Abstract
BACKGROUND
Hirayama disease (HD), also known as juvenile spinal muscular atrophy, is a rare condition in which flexion of the cervical neck causes compression and ischemic changes to the anterior horns of the spinal cord. Here the authors presented the first reported case of HD in North America that was successfully treated via surgical intervention.
OBSERVATIONS
The patient was a 15-year boy with insidious onset upper limb weakness and atrophy. His findings were a classic presentation of HD although his complex history and relative rarity of the disease caused him to remain undiagnosed for months. After conservative management via cervical collar failed, the patient was successfully treated via C5-C7 anterior cervical discectomy and fusion. The patient’s symptoms stabilized by the 3-month follow-up.
LESSONS
The diagnosis of HD is easy to miss because of the lack of reporting and widespread knowledge of this condition in North America. Thus, when presented with a case of insidious onset limb weakness in a juvenile patient, HD should be placed on the differential list and verified with cervical flexion magnetic resonance imaging. Additionally, surgical intervention should be considered a safe and effective option for HD when conservative methods have failed.
Keywords: Hirayama disease, spinal muscular atrophy, ACDF, anterior cervical discectomy and fusion; surgical outcomes
ABBREVIATIONS : HD = Hirayama disease, MRI = magnetic resonance imaging
Progressive neurodegenerative disorders cover a broad range of pathology, including monomelic amyotrophy. Hirayama disease (HD), also known as juvenile spinal muscular atrophy, is one form of monomelic amyotrophy. This disease is characterized by compression and progressive ischemic changes to the anterior horns of the spinal cord upon neck flexion.1 First described in a series of 12 patients by Hirayama et al. in 1959, this condition predominately occurs in young adult males of Chinese and Indian descent.1–6 Patients most often present with insidious onset unilateral or bilateral atrophy of the muscles of the arms and hands although lower extremities can be involved as well.3,4,7,8 Magnetic resonance imaging (MRI) of this condition demonstrates largely normal appearance, causing patients to be undiagnosed for several months.9 Flexion MRI in these patients demonstrates expansion of the posterior epidural space resulting in compression of the dorsal dura and spinal cord. There is resultant, disproportionate dysfunction in the anterior horns of the spinal cord.10 These radiographic findings alongside a patient’s clinical presentation allow for a confident diagnosis of HD.
HD is commonly a self-limiting condition that is predominately treated via conservative methods such as a cervical collar.11 Nonetheless, disease progression can occur despite conservative management, leading to considerable limb dysfunction.1,5,8,11 A limited number of case reports have previously described surgical intervention for this disorder although none have been reported in North America thus far. Here we present the first reported case of HD in North America that was successfully treated via surgical intervention.
Illustrative Case
A 15-year-old boy presented to the clinic with a 6-month history of progressive right-hand weakness and atrophy. His past medical history was significant for bilateral distal radial and ulnar metaphyseal fractures from an all-terrain vehicle accident at age 13 years. After the accident, his hands were pinned and casted from the elbows down to the proximal interphalangeal joints. He did not report any sensory symptoms while in the cast or immediately after removal. One year later, the patient began experiencing weakness with flexion and extension of the third through fifth digits on the right hand. He experienced progressive right-hand weakness and functional impairment without sensory symptoms.
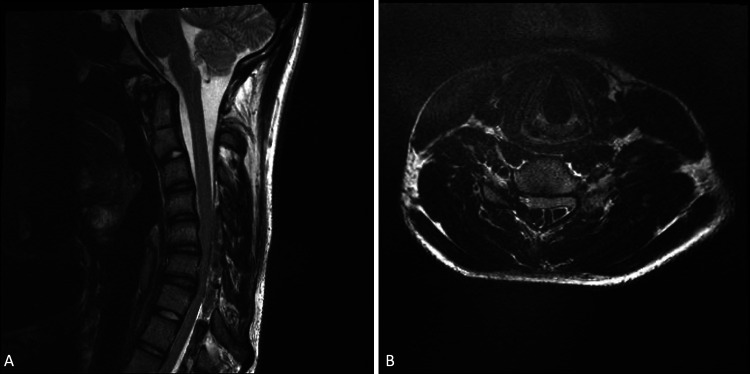
Physical examination revealed significant weakness in his right upper extremity as well as severe atrophy of his right hand and forearm. The patient was full strength (5/5) in his proximal right upper extremity, including deltoid and biceps, decreasing distally to 2/5 in his intrinsic hand muscles. Pectoralis asymmetry was also noted, with slightly smaller musculature on the right side. Patellar reflexes were increased bilaterally. No fasciculations were detected, and the patient did not report any upper extremity pain. Additionally, no family history of neuromuscular or peripheral nerve disorders was reported. The patient saw several providers, but no diagnosis was reached because his static cervical spine MRI appeared normal (Fig. 1). Electromyographic examination revealed marked low amplitudes of the right median and ulnar muscle action potentials with normal sensory nerve potentials, indicative of a chronic neurogenic process. Based on clinical suspicion, a flexion MRI was performed, and it revealed significant anterior displacement of the thecal sac, effacement of the subarachnoid space from C5 to C7, and engorgement of the posterior, epidural veins. A diagnosis of HD was confirmed (Fig. 2A and B).
FIG. 1.

Nonflexion sagittal T2-weighted cervical MRI appearing largely normal with some slight narrowing in the C4-C6 region.
FIG. 2.
A: Flexion sagittal T2-weighted MRI showing extreme compression of the C4-C7 region. B: Flexion axial MRI showing compression of C4-C7 region.
Initially, the patient was placed in a Miami J collar (Össur) with hopes that immobilization would suspend disease progression. Unfortunately, the patient’s symptoms worsened at 1-month follow-up, and surgical intervention was recommended. A standard C5-C7 anterior cervical discectomy and fusion was performed (Fig. 3A and B). The patient tolerated the procedure well and had a stable neurological examination after surgery. The patient completed a course of postoperative physical therapy. At the 12-month follow-up, the patient had no further progression of disease. His intrinsic hand muscles remained atrophied, and his strength and dexterity did not improve.
FIG. 3.
A: Postoperative coronal radiograph displaying successful anterior cervical discectomy and fusion from C5 to C7. B: Postoperative sagittal radiograph displaying successful anterior cervical discectomy and fusion from C5 to C7.
Discussion
Observations
The patient is an adolescent boy with insidious-onset upper limb weakness and atrophy. Although he had a classic presentation for HD, his diagnosis was missed by several providers. The delayed diagnosis may be attributed to unfamiliarity with this rare entity in North America. Although no global epidemiological studies on HD have been conducted, Tashiro et al. completed the largest nationwide survey of HD to date in Japan. Of the 2,260 facilities they queried, only 333 cases of HD were reported.11 These rates are likely even higher than what would be seen in Western countries given the paucity of reports on HD to come out of Europe and North America. Presently, only a single case series exists on patients with HD in North America. Ghosh et al. performed a retrospective review of pediatric patients at the Cleveland Clinic over 10 years and found a total of six children with a confirmed diagnosis of HD.12 All patients had the classic physical examination findings of unilateral or bilateral upper extremity weakness without sensory loss, and they were successfully managed via cervical collar. Because of the high likelihood of misdiagnosed and undiagnosed patients with HD, it is uncertain how prevalent this condition is in the United States. It is clear that continued reporting and education on this topic are essential when it comes to patient care.
The patient’s past medical history of bilateral radial and ulnar fractures may have distracted from the cardinal features of HD and delayed diagnosis. Although the timing and location of the trauma coincided closely with the patient’s symptomatology, the insidious unilateral arm weakness and lack of sensory symptoms should have raised suspicion for HD. The workup for HD first begins with a thorough physical examination. Symptoms are most often unilateral, but bilateral presentation is possible.13 In most patients, deep tendon reflexes are normal although hyperreflexia can be present, as in this case.13 Electrophysiological studies can be helpful for diagnosis. The results of these studies most often display high amplitude polyphasic action potentials in the tested nerves.14 A flexion MRI is perhaps the most conclusive diagnostic tool in HD workup. With the patient’s neck flexed forward, MRI reveals the pathognomonic anterior displacement of the lower cervical dural sac with flattening of the cervical cord.15 Therefore, if HD is on the differential, flexion MRI is critical to establish the diagnosis.
The prevailing pathophysiological theory for the findings seen on flexion MRI is that disproportionate growth may occur during adolescent development, with vertebral growth outpacing that of the spinal cord. It leads to chronic compression and ischemic changes to the cervical cord during neck flexion. This condition is largely self-limiting and resolves within 1 to 3 years in most patients.15 Currently, first-line treatment consists of conservative management via a cervical collar. In cases in which conservative management fails to arrest disease progression, surgical intervention is pursued. The necessity of surgical intervention in HD is still debated, and few reports exist in the literature. A literature search on this topic yielded 16 reports encompassing 110 patients with HD treated via surgical methods.13,16–30 Of these 110 patients, 104 (94.5%) were male and 6 (5.5%) were female. The average age for the surgical patient was 19.5 years. Surgically treated patients were Chinese (82.7%), Indian (6.4%), Taiwanese (4.5%), Japanese (2.7%), French (2.7%), and Spanish (0.9%). In all cases, patients reported stabilized or improved strength in the affected limb(s). Although there may be a reporting bias, the generally favorable outcomes in the literature as well as our own case indicate that surgical intervention is a safe and effective option for refractory HD.
Lessons
HD is easy to miss because of the lack of reporting and widespread knowledge of this condition in North America. It is likely that numerous cases of HD have gone undiagnosed and untreated due to a lack of awareness on this topic. Reports have indicated that early recognition and intervention are crucial in preventing severe and irreversible damage to the cervical spinal cord.31–33 Thus, when presented with a case of insidious-onset limb weakness in a juvenile patient, HD should be considered and verified with cervical flexion MRI.
Surgical intervention should be considered a safe and effective option in treating refractory cases of HD. Whereas conservative therapy with a cervical collar is a first-line treatment, numerous reports have demonstrated successful surgical treatment for refractory cases.17,18,26,28 Several techniques have been used successfully to treat this condition, including anterior cervical decompression and fusion, posterior instrumented fusion, and laminectomy.34 Among these treatments, anterior cervical discectomy and fusion is the most commonly reported, likely because of its high efficacy and low complication rates.30 It is also important to note that early surgical intervention can help prevent permanent neurological sequelae.35 Surgical intervention should be considered early in patients with progressive neurological changes.
Conservative methods are still the primary line of treatment. One reason these approaches may fail is due to noncompliance. If this is the case, improved patient education and a heightened emphasis on the importance of a strict treatment regimen may avoid the unnecessary risks of surgery.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Patel. Acquisition of data: Hudson, Patel. Analysis and interpretation of data: Hudson, Patel. Drafting the article: Singh, Hudson, Neal, Patel. Critically revising the article: Hudson, Neal, Patel. Reviewed submitted version of manuscript: all authors. Statistical analysis: Patel. Administrative/technical/material support: Meyer. Study supervision: Patel.
Supplemental Information
Previous Presentations
Previously presented virtually as a poster at the AANS Annual Scientific Meeting, August 21–25, 2021.
References
- 1. Kieser DC, Cox PJ, Kieser SCJ. Hirayama disease. Eur Spine J. 2018;27(6):1201–1206. doi: 10.1007/s00586-018-5545-9. [DOI] [PubMed] [Google Scholar]
- 2.Lay SE, Sharma S. Hirayama Disease. StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 3. Zhou F, Zhang Y, Sun Y, Pan S, Zhang F, Zhang L. Correlation of pre-operative factors with the outcome of surgical treatment of Hirayama disease. Article in Chinese. Zhonghua Yi Xue Za Zhi. 2016;96(5):349–353. doi: 10.3760/cma.j.issn.0376-2491.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 4. Sun Y, Liu X, Fan DS, et al. Midterm clinical outcomes and radiological results of surgical treatment for Hirayama disease. Article in Chinese. Beijing Da Xue Xue Bao. 2017;49(6):1019–1026. [PubMed] [Google Scholar]
- 5. Fu Y, Qin W, Sun QL, Fan DS. Investigation of the compliance of cervical collar therapy in 73 patients with Hirayama disease. Article in Chinese. Zhonghua Yi Xue Za Zhi. 2016;96(43):3485–3488. doi: 10.3760/cma.j.issn.0376-2491.2016.43.009. [DOI] [PubMed] [Google Scholar]
- 6. Hirayama K. Juvenile muscular atrophy of unilateral upper extremity (Hirayama disease): half-century progress and establishment since its discovery. Article in Japanese. Brain Nerve. 2008;60(1):17–29. [PubMed] [Google Scholar]
- 7. Das A, Pradhan S. Cardiovascular and sudomotor dysfunction in Hirayama disease. Acta Neurol Belg. 2021;121(2):545–553. doi: 10.1007/s13760-019-01253-w. [DOI] [PubMed] [Google Scholar]
- 8. Ozturker C, Kara K, Incedayi M, Sonmez G, Mutlu H. Hirayama disease. Spine J. 2016;16(5):e299–e300. doi: 10.1016/j.spinee.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 9. Kapetanakis S, Chourmouzi D, Terzoudi A, Georgiou N, Giovannopoulou E. Hirayama disease: diagnostic essentials in neuroimaging. Clin Case Rep. 2017;5(12):2151–2152. doi: 10.1002/ccr3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta K, Sood S, Modi J, Gupta R. Imaging in Hirayama disease. J Neurosci Rural Pract. 2016;7(1):164–167. doi: 10.4103/0976-3147.172174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tashiro K, Kikuchi S, Itoyama Y, et al. Nationwide survey of juvenile muscular atrophy of distal upper extremity (Hirayama disease) in Japan. Amyotroph Lateral Scler. 2006;7(1):38–45. doi: 10.1080/14660820500396877. [DOI] [PubMed] [Google Scholar]
- 12. Ghosh PS, Moodley M, Friedman NR, Rothner AD, Ghosh D. Hirayama disease in children from North America. J Child Neurol. 2011;26(12):1542–1547. doi: 10.1177/0883073811409226. [DOI] [PubMed] [Google Scholar]
- 13. Lin MS, Kung WM, Chiu WT, Lyu RK, Chen CJ, Chen TY. Hirayama disease. J Neurosurg Spine. 2010;12(6):629–634. doi: 10.3171/2009.12.SPINE09431. [DOI] [PubMed] [Google Scholar]
- 14. Baumann M, Finsterer J, Gizewski ER, Löscher WN. Early-onset Hirayama disease in a female. SAGE Open Med Case Rep. 2017;5:2050313X16686710. doi: 10.1177/2050313X16686710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Y-L, Chen C-J. Hirayama disease. Neuroimaging Clin N Am. 2011;21(4):939–950. doi: 10.1016/j.nic.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 16. Goel A, Jadhav N, Shah A, Rai S, Vutha R. Chiari malformation and syringomyelia associated with Hirayama disease. World Neurosurg. 2020;135:241–244. doi: 10.1016/j.wneu.2019.12.101. [DOI] [PubMed] [Google Scholar]
- 17. Dohzono S, Toyoda H, Tamura A, Hayashi K, Terai H, Nakamura H. Surgical treatment of a patient with prolonged exacerbation of Hirayama disease. Spine Surg Relat Res. 2018;3(1):95–97. doi: 10.22603/ssrr.2018-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang H, Wang S, Li Z, et al. Anterior cervical surgery for the treatment of Hirayama disease. World Neurosurg. 2019;127:e910–e918. doi: 10.1016/j.wneu.2019.03.295. [DOI] [PubMed] [Google Scholar]
- 19. Wang HL, Wu YW, Song J, et al. Cortical activation changes in Hirayama disease after anterior cervical decompression and fusion. World Neurosurg. 2018;116:e588–e594. doi: 10.1016/j.wneu.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 20. Brandicourt P, Sol JC, Aldéa S, Bonneville F, Cintas P, Brauge D. Cervical laminectomy and micro resection of the posterior venous plexus in Hirayama disease. Neurochirurgie. 2018;64(4):303–309. doi: 10.1016/j.neuchi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 21. Luo J, Yang K, Zhong Y, et al. Hirayama disease treated by anterior cervical diskectomy and fusion: case report and literature review. World Neurosurg. 2020;141:171–174. doi: 10.1016/j.wneu.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 22. Xu Q, Gu R, Zhu Q, Suya D. A severe case of Hirayama disease successfully treated by posterior cervical fixation without decompression and fusion. World Neurosurg. 2019;122:326–330. doi: 10.1016/j.wneu.2018.10.157. [DOI] [PubMed] [Google Scholar]
- 23. Chiba S, Yonekura K, Nonaka M, Imai T, Matumoto H, Wada T. Advanced Hirayama disease with successful improvement of activities of daily living by operative reconstruction. Intern Med. 2004;43(1):79–81. doi: 10.2169/internalmedicine.43.79. [DOI] [PubMed] [Google Scholar]
- 24. Goel A, Dhar A, Shah A. Multilevel spinal stabilization as a treatment for Hirayama disease: report of an experience with five cases. World Neurosurg. 2017;99:186–191. doi: 10.1016/j.wneu.2016.11.143. [DOI] [PubMed] [Google Scholar]
- 25. Fujimori T, Tamura A, Miwa T, Iwasaki M, Oda T. Severe cervical flexion myelopathy with long tract signs: a case report and a review of literature. Spinal Cord Ser Cases. 2017;3:17016. doi: 10.1038/scsandc.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu W, Wang S, Lin J. A 34-year-old female patient with Hirayama disease complicated by severe spinal cord injury. World Neurosurg. 2019;130:84–88. doi: 10.1016/j.wneu.2019.06.208. [DOI] [PubMed] [Google Scholar]
- 27. Srivastava SK, Marathe N, Raj A, Bhosale S, Dhole K. Surgical management of Hirayama disease: a rare entity with unusual clinical features. Asian J Neurosurg. 2020;15(2):405–408. doi: 10.4103/ajns.AJNS_291_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paredes I, Esteban J, Ramos A, Gonzalez P, Rivas JJ. A severe case of Hirayama disease successfully treated by anterior cervical fusion. J Neurosurg Spine. 2014;20(2):191–195. doi: 10.3171/2013.10.SPINE13508. [DOI] [PubMed] [Google Scholar]
- 29. Guo X, Lu M, Xie N, Guo Q, Ni B. Multilevel anterior cervical discectomy and fusion with plate fixation for juvenile unilateral muscular atrophy of the distal upper extremity accompanied by cervical kyphosis. J Spinal Disord Tech. 2014;27(7):E241–E246. doi: 10.1097/BSD.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 30. Lu F, Wang H, Jiang J, et al. Efficacy of anterior cervical decompression and fusion procedures for monomelic amyotrophy treatment: a prospective randomized controlled trial: clinical article. J Neurosurg Spine. 2013;19(4):412–419. doi: 10.3171/2013.4.SPINE12575. [DOI] [PubMed] [Google Scholar]
- 31. Hosokawa T, Fujieda M, Wakiguchi H, Oosaki Y. Pediatric Hirayama disease. Pediatr Neurol. 2010;43(2):151–153. doi: 10.1016/j.pediatrneurol.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 32. Yang G, Yang X, Zhang M, et al. Hirayama disease in children from mainland of China. J Child Neurol. 2014;29(4):509–513. doi: 10.1177/0883073813482770. [DOI] [PubMed] [Google Scholar]
- 33. Martínez-Cayuelas E, Martínez-Salcedo E, Alarcón-Martínez H, et al. Hirayama disease in paediatrics: a clinical case report and review of the literature. Article in Spanish. Rev Neurol. 2015;60(7):309–315. [PubMed] [Google Scholar]
- 34. Wang H, Sun C, Yang S, et al. Dynamic cervical radiographs in patients with Hirayama disease: an unneglectable factor on the choice of surgery options. World Neurosurg. 2018;114:e433–e440. doi: 10.1016/j.wneu.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 35. Song J, Wang HL, Zheng CJ, Jiang JY. Risk factors for surgical results of Hirayama disease: a retrospective analysis of a large cohort. World Neurosurg. 2017;105:69–77. doi: 10.1016/j.wneu.2017.05.097. [DOI] [PubMed] [Google Scholar]