Abstract
The spread of SARS-CoV-2 over the entire world is more commonly known as COVID-19. COVID-19 has impacted society in every aspect of routine life. SARS-CoV-2 infection is often misdiagnosed as influenza or seasonal upper respiratory tract viral infections. General diagnostic tools can detect the viral antigen or isotypes of antibodies. However, inter- and intraindividual variations in antibody levels can cause false negatives in antibody immunoassays. On the contrary, the false-positive test results can also occur due to either cross-reactivity of the viral antigens or some other patient-related autoimmune factors. There is need for a cogent diagnostic tool with more specificity, selectivity, and reliability. Here, we have described the potential of convalescent serum-derived exosome as a diagnostic tool for the detection of SARS-CoV-2, even in asymptomatic patients, which is a limitation for currently practiced diagnostic tests throughout the globe. In addition, its potential as a vehicle for messenger RNA (mRNA) delivery is also emphasized.
Keywords: Convalescent serum, COVID-19, exosomes, SARS-CoV-2, diagnostic tool, mRNA
Impact Statement
SARS-CoV-2 has spread across the world like a wildfire since its emergence in late 2019. COVID-19 symptoms overlap with several commonly occurring viral infections including influenza. Several cases even go undetected due to the asymptomatic nature of the disease. Diagnostic tools with higher sensitivity and accuracy are urgently needed to tackle this issue.
Background about COVID-19
The presence of the deadly coronavirus disease 2019 (COVID-19) was noted in December 2019 in Wuhan City of China. COVID-19 has gained major attention in the therapeutic arena since it was declared a pandemic and it has impacted all aspects of life.1 –3 It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).4 –6 It is the seventh coronavirus to infect humans and is named COVID-19 by the World Health Organization (WHO).7,8 Currently, there are more than 443 million confirmed cases with more than 5.9 million deaths registered globally due to the SARS-CoV-2 infection. 9 Once the viral genome was encoded and the structural proteins were identified (Figure 1), the efforts were focused on the development of therapeutic agents, vaccines, as well as reliable and sensitive diagnostic tests.
Figure 1.
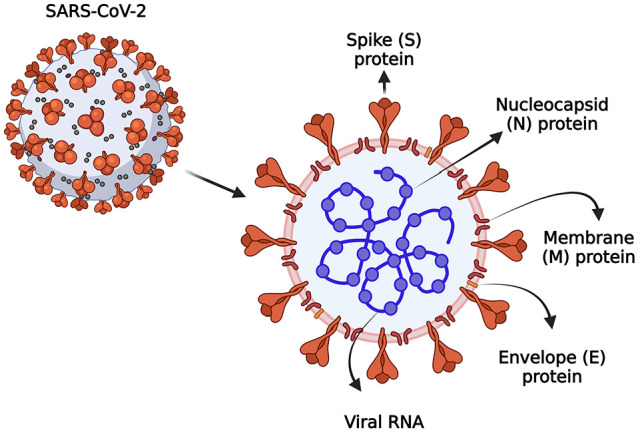
The structural representation of the SARS-CoV-2. SARS-CoV-2 is an RNA virus composed of various structural proteins such as nucleocapsid (N), envelope (E), membrane (M), and spike (S), which facilitate viral entry into the host, replication, assembly of virions, and pathogenesis, created with Biorender.com. (A color version of this figure is available in the online journal.)
The symptoms of COVID-19 include fever, body aches, sore throat, cough, body pain, difficulty in breathing, confusion, anosmia, loss of taste, and headache. 10 However, there are a lot of asymptomatic carriers globally which are difficult to identify. The new strain recently identified in England is responsible for additional symptoms like lethargy, dizziness, and diarrhea. The strain identified in Africa has modified viral spike proteins and is spreading at a much faster rate in various African regions. Although the majority of cases are mild, some can progress to pneumonia and lead to multiorgan failure. Various diagnostic tools that are being implemented for the detection of COVID-19 include serological tests, computerized tomography (CT) scans, magnetic resonance imaging (MRI), and the detection of viral antigens.11,12 As the symptoms overlap with those of influenza and dengue, there is need for an effective diagnostic tool that can detect the presence of COVID-19 in symptomatic as well as asymptomatic patients. The present study describes currently used tools for COVID-19 detection, their limitations, and the potential use of exosomes for diagnosis as well as delivery of mRNA-based therapeutics.
Types of SARS-CoV-2 variants in COVID-19
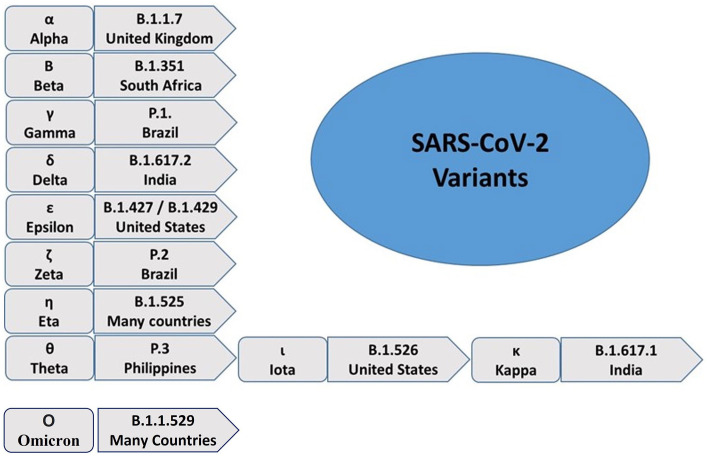
After the emergence of SARS-CoV-2 in 2019, several variants of SARS-CoV-2 have been observed all over the world, including South Africa, India, the United Kingdom, Brazil, and so on. 6 SARS-CoV-2 variants complicate the recovery of COVID-19 patients. Therefore, there is a need to understand the biology of these variants. The genetic mutations in SARS-CoV-2 are responsible for the generation of new variants. The mutation rate is generally higher in RNA viruses like coronaviruses. B.1.1.7 is a UK variant and it is resistant to the monoclonal antibodies against N-terminal of the spike protein. A total of 23 mutations were found in the UK variant. The South African variant (B.1.351) has shown 21 mutations, with many variations in the spike protein. The Brazil variant (P.1) was detected with 17 mutations and has a higher transmission rate. Moreover, other variants of SARS-CoV-2 have been emerging, including B.1.617 in India (Figure 2). 13
Figure 2.
The reported variants of SARS-CoV-2. Several SARS-CoV-2 variants have been reported across the world since its emergence in December 2020. The most commonly found variants include B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.427, B.1.429, P.2, B.1.525, P.3, B.1.526, B.1.617.1, and B.1.1.529. They have been designated with Greek letters ranging from Alpha to Kappa in that order. (A color version of this figure is available in the online journal.)
Diagnostic and detection methods for COVID-19
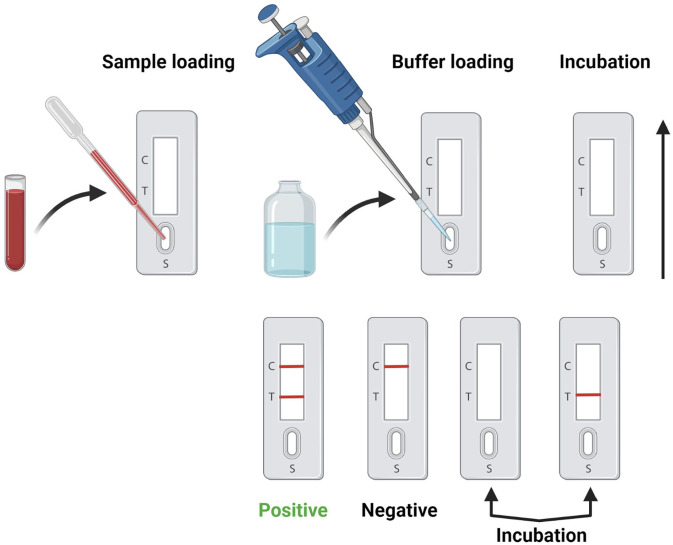
Following the onset of COVID-19 symptoms, the first step is to detect the presence or absence of SARS-CoV-2. Many patients test positive despite being asymptomatic, due to testing requirements as per the government norms. Rapid antigen detection test is one of the easiest first-line detection mechanisms but its reliability has been questioned. Therefore, real-time reverse transcription-polymerase chain reaction (RT–PCR) is recommended for the cogent early detection (Figure 3).14,15
Figure 3.
SARS-CoV-2 serological testing. Serological testing for SARS-CoV-2 detection involves a series of steps including sample/buffer loading, incubation, antibody-antigen recognition, SARS-CoV-2 / control antibody detection, and data interpretation, created with Biorender.com. (A color version of this figure is available in the online journal.)
RT-PCR test mainly focuses on the detection of the viral nucleic acid or its fragments present in the upper respiratory tract of the patient. Improving test sensitivity and specificity is essential to reduce the number of false-positive or false-negative cases. 16 More sophisticated enzyme-linked immunosorbent assay (ELISA) can detect viral spike protein as well as the presence of IgG and IgM. 17 In addition, there are next-generation sequencing-based assays that are commercially viable and more reliable than the other 430 different types of nucleic acid detection assays in the market. More than 170 and 430 immunoassays are being developed for the detection of viral antigens and antibodies, respectively.
Limitations of current diagnostic tools
Although there are several immunoassays in development for the diagnosis of COVID-19, most of them have shown very poor sensitivity or reliability of the test outcome. 18 Assay formats, antigens to target (S and N proteins, as well as the subunits of SARS-CoV-2), isotypes of antibodies to detect (IgA, IgM, IgG, and complete antibodies), the diagnostic testing window, as well as inter- and intraindividual variation in antibody levels can all cause false negatives in antibody immunoassays. 19 Moreover, false-positive test results are mainly due to either cross-reactivity of the viral antigens or some other patient-related autoimmune factors (Figure 3).
In order to improve the false-positive and false-negative results, there is need for a highly sensitive and specific diagnostic test. CRISPRCas13a has recently obtained a lot of attention because of its ability to remove amplification steps while also shedding light on point-of-care (POC) diagnostics and, as a result, removing any potential background signal from the amplification method. Selection of the particular epitope and isotope is also very crucial for the development of a reliable and specific diagnostic test (Figure 4).20 –22 Combining RT-PCR and IgM-IgG antibody tests would result in more reliable and sensitive methods to detect the SARS-CoV-2 virus. However, there is a need to think outside the box and develop some diagnostic tools having more accuracy and sensitivity of detection with added advantages of the existing methods. Currently, none of the clinical or laboratory approaches available are reliable or precise enough to question the existing COVID-19 strain.
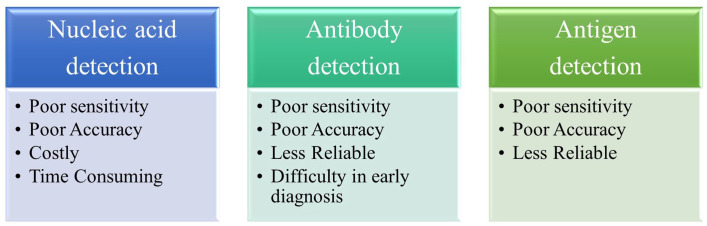
Figure 4.
Limitations of current diagnostic tools. Current diagnostic tools for nucleic acid and, antibody and antigen detection have limited sensitivity, accuracy, and reliability. They are also expensive, time-consuming, and lack early detection capabilities. (A color version of this figure is available in the online journal.)
Exosome as a futuristic diagnostic tool for COVID-19
As there is no vaccine or a therapeutic regimen to cure COVID-19, convalescent plasma therapy is usually the first choice of treatment for patients. Convalescent serum is derived from recovered COVID-19 patients with high neutralizing antibody titers. Although the clinical advantage of convalescent plasma therapy in COVID-19 is still unknown, using antibody-containing plasma from recovered patients is a near-term choice that can be introduced rapidly.23,24 It is a kind of passive immunization as opposed to vaccines, which trigger antibody production. Convalescent plasma provides immediate immunity. However, the mechanism of action is not very clear. Plasma also contains exosomes, other vesicles, proteins, lipoproteins, and so on, in addition to neutralizing antibodies.
Exosomes are formed through multivesicular bodies (MVB), which appear along the endocytic pathway, and are characterized by the presence of vesicles in their lumen (i.e. intraluminal vesicles) formed by inward budding from the limiting membrane. Exosomes contain various biological materials such as proteins, lipids, miRNA, mRNA, snRNA, and DNA.25 –27 Exosomes, in particular, are extracellular vesicles (EVs) that can carry viral DNA, RNA, and other biologic payloads,25 –31 and have great potential to be used as a diagnostic tool.32,33 Several methods have been used to isolate exosomes, which are present in many extracellular body fluids such as serum, plasma, urine, and saliva. Exosomes are isolated from differential ultracentrifugation, ultrafiltration, precipitation, immunoaffinity capture, and size-exclusion chromatography. 34 Although many methods have been used, ultrafiltration is considered to be the gold standard. 35 Following the recovery of convalescent serum from patients, ultracentrifugation and filtration are carried out in order to isolate the exosomes. Convalescent serum-derived exosomes can be a cogent diagnostic tool for futuristic COVID-19 detection with more selectivity and specificity. Exosomes in the blood play a key role in cell-to-cell communication and are a promising new source of biomarkers for disease diagnosis and prognosis. Furthermore, exosomes have shown promise as biomarkers for disease status and treatment outcomes.36,37 Proteomic profiling of exosomes may help identify the differential biomarkers. Furthermore, cluster analysis can pinpoint the differentially expressed proteins which could be the potential biomarkers for noninvasive COVID-19 detection. A growing body of evidence suggests that exosomes proved to be a potential biomarker for conditions like cancers, viral diseases, and some autoimmune disorders.38 –40 Proteomic analysis of patient-derived exosomes have detected many components involved in the immune response, pathology, and activation of the coagulation, and complement pathways associated with COVID-19-associated tissue damage and multiple organ dysfunctions. 41 It can also detect the asymptomatic patients acting as disease spreaders in society. In sum, the exosome will be the potential candidate for the rapid and more reliable diagnostic method development for COVID-19.
Exosome-mediated mRNA delivery
To have therapeutic effects, mRNA molecules must reach certain target cells and create a sufficient amount of the desired proteins. However, targeted distribution and endosomal escape continue to be difficult challenges for mRNA delivery systems, emphasizing the need for safe and efficient mRNA delivery materials. Notably, lipid nanoparticles are used to deliver antigen mRNA in two approved coronavirus disease 2019 (COVID-19) vaccines: mRNA-1273 and BNT162b. Lipid nanoparticle–mRNA compositions must overcome various extracellular and intracellular obstacles in order to work in vivo. First, mRNA in physiological fluids must be shielded against nuclease destruction. Second, upon systemic injection, the formulation should avoid interception by the mononuclear phagocyte system and clearance by renal glomerular filtration. Third, lipid nanoparticle–mRNA systems must enter target tissues before being internalized by target cells. Finally, mRNA molecules must avoid endosomes in order to enter the cytoplasm, where translation takes place. All these features can be achieved by exosomes with least side effects and mRNA loss, as these vesicles are derived from humans.42 –46
Exosomes have gained popularity in recent years due to their ability to facilitate intercellular communication via the targeted delivery of multimolecular cargo.47,48 They are particularly useful in transporting mRNA molecules which are prone to degradation inside the cell. Other vehicles such as liposomes or nanoparticles have been previously used to carry mRNAs. 49 But these vehicles were found to have serious side effects. 50 On the contrary, exosomes are a part of the cell secretome, and are ubiquitous in the human body 51 and thus may serve as suitable candidates for delivering RNA-based therapeutics. 52
In a recent study conducted by Tsai et al., exosomes purified from cultured human cells were packed with mRNA encoding a carrier protein and multiple SARS-CoV-2 proteins including spike protein, before delivering them into in vitro and in vivo models at a dosage of 0.25 μg or 4 μg mRNA every 3 weeks (total three doses). Harvested tissues showed a prolonged (although modest, possibly due to the lower dose) CD4+ and CD8+ T-cell-mediated immune response against the N and S proteins for almost 8 weeks, following the final booster dose. In addition, CD4+ T cells demonstrated increased expression of interferon-gamma and lower expression of interleukin-4, indicating Th1-linked response. 53 While this study is yet to be peer-reviewed, it opens up a new avenue for the potential use of exosomes as delivery vehicles for mRNA-based drugs, including optimization of the exosome-RNA formulation conditions (Figure 5), scale-up of the dosage for studies in the larger animals, and determination of side effects.
Figure 5.
Schema for exosomal biomarker development. The optimization of the exosome-RNA formulation conditions. (A color version of this figure is available in the online journal.)
Exosomes: advantages and importance
Consistent evolution of the viral genome has made COVID-19 management quite difficult over the past few years. Based on the severity of disease, COVID-19 can be divided into asymptomatic or presymptomatic infection, mild, moderate, severe, and critical illness which is associated with intensive care unit (ICU) admission and mortality.54,55 Considering the current devastating global scenario of COVID-19, there is an urgent need for the development of therapeutics that will be helpful in dealing with severe cases of COVID-19 with higher efficacy and recovery rates.56,57 Furthermore, there is also an urge for a cogent diagnostic tool with more reliability, specificity, and sensitivity. Exosomes are becoming more widely accepted as contributors to a variety of diseases, and their potential as biomarkers and therapeutics is steadily gaining traction.58,59
The exosome is a bilayer structure with the ability to carry the genetic material as well as the drug molecules for targeted delivery. Exosomes play a role in immune protection by activating immune responses. The exosomes produced in diseased cells have been shown to express specific proteins that can differentiate them from normal cells. In this way, exosomes may serve as useful biomarkers for disease diagnosis and monitoring (Figures 6 and 7).60,61
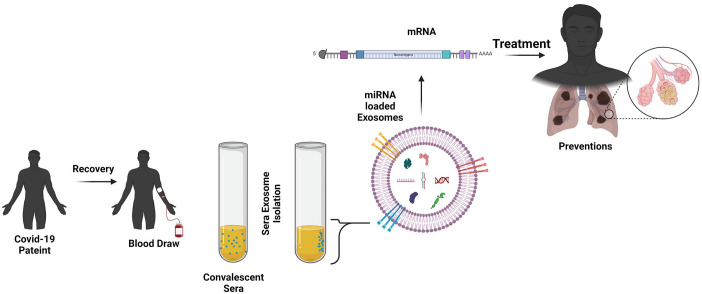
Figure 6.
Convalescent serum-derived exosome-mediated mRNA delivery. Exosomes are isolated using convalescent serum obtained from a recovered COVID-19 patient, loaded with mRNA, and delivered to a patient as a potential treatment, created with Biorender.com. (A color version of this figure is available in the online journal.)
Figure 7.
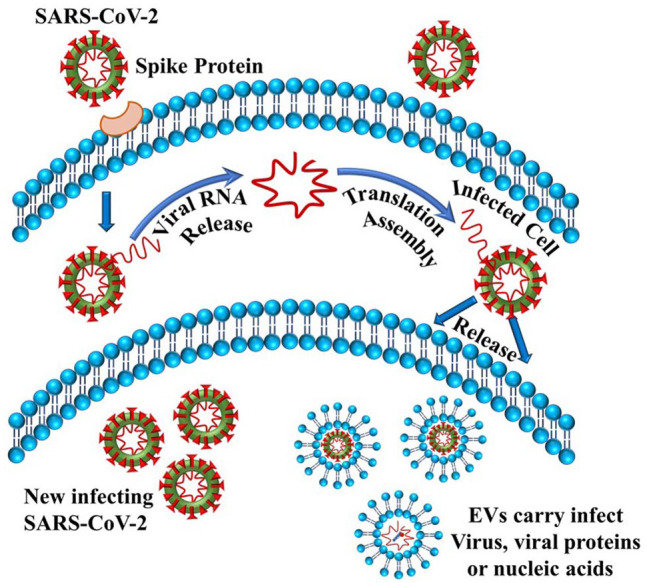
SARS-CoV-2 replication process using elements of exosomes. Viral replication occurs upon its entry into the host cell. Extracellular vesicles (EVs) such as exosomes capture these virions along with any viral protein or nucleic acids. (A color version of this figure is available in the online journal.)
Exosomes and viruses share similar endosomal sorting pathways and mechanisms, giving them the ability to be used as a therapeutic to target, bind, and suppress the cellular uptake of a variety of viruses, including the novel SARS-CoV-2. Exosomal contents are relatively stable and safe against foreign proteases and other enzymes, making them valuable diagnostic tools. Accumulating evidence indicates that exosomal miRNA and lncRNA profiles in patients with particular pathologies vary from those in healthy people.62,63
Exosomes that exist naturally are best tolerated, have low immunogenicity and high extracellular fluid resilience.57,64 These “naturally-equipped” nanovesicles could be used as drug delivery systems or be therapeutically targeted.65 –67 Exosomes have the potential to cross the blood–brain barrier, at least in certain circumstances, and may be used to deliver a variety of therapies such as small molecules, RNA therapies, proteins, viral gene therapy, and CRISPR gene editing. 68
Exosomes in clinical trials for the treatment of COVID-19
There are a total of 15 ongoing clinical trials, of which 7 are in the United States and 8 in Europe, where the potential of exosomes is being evaluated for the treatment of COVID-19. The virus-specific T cells (VSTs) in conjunction with interferon-gamma are engulfed in exosomes and evaluated in COVID-19 patients with pulmonary complications. 69
Another clinical trial, a non-randomized open-label cohort study, was conducted to determine the safety and efficacy of exosomes (ExoFlo™) derived from allogeneic bone marrow mesenchymal stem cells as a treatment for severe COVID-19. From days 1–14, patients were tested for both protection and efficacy after receiving a single 15 mL intravenous dose of ExoFlo™. Within 72 h of ExoFlo administration, all safety endpoints were reached, with no adverse effects reported. The survival rate was found to be 83%. ExoFlo is a promising therapeutic candidate for extreme COVID-19 because of its safety profile and ability to restore oxygenation, downregulate cytokine storms, and restore immunity. ExoFlo’s therapeutic potential might be assessed in future randomized controlled trials (RCTs). 68 Exosomes can control inflammation and regenerative processes by changing the concentration of anti-inflammatory cytokines and moving the immune cells to a regenerative secretome. Exosome inhalation has been shown to minimize inflammation and damage to lung tissue while also promoting regenerative processes. A pilot clinical trial involving the use of mesenchymal stem cells (MSCs) exosomes to treat severe novel coronavirus pneumonia (NCP) is ongoing in Wuhan, China (NCT04276987). This procedure aims to see whether exosome aerosol inhalation is safe and effective in treating critical patients hospitalized with NCP.
Exosomes as therapeutics
Experimental research has shown that MSCs can minimize lung inflammation and pathological impairment caused by various forms of lung injury. Many researchers attribute MSC’s anti-inflammatory properties to their secretome, which includes exosomes derived from MSC. Furthermore, exosomes have a powerful regenerative effect on various wounds, implying their usefulness in treating COVID-19 patients.
The use of convalescent blood products such as whole blood, plasma, or serum, pooled human immunoglobulin IgG, and polyclonal and monoclonal antibodies have also gained prominence.38,70 Improvement of clinical symptoms, increased oxygen saturation, higher neutralizing antibody titers, reduced lung consolidation, and reduced viral load have been observed in response to the administration of convalescent blood products.39,40 However, the mechanism of action remains unknown. Some authors refer to it as an “empirical therapy” since most of the findings come from case studies with few participants, and the results, while encouraging, may be skewed due to the lack of a well-designed experimental setup. The presence of EVs in convalescent blood products could explain some of the therapeutic benefits including immunomodulation and wound healing in the lungs. Neutralizing antibodies, growth factors, and EVs can be passed to patients during plasma apheresis.41,54,71 In addition, exosomes can induce the activation of intracellular signaling pathways which will help in the theranostics of COVID-19.
Platelet-derived EVs were shown to enhance cell proliferation, migration, and angiogenesis in wound healing models by activating phosphoinositide 3-kinase (PI3K)-mitogen-activated protein kinase (Akt) and mitogen-activated protein kinase (MAPK) TGF- and yes-associated protein interaction, as well as -Erk signaling (YAP),39,41 which could help elucidate the mechanism by which convalescent plasma shows positive results. Another study found that engineered platelet-derived EVs loaded with TPCA-1 were successful in treating pneumonia. 55 In a mouse model targeting inflammatory sites, this treatment suppressed inflammation and reduced the local cytokine storm. Thus, platelet-derived EVs may be useful in developing new therapeutic strategies for COVID-19.
Conclusions
The SARS-CoV-2 pandemic is on an unsettling course. The health, humanitarian, social, and economic policies of each country will affect the pace and intensity of recovery. Treatment for COVID-19 is currently very limited, and there is no curative solution available. Rapid and early laboratory diagnosis is critical to detect infection and control the transmission. As per WHO, RT-PCR is the standard test for COVID-19 detection. Mismatches between primers, probes, and target sequences may result in reduced detection performance and false-negative results, although RT-PCR is optimized for conserved regions of the viral genome. Serological surveys may assist with the investigation of an existing epidemic as well as the retrospective evaluation of an attack intensity or spectrum of the outbreak. Rapid diagnosis is expected due to appropriate treatment strategies and necessary containment. While the general diagnostic tools have the capability to detect viral antigens or isotypes of antibodies, the diagnostic testing window, interindividual variation, and intraindividual variation in antibody levels can all cause false negatives in antibody immunoassays. On the contrary, the false-positive test results are mainly due to either cross-reactivity of the viral antigens or some other patient-related autoimmune factors. There is need for a cogent diagnostic tool with more specificity, selectivity, and reliability. Here, we have attempted to describe the potential of convalescent serum-derived exosome as a diagnostic tool for the detection of SARS-CoV-2 that can detect even asymptomatic patients, which is the limitation for the currently practiced diagnostic tests throughout the globe. There is a consistent change in the virus structure due to mutation which makes it difficult to detect and even cure by generating passive immunity by the vaccine. As the exosomes are derived from the serum of the COVID-19 recovered patient, it can differentially express the protein, a potential biomarker for the detection of COVID-19 infections even if the person is asymptomatic. The differential protein expression by the exosomes can be identified by the proteomic analysis for its potential use as a biomarker, checking the drug repurposing status, and evaluation of the newer therapeutic moieties. On the contrary, the lipid bilayer vesicle can also be used as a vehicle for drug delivery as well as non-immunogenic vaccine delivery, either DNA or mRNA. It has the machinery to easily carry the nucleic acid material with more stability which will become a boon for vaccine delivery. As indicated by Tsai et al., 53 exosome-mediated mRNA delivery may soon turn into a reality as the second-generation vaccine for COVID-19 with fewer side effects and immunogenic complications.
Footnotes
Authors’ Contributions: AK and PG equally contributed to the manuscript. VPC, MPJ, RM, DV, CV, PR, AL, NKK, and B-CA contributed to the preparation of the initial draft and subsequent revisions. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Prakash Gangadaran  https://orcid.org/0000-0002-0658-4604
https://orcid.org/0000-0002-0658-4604
Manasi P Jogalekar  https://orcid.org/0000-0003-1307-4829
https://orcid.org/0000-0003-1307-4829
Prasanna Ramani  https://orcid.org/0000-0002-5236-7141
https://orcid.org/0000-0002-5236-7141
Byeong-Cheol Ahn  https://orcid.org/0000-0001-7700-3929
https://orcid.org/0000-0001-7700-3929
References
- 1. Chavda VP, Vora LK, Vihol DR. COVAX-19® vaccine: completely blocks virus transmission to non-immune individuals. Clin Complement Med Pharmacol 2021;1:100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goudouris ES. Laboratory diagnosis of COVID-19. J Pediatr 2021;97:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol 2020;2: e428–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felsenstein S, Hedrich CM. SARS-CoV-2 infections in children and young people. Clin Immunol 2020;220:108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jogalekar MP, Veerabathini A, Gangadaran P. Novel 2019 coronavirus: genome structure, clinical trials, and outstanding questions. Exp Biol Med 2020;245:964–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jogalekar MP, Veerabathini A, Gangadaran P. SARS-CoV-2 variants: a double-edged sword? Exp Biol Med 2021;246:1721–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chavda VP, Feehan J, Apostolopoulos V. A Veterinary vaccine for SARS-CoV-2: the first COVID-19 vaccine for animals. Vaccines 2021; 9:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today 2021;26:2619–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moulahoum H, Ghorbanizamani F, Zihnioglu F, Turhan K, Timur S. How should diagnostic kits development adapt quickly in COVID 19-like pandemic models? Pros and cons of sensory platforms used in COVID-19 sensing. Talanta 2021;222:121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, Chen H, Mubareka S, Gubbay JB, Chan WCW. Diagnosing COVID-19: the disease and tools for detection. ACS Nano 2020;14:3822–35 [DOI] [PubMed] [Google Scholar]
- 13. Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med 2021;27:1131–3 [DOI] [PubMed] [Google Scholar]
- 14. Beigel JH, Voell J, Kumar P, Raviprakash K, Wu H, Jiao J-A, Sullivan E, Luke T, Davey RT., Jr. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis 2018;18:410–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 2020;55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 2020;505: 172–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, Long X, Guo S, Zhao Z, Liu Y, Hu H, Xue H, Li Y. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 2020;81:e28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette J-L, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sens 2021;6:593–612 [DOI] [PubMed] [Google Scholar]
- 20. Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DBT, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016;353:aaf5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017;356:438–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nouri R, Tang Z, Dong M, Liu T, Kshirsagar A, Guan W. CRISPR-based detection of SARS-CoV-2: a review from sample to result. Biosens Bioelectron 2021;178:113012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, Pekosz A, Lau B, Wesolowski A, Katz L, Shan H, Auwaerter PG, Thomas D, Sullivan DJ, Paneth N, Gehrie E, Spitalnik S, Hod EA, Pollack L, Nicholson WT, Pirofski L-A, Bailey JA, Tobian AA. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020;130:2757–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng HT, Rhind SG, Beckett A. Convalescent plasma for the prevention and treatment of COVID-19: a systematic review and quantitative analysis. JMIR Public Health Surveill 2021;7:e25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gangadaran P, Ahn B-C. Extracellular vesicle- and extracellular vesicle mimetics-based drug delivery systems: new perspectives, challenges, and clinical developments. Pharmaceutics 2020;12:E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gangadaran P, Rajendran RL, Oh JM, Hong CM, Jeong SY, Lee S-W, Lee J, Ahn B-C. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp Cell Res 2020;394:112146. [DOI] [PubMed] [Google Scholar]
- 27. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest 2016;126:1139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 2016;30:836–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gangadaran P, Rajendran RL, Oh JM, Oh EJ, Hong CM, Chung HY, Lee J, Ahn B-C. Identification of angiogenic cargo in extracellular vesicles secreted from human adipose tissue-derived stem cells and induction of angiogenesis in vitro and in vivo. Pharmaceutics 2021;13:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 2019;86:1–14. [DOI] [PubMed] [Google Scholar]
- 31. Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med 2019;11:eaav8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giró O, Jiménez A, Pané A, Badimon L, Ortega E, Chiva-Blanch G. Extracellular vesicles in atherothrombosis and cardiovascular disease: friends and foes. Atherosclerosis 2021;330:61–75 [DOI] [PubMed] [Google Scholar]
- 33. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci 2020;21:6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toma C-M, Imre S, Vari C-E, Muntean D-L, Tero-Vescan A. Ultrafiltration method for plasma protein binding studies and its limitations. Processes 2021;9:382 [Google Scholar]
- 36. Ruhen O, Meehan K. Tumor-derived extracellular vesicles as a novel source of protein biomarkers for cancer diagnosis and monitoring. Proteomics 2019;19:e1800155 [DOI] [PubMed] [Google Scholar]
- 37. Urabe F, Kosaka N, Ito K, Kimura T, Egawa S, Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol 2020;318:C29–39 [DOI] [PubMed] [Google Scholar]
- 38. Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, Zhao ZJ, Blick KE, Dooley WC, Ding WQ. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res BCR 2016;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang A, Dong J, Li S, Wang C, Ding H, Li H, Su X, Ge X, Sun L, Bai C, Shen X, Fang T, Li J, Shao N. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci 2015;11:961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 2015;4:26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiao Y-J, Jin D-D, Jiang F, Liu J-X, Qu L-S, Ni W-K, Liu Z-X, Lu C-H, Ni R-Z, Zhu J, Xiao M-B. Characterization and proteomic profiling of pancreatic cancer-derived serum exosomes. J Cell Biochem 2019;120:988–99 [DOI] [PubMed] [Google Scholar]
- 42. Rubin EJ, Longo DL. Covid-19 mRNA vaccines—six of one, half a dozen of the other. N Engl J Med 2022;386:183–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chavda VP, Kapadia C, Soni S, Prajapati R, Chauhan SC, Yallapu MM, Apostolopoulos V. A global picture: therapeutic perspectives for COVID-19. Immunotherapy 2022;14:351–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 2021;6:1078–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dolgin E. The tangled history of mRNA vaccines. Nature 2021;597:318–24 [DOI] [PubMed] [Google Scholar]
- 46. Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater 2017;2:1–17 [Google Scholar]
- 47. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017;546:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020;21:585–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu A-M, Choi YH, Tu M-J. RNA drugs and RNA targets for small molecules: principles, progress, and challenges. Pharmacol Rev 2020;72: 862–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong DS, Kang Y-K, Borad M, Sachdev J, Ejadi S, Lim HY, Brenner AJ, Park K, Lee J-L, Kim T-Y, Shin S, Becerra CR, Falchook G, Stoudemire J, Martin D, Kelnar K, Peltier H, Bonato V, Bader AG, Smith S, Kim S, O’Neill V, Beg MS. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 2020;122:1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mäger I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R. Methodological guidelines to study extracellular vesicles. Circ Res 2017;120:1632–48 [DOI] [PubMed] [Google Scholar]
- 52. Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab 2018;27:714–39 [DOI] [PubMed] [Google Scholar]
- 53. Tsai SJ, Atai NA, Cacciottolo M, Nice J, Salehi A, Guo C, Sedgwick A, Kanagavelu S, Gould SJ. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J Biol Chem 2021;297:101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chrzanowski W, Kim SY, McClements L. Can stem cells beat COVID-19: advancing stem cells and extracellular vesicles toward mainstream medicine for lung injuries associated with SARS-CoV-2 infections. Front Bioeng Biotechnol 2020;8:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Slonchak A, Clarke B, Mackenzie J, Amarilla AA, Setoh YX, Khromykh AA. West Nile virus infection and interferon alpha treatment alter the spectrum and the levels of coding and noncoding host RNAs secreted in extracellular vesicles. BMC Genomics 2019;20:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 2015;77:13–27 [DOI] [PubMed] [Google Scholar]
- 57. Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci 2016;4: 910–21. [DOI] [PubMed] [Google Scholar]
- 58. Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, Piyachaturawat P, Chairoungdua A. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta 2015;1852:1989–99 [DOI] [PubMed] [Google Scholar]
- 59. Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci 2014;105:1384–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T. Donor-derived exosomes with lung self-antigens in human lung allograft rejection. Am J Transplant 2017;17:474–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hassanpour M, Rezaie J, Nouri M, Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect Genet Evol 2020;85:104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther 2019;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoshimura A, Sawada K, Kimura T. Is the exosome a potential target for cancer immunotherapy? Ann Transl Med 2017;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weidle UH, Birzele F, Kollmorgen G, Rüger R. The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics 2017;14:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 2012;82:1024–32 [DOI] [PubMed] [Google Scholar]
- 66. Livshits MA, Livshts MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 2015;5:17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu L-L, Zhu J, Liu J-X, Jiang F, Ni W-K, Qu L-S, Ni R-Z, Lu C-H, Xiao M-B. A comparison of traditional and novel methods for the separation of exosomes from human samples. Biomed Res Int 2018;2018:3634563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020;29:747–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sattler A, Angermair S, Stockmann H, Heim KM, Khadzhynov D, Treskatsch S, Halleck F, Kreis ME, Kotsch K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest 2020;130:6477–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hicar MD. Antibodies and immunity during Kawasaki disease. Front Cardiovasc Med 2020;7:94, https://www.frontiersin.org/article/10.3389/fcvm.2020.00094 (accessed 15 March 2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rodríguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, Sandvig K, Linē A, Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer 2017;16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]