptosis, and secretion of antifibrotic cytokines, thus attenuating fibrosis.
Table 1.
Different therapeutic interventions for liver fibrosis.
| Agents or methods | Target effects | Reference |
|---|---|---|
| siRNA targeting KIR | Increases the degranulation, activation and antifibrogenic function of NK cells; attenuates HSCs activation, serum alanine aminotransferase levels and hepatic fibrosis | Muhanna et al. 40 |
| IFN-α | Increases the TRAIL expression of NK cells, and controls virus replication | Stegmann et al. 69 |
| Mycelium cordyceps sinensis | Increases the frequency of hepatic NK cells expressed high level of NKG2D and the apoptosis of HSCs, improves liver function, and attenuates liver inflammation and fibrosis | Peng et al. 99 |
| Salvia Miltiorrhiza | Increases the frequency of NK cells, and activities of NKG2D and Nkp46 on NK cells, inhibits activation of HSCs in vivo and in vitro, and promotes the activities of NK cells by increasing the expressions of IFN-γ | Peng et al. 100 |
| Yu Gan Long | Decreases liver fibrosis biomarkers including collagen IV, type III precollagen, hyaluronuc acid and laminin, α-smooth muscle actin, and liver fibrosis proteins such as p-Smad2, p-Smad3 and Smad4; inhibits the expression of IL-1β, IL-6, IL-4, IL-17α but promotes IFN-γ expression | Xia et al. 101 |
| IFN-γ | Increases the antifibrotic potency and improves the general safety profile in vivo by HSC-specific delivery of IFN-γ | van Dijk et al. 102 |
Although increasing studies have investigated the effects of NK cells in liver fibrosis, a number of issues remain to be resolved. (1) Liver fibrosis can also be influenced by other cells, such as portal fibroblasts, bone marrow-derived progenitor cells and immune cells. Moreover, Kupffer cells are the major innate immune cells in the liver and play a vital role in liver inflammation and fibrosis. Activated Kupffer cells create a proinflammatory microenvironment that promotes HSC activation. Whether NK cells target these cells? If so, what is the meaning for fibrogenesis? (2) There exist differences in the phenotype and function between circulating and intrahepatic NK cells. Clarifying roles of these two kinds of NK cells contributes to the development of activating NK cell therapies for liver fibrosis. (3) The activation of NK cells is modulated by activating and inhibitory receptors. Is there any other activating and inhibitory receptors expressed on NK cells? Which activating receptor is predominated in the killing of target cells? Whether these receptors affect each other? (4) IFN-γ produced by NK cells serves as the antifibrotic factor. If there are any other antifibrotic cytokines are secreted by NK cells? Whether their functions are influenced by the inflammatory environment? (5) Given TRAIL (+) NK cells can kill normal hepatocytes, activating NK cell therapies should make a distinction between TRAIL (+) and TRAIL (-) NK cells. Also, preclinical settings about the antifibrotic effects of NK should evaluate the toxicity of NK cells to hepatocytes. (6) Functional exhaustion of NK cells may aggravate fibrosis at the advanced stage of hepatic diseases. Therefore, clarifying the mechanism of dysfunction in NK cells to develop novel therapies to halt fibrosis, merits further research and investigations.
Footnotes
Authors’ Contributions: YW, WBY and YL wrote the manuscript; YXX also planned and supervised the writing of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Youth project: 81904182); the Natural Science Foundation of Hunan Province (2020JJ5441); the TCM research projects of Heilongjiang Province (ZHY18-029, ZHY 19-062, ZHY 2020-041).
ORCID iD: Yuan Xingxing  https://orcid.org/0000-0002-9894-4127
https://orcid.org/0000-0002-9894-4127
References
- 1. Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol 2009;130:16–26 [DOI] [PubMed] [Google Scholar]
- 2. Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis 2010; 30:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lian RH, Kumar V. Murine natural killer cell progenitors and their requirements for development. Semin Immunol 2002;14:453–60 [DOI] [PubMed] [Google Scholar]
- 4. Zheng M, Sun H, Tian Z. Natural killer cells in liver diseases. Front Med 2018;12:269–79 [DOI] [PubMed] [Google Scholar]
- 5. Gao B, Radaeva S, Jeong WI. Activation of natural killer cells inhibits liver fibrosis: a novel strategy to treat liver fibrosis. Expert Rev Gastroenterol Hepatol 2007;1:173–80 [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Wang Y, Quan J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Hum Cell 2020;33:582–9 [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Wang Y, Quan J. Exosomal miR-223 derived from natural killer cells inhibits hepatic stellate cell activation by suppressing autophagy. Mol Med 2020;26:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018;155:282–302 [DOI] [PubMed] [Google Scholar]
- 9. Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59:898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol 2014;61:1376–84 [DOI] [PubMed] [Google Scholar]
- 11. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 2015;61:1066–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. PANS 2014;111:E3297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishio T, Hu R, Koyama Y, Liang S, Rosenthal SB, Yamamoto G, Karin D, Baglieri J, Ma HY, Xu J, Liu X, Dhar D, Iwaisako K, Taura K, Brenner DA, Kisseleva T. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol 2019;71:573–85 [DOI] [PubMed] [Google Scholar]
- 14. Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 2006;43:435–43 [DOI] [PubMed] [Google Scholar]
- 15. Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 2007;46:1509–18 [DOI] [PubMed] [Google Scholar]
- 16. Wan M, Han J, Ding L, Hu F, Gao P. Novel immune subsets and related cytokines: emerging players in the progression of liver fibrosis. Front Med (Lausanne) 2021;8:604894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 2019;65:37–55 [DOI] [PubMed] [Google Scholar]
- 18. Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, Pena CA, Geisler LJ, Papouchado BG, Hoffman HM, Feldstein AE. NLRP3 inflammasome driven liver injury and fibrosis: roles of IL-17 and TNF in mice. Hepatology 2018;67:736–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol 2017;12:153–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermijlen D, Luo D, Froelich CJ, Medema JP, Kummer JA, Willems E, Braet F, Wisse E. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol 2002;72:668–76 [PubMed] [Google Scholar]
- 21. Mikulak J, Bruni E, Oriolo F, Di Vito C, Mavilio D. Hepatic natural killer cells: organ-specific sentinels of liver immune homeostasis and physiopathology. Front Immunol 2019;10:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001;97:3146–51 [DOI] [PubMed] [Google Scholar]
- 23. Brusilovsky M, Rosental B, Shemesh A, Appel MY, Porgador A. Human NK cell recognition of target cells in the prism of natural cytotoxicity receptors and their ligands. J Immunotoxicol 2012;9:267–74. [DOI] [PubMed] [Google Scholar]
- 24. Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 2011;89:216–24 [DOI] [PubMed] [Google Scholar]
- 25. Lanier LL. NK cell recognition. Annu Rev Immunol 2005;23:225–74 [DOI] [PubMed] [Google Scholar]
- 26. Radaeva S, Wang L, Radaev S, Jeong WI, Park O, Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol 2007;293:G809–16 [DOI] [PubMed] [Google Scholar]
- 27. Jin H, Jia Y, Yao Z, Huang J, Hao M, Yao S, Lian N, Zhang F, Zhang C, Chen X, Bian M, Shao J, Wu L, Chen A, Zheng S. Hepatic stellate cell interferes with NK cell regulation of fibrogenesis via curcumin induced senescence of hepatic stellate cell. Cell Signal 2017;33:79–85 [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Wang W, Shen C, Wang Y, Jiao M, Yu W, Yin H, Shang X, Liang Q, Zhao C. NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection. Sci Rep 2017;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stiglund N, Strand K, Cornillet M, Stal P, Thorell A, Zimmer CL, Naslund E, Karlgren S, Nilsson H, Mellgren G, Ferno J, Hagstrom H, Bjorkstrom NK. Retained NK cell phenotype and functionality in non-alcoholic fatty liver disease. Front Immunol 2019;10:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahraman A, Schlattjan M, Kocabayoglu P, Yildiz-Meziletoglu S, Schlensak M, Fingas CD, Wedemeyer I, Marquitan G, Gieseler RK, Baba HA, Gerken G, Canbay A. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology 2010;51:92–102 [DOI] [PubMed] [Google Scholar]
- 31. Laso FJ, Madruga JI, Giron JA, Lopez A, Ciudad J, San Miguel JF, Alvarez-Mon M, Orfao A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology 1997;25:1096–100 [DOI] [PubMed] [Google Scholar]
- 32. Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, Mandelboim O. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut 2012;61:885–93 [DOI] [PubMed] [Google Scholar]
- 33. Li X, Zhang M, Liu J, Huang Z, Zhao Q, Huang Y, Li X, Gao Z. Intrahepatic NK cells function suppressed in advanced liver fibrosis. Eur J Clin Invest 2016;46:864–72 [DOI] [PubMed] [Google Scholar]
- 34. Li W, Jiang Y, Wang X, Jin J, Qi Y, Chi X, Zhang H, Feng X, Niu J. Natural killer p46 controls hepatitis B virus replication and modulates liver inflammation. PLoS ONE 2015;10:e0135874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006;55:869–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer B, Korner C, Kebschull M, Glassner A, Eisenhardt M, Nischalke HD, Alexander M, Sauerbruch T, Spengler U, Nattermann J. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology 2012;56:1201–13 [DOI] [PubMed] [Google Scholar]
- 37. Nel I, Lucar O, Petitdemange C, Béziat V, Lapalus M, Bédossa P, Debré P, Asselah T, Marcellin P, Vieillard V. Accumulation of intrahepatic TNF-α-producing NKp44+ NK cells correlates with liver fibrosis and viral load in chronic HCV infection. Medicine (Baltimore) 2016;95:e3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wijaya RS, Read SA, Schibeci S, Eslam M, Azardaryany MK, El-Khobar K, van der Poorten D, Lin R, Yuen L, Lam V, George J, Douglas MW, Ahlenstiel G. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J Hepatol 2019;71:252–64 [DOI] [PubMed] [Google Scholar]
- 39. Abu-Tair L, Axelrod JH, Doron S, Ovadya Y, Krizhanovsky V, Galun E, Amer J, Safadi R. Natural killer cell-dependent anti-fibrotic pathway in liver injury via toll-like receptor-9. PLoS ONE 2013;8:e82571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muhanna N, Abu Tair L, Doron S, Amer J, Azzeh M, Mahamid M, Friedman S, Safadi R. Amelioration of hepatic fibrosis by NK cell activation. Gut 2011;60:90–8 [DOI] [PubMed] [Google Scholar]
- 41. Krueger PD, Lassen MG, Qiao H, Hahn YS. Regulation of NK cell repertoire and function in the liver. Crit Rev Immunol 2011;31:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogbomo H, Mody CH. Granule-dependent natural killer cell cytotoxicity to fungal pathogens. Front Immunol 2016;7:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ 2008;15:251–62 [DOI] [PubMed] [Google Scholar]
- 44. Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol 2006;45:60–71 [DOI] [PubMed] [Google Scholar]
- 45. Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 2009;49:1683–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 2013;32:1971–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun 2010;35:424–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fan Y, Zhang W, Wei H, Sun R, Tian Z, Chen Y. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int 2020;40:598–608 [DOI] [PubMed] [Google Scholar]
- 49. Fugier E, Marche H, Thélu MA, Macek Jilkova Z, Van Campenhout N, Dufeu-Duchesne T, Leroy V, Zarski JP, Sturm N, Marche PN, Jouvin-Marche E. Functions of liver natural killer cells are dependent on the severity of liver inflammation and fibrosis in chronic hepatitis C. PLoS ONE 2014;9:e95614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006;130:435–52 [DOI] [PubMed] [Google Scholar]
- 51. Li T, Yang Y, Song H, Li H, Cui A, Liu Y, Su L, Crispe IN, Tu Z. Activated NK cells kill hepatic stellate cells via p38/PI3K signaling in a TRAIL-involved degranulation manner. J Leukoc Biol 2019;105:695–704 [DOI] [PubMed] [Google Scholar]
- 52. Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 2006;44:1441–51 [DOI] [PubMed] [Google Scholar]
- 53. Ma PF, Gao CC, Yi J, Zhao JL, Liang SQ, Zhao Y, Ye YC, Bai J, Zheng QJ, Dou KF, Han H, Qin HY. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol 2017;67:770–9 [DOI] [PubMed] [Google Scholar]
- 54. Glassner A, Eisenhardt M, Kramer B, Korner C, Coenen M, Sauerbruch T, Spengler U, Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest 2012;92:967–77 [DOI] [PubMed] [Google Scholar]
- 55. Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, Michelone G, Alessiani M, Rosati R, Montorsi M, Mondelli MU. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology 2012;56:841–9 [DOI] [PubMed] [Google Scholar]
- 56. Martínez-Chantar ML, Delgado TC, Beraza N. Revisiting the role of natural killer cells in non-alcoholic fatty liver disease. Front Immunol 2021;12:640869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pan HN, Sun R, Jaruga B, Hong F, Kim WH, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res 2006;30:1615–23 [DOI] [PubMed] [Google Scholar]
- 58. Zou Y, Chen T, Han M, Wang H, Yan W, Song G, Wu Z, Wang X, Zhu C, Luo X, Ning Q. Increased killing of liver NK cells by Fas/Fas ligand and NKG2D/NKG2D ligand contributes to hepatocyte necrosis in virus-induced liver failure. J Immunol 2010;184:466–75 [DOI] [PubMed] [Google Scholar]
- 59. Wan Z, Xie G, Wu Y, Liu F, Xin S, You S, Liu H, Li C, Li D. Cytokines elevated in patients with HBV-related acute-on-chronic liver failure promote NK cell mediated cytotoxicity through TRAIL. Dig Liver Dis 2016;48:528–35 [DOI] [PubMed] [Google Scholar]
- 60. Oh Y, Park O, Swierczewska M, Hamilton JP, Park JS, Kim TH, Lim SM, Eom H, Jo DG, Lee CE, Kechrid R, Mastorakos P, Zhang C, Hahn SK, Jeon OC, Byun Y, Kim K, Hanes J, Lee KC, Pomper MG, Gao B, Lee S. Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology 2016;64:209–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology 1992;16:776–84 [DOI] [PubMed] [Google Scholar]
- 62. Hou X, Yu F, Man S, Huang D, Zhang Y, Liu M, Ren C, Shen J. Negative regulation of Schistosoma japonicum egg-induced liver fibrosis by natural killer cells. PLoS Negl Trop Dis 2012;6:e1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weng HL, Feng DC, Radaeva S, Kong XN, Wang L, Liu Y, Li Q, Shen H, Gao YP, Mullenbach R, Munker S, Huang T, Chen JL, Zimmer V, Lammert F, Mertens PR, Cai WM, Dooley S, Gao B. IFN-gamma inhibits liver progenitor cell proliferation in HBV-infected patients and in 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-fed mice. J Hepatol 2013;59:738–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tosello-Trampont AC, Krueger P, Narayanan S, Landes SG, Leitinger N, Hahn YS. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 2016;63:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cuff AO, Sillito F, Dertschnig S, Hall A, Luong TV, Chakraverty R, Male V. The obese liver environment mediates conversion of NK cells to a less cytotoxic ILC1-like phenotype. Front Immunol 2019;10:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Luo XY, Takahara T, Kawai K, Fujino M, Sugiyama T, Tsuneyama K, Tsukada K, Nakae S, Zhong L, Li XK. IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am J Physiol Gastrointest Liver Physiol 2013;305:G891–9 [DOI] [PubMed] [Google Scholar]
- 67. Horras CJ, Lamb CL, Mitchell KA. Regulation of hepatocyte fate by interferon-gamma. Cytokine Growth Factor Rev 2011;22:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morita M, Watanabe Y, Akaike T. Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology 1995;21:1585–93 [PubMed] [Google Scholar]
- 69. Stegmann KA, Björkström NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, Schlaphoff V, Fytili P, Cornberg M, Manns MP, Geffers R, Pietschmann T, Guzmán CA, Ljunggren HG, Wedemeyer H. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology 2010;138:1885–97 [DOI] [PubMed] [Google Scholar]
- 70. Choi WM, Ryu T, Lee JH, Shim YR, Kim MH, Kim HH, Kim YE, Yang K, Kim K, Choi SE, Kim W, Kim SH, Eun HS, Jeong WI. Metabotropic glutamate receptor 5 in natural killer cells attenuates liver fibrosis by exerting cytotoxicity to activated stellate cells. Hepatology 2021;74:2170–85 [DOI] [PubMed] [Google Scholar]
- 71. Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, Blanchard S, Fremaux I, Croue A, Fouchard I, Lunel-Fabiani F, Boursier J, Roingeard P, Cales P, Delneste Y, Jeannin P. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology 2014;60:1879–90 [DOI] [PubMed] [Google Scholar]
- 72. Yi HS, Lee YS, Byun JS, Seo W, Jeong JM, Park O, Duester G, Haseba T, Kim SC, Park KG, Gao B, Jeong WI. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology 2014;60:1044–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yi HS, Eun HS, Lee YS, Jung JY, Park SH, Park KG, Choi HS, Suh JM, Jeong WI. Treatment with 4-methylpyrazole modulated stellate cells and natural killer cells and ameliorated liver fibrosis in mice. PLoS ONE 2015;10:e0127946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology 2008;134:248–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res 1996;20:900–7 [DOI] [PubMed] [Google Scholar]
- 76. Wei X, Qian J, Yao W, Chen L, Guan H, Chen Y, Xie Y, Lu H, Zhang Z, Shi L, Lin X. Hyperactivated peripheral invariant natural killer T cells correlate with the progression of HBV-relative liver cirrhosis. Scand J Immunol 2019;90:e12775 [DOI] [PubMed] [Google Scholar]
- 77. Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 2011;53:219–29 [DOI] [PubMed] [Google Scholar]
- 78. Rehermann B. Natural killer cells in viral hepatitis. Cell Mol Gastroenterol Hepatol 2015;1:578–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology 2007;46:706–15 [DOI] [PubMed] [Google Scholar]
- 80. Zhao J, Li Y, Jin L, Zhang S, Fan R, Sun Y, Zhou C, Shang Q, Li W, Zhang Z, Wang FS. Natural killer cells are characterized by the concomitantly increased interferon-gamma and cytotoxicity in acute resolved hepatitis B patients. PLoS ONE 2012;7:e49135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology 2010;138:1536–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu WH, Cosgrove C, Berger CT, Cheney PC, Krykbaeva M, Kim AY, Lewis-Ximenez L, Lauer GM, Alter G. ADCC-mediated CD56(DIM) NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog Immun 2018;3:2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lunemann S, Schobel A, Kah J, Fittje P, Holzemer A, Langeneckert AE, Hess LU, Poch T, Martrus G, Garcia-Beltran WF, Korner C, Ziegler AE, Richert L, Oldhafer KJ, Schulze Zur Wiesch J, Schramm C, Dandri M, Herker E, Altfeld M. Interactions between KIR3DS1 and HLA-F activate natural killer cells to control HCV replication in cell culture. Gastroenterology 2018;155:1366–71 [DOI] [PubMed] [Google Scholar]
- 84. Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009;137:1151–60 [DOI] [PubMed] [Google Scholar]
- 85. Fisicaro P, Rossi M, Vecchi A, Acerbi G, Barili V, Laccabue D, Montali I, Zecca A, Penna A, Missale G, Ferrari C, Boni C. The good and the bad of natural killer cells in virus control: perspective for anti-HBV therapy. Int J Mol Sci 2019;20:5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, Humphreys E, Afford S, Adams DH, Bertoletti A, Maini MK. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 2007;204:667–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Beraza N, Malato Y, Sander LE, Al-Masaoudi M, Freimuth J, Riethmacher D, Gores GJ, Roskams T, Liedtke C, Trautwein C. Hepatocyte-specific NEMO deletion promotes NK/NKT cell- and TRAIL-dependent liver damage. J Exp Med 2009;206:1727–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fernandez-Alvarez S, Gutierrez-de Juan V, Zubiete-Franco I, Barbier-Torres L, Lahoz A, Pares A, Luka Z, Wagner C, Lu SC, Mato JM, Martinez-Chantar ML, Beraza N. TRAIL-producing NK cells contribute to liver injury and related fibrogenesis in the context of GNMT deficiency. Lab Invest 2015;95:223–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jiang Y, Qin S, Wei X, Liu X, Guan J, Zhu H, Chang G, Chen Y, Lu H, Qian J, Wang Z, Shen M, Lin X. Highly activated TRAIL(+) CD56(bright) NK cells are associated with the liver damage in HBV-LC patients. Immunol Lett 2021;232:9–19 [DOI] [PubMed] [Google Scholar]
- 90. Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, Ishiyama K, Zhou W, Tanaka Y, Asahara T. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology 2004;39:1321–31 [DOI] [PubMed] [Google Scholar]
- 91. Eisenhardt M, Glassner A, Kramer B, Korner C, Sibbing B, Kokordelis P, Nischalke HD, Sauerbruch T, Spengler U, Nattermann J. The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C. PLoS ONE 2012;7:e38846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Polo ML, Ghiglione YA, Salido JP, Urioste A, Poblete G, Sisto AE, Martinez A, Rolon MJ, Ojeda DS, Cahn PE, Turk GJ, Laufer NL. Liver cirrhosis in HIV/HCV-coinfected individuals is related to NK cell dysfunction and exhaustion, but not to an impaired NK cell modulation by CD4(+) T-cells. J Int AIDS Soc 2019;22:e25375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perpinan E, Perez-Del-Pulgar S, Londono MC, Marino Z, Bartres C, Gonzalez P, Garcia-Lopez M, Pose E, Lens S, Maini MK, Forns X, Koutsoudakis G. Cirrhosis hampers early and rapid normalization of natural killer cell phenotype and function in hepatitis C patients undergoing interferon-free therapy. Front Immunol 2020;11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li Y, Wang JJ, Gao S, Liu Q, Bai J, Zhao XQ, Hao YH, Ding HH, Zhu F, Yang DL, Zhao XP. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS ONE 2014;9:e86927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hu Y, Wang X, Wei Y, Liu H, Zhang J, Shen Y, Cao J. Functional inhibition of natural killer cells in a BALB/c mouse model of liver fibrosis induced by schistosoma japonicum infection. Front Cell Infect Microbiol 2020;10:598987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chuang WL, Liu HW, Chang WY, Chen SC, Hsieh MY, Wang LY. Natural killer cell activity in patients with liver cirrhosis relative to severity of liver damage. Dig Dis Sci 1991;36:299–302 [DOI] [PubMed] [Google Scholar]
- 97. Shi J, Zhao J, Zhang X, Cheng Y, Hu J, Li Y, Zhao X, Shang Q, Sun Y, Tu B, Shi L, Gao B, Wang FS, Zhang Z. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-beta-dependent emperipolesis in HBV cirrhotic patients. Sci Rep 2017;7:44544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Amer J, Salhab A, Noureddin M, Doron S, Abu-Tair L, Ghantous R, Mahamid M, Safadi R. Insulin signaling as a potential natural killer cell checkpoint in fatty liver disease. Hepatol Commun 2018;2:285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peng Y, Huang K, Shen L, Tao YY, Liu CH. Cultured mycelium cordyceps sinensis alleviates CCl4-induced liver inflammation and fibrosis in mice by activating hepatic natural killer cells. Acta Pharmacol Sin 2016;37:204–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Peng Y, Yang T, Huang K, Shen L, Tao Y, Liu C. Salvia miltiorrhiza ameliorates liver fibrosis by activating hepatic natural killer cells in vivo and in vitro. Front Pharmacol 2018;9:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xia Y, Yu B, Ma C, Tu Y, Zhai L, Yang Y, Liu D, Liu Y, Wu H, Dan H, You P. Yu Gan long reduces rat liver fibrosis by blocking TGF-beta1/Smad pathway and modulating the immunity. Biomed Pharmacother 2018;106:1332–8 [DOI] [PubMed] [Google Scholar]
- 102. van Dijk F, Olinga P, Poelstra K, Beljaars L. Targeted therapies in liver fibrosis: combining the best parts of platelet-derived growth factor BB and interferon gamma. Front Med (Lausanne) 2015;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]