Abstract
Introduction
Typhoid fever is a public-health problem in Harare, the capital city of Zimbabwe, with seasonal outbreaks occurring annually since 2010. In 2019, the Ministry of Health and Child Care (MOHCC) organized the first typhoid conjugate vaccination campaign in Africa in response to a recurring typhoid outbreak in a large urban setting.
Method
As part of a larger public health response to a typhoid fever outbreak in Harare, Gavi approved in September 2018 a MOHCC request for 340,000 doses of recently prequalified Typbar-TCV to implement a mass vaccination campaign. To select areas for the campaign, typhoid fever surveillance data from January 2016 until June 2018 was reviewed. We collected and analyzed information from the MOHCC and its partners to describe the vaccination campaign planning, implementation, feasibility, administrative coverage and financial costs.
Results
The campaign was conducted in nine high-density suburbs of Harare over eight days in February–March 2019 and targeted all children aged 6 months–15 years; however, the target age range was extended up to 45 years in one suburb due to the past high attack rate among adults. A total of 318,698 people were vaccinated, resulting in overall administrative coverage of 85.4 percent. More than 750 community volunteers and personnel from the MOHCC and the Ministry of Education were trained and involved in social mobilization and vaccination activities. The MOHCC used a combination of vaccination strategies (i.e., fixed and mobile immunization sites, a creche and school-based strategy, and door-to-door activities). Financial costs were estimated at US$ 2.39 per dose, including the vaccine and vaccination supplies (US$ 0.79 operational costs per dose excluding vaccine and vaccination supplies).
Conclusion
A mass targeted campaign in densely populated urban areas in Harare, using the recently prequalified typhoid conjugate vaccine, was feasible and achieved a high overall coverage in a short period of time.
Keywords: Typhoid fever, Typhoid conjugate vaccine, Mass vaccination campaign, Outbreak response, Financial costs, Zimbabwe
1. Introduction
Typhoid fever, an infection caused by the bacterium Salmonella enterica serovar Typhi (S. Typhi), is highly endemic in many parts of the world. Each year, typhoid fever causes an estimated 11─21 million cases and 128,000─161,000 deaths globally [1]. High incidence (i.e., at least 100 cases for 100,000 person per year) is found in South Asia and sub-Saharan Africa, particularly among children aged 5 to <15 years [2], [3], [4], [5]. The infection is transmitted via the fecal-oral route and is more common in populations that lack access to safe and adequate water, sanitation, and hygiene (WaSH) [6]. Typhoid fever outbreaks still remain an important public health issue in resource limited settings; a recent review found at least 48 outbreaks documented in 25 countries, accounting for over 45,215 cases during the years 1989–2018 [7]. Complications, including intestinal perforations and neurologic manifestations, are well-documented and occur among 10–15 % of hospitalized patients, with case fatality rate estimated at 1–4 % [1], [5], [8]. Adequate treatment requires appropriate antibiotics; however, increasing antimicrobial resistance globally is limiting treatment options, thereby potentially increasing the risk of severe disease and death attributable to severe typhoid fever [9], [10], [11]. In addition to increasing resistance to cotrimoxazole, ampicillin, and chloramphenicol, which is defined as multi-drug resistance typhoid fever, and fluoroquinolones, recent outbreak in Pakistan and in Bangladesh highlighted the occurrence of extensive drug resistance, which includes additional resistance to the third-generation cephalosporins [12], [13].
Access to safe WaSH is the cornerstone for typhoid fever prevention and control. While infrastructure improvements are required to disrupt the transmission long-term, these are unlikely to provide a timely solution given their financial costs as well as the enormous challenges in many countries with rapid population growth and urbanization [1], [6], [14], [15]. Typhoid vaccination is an important complementary tool in the short- to medium-term, particularly in light of the increasing threat of antimicrobial resistance. Safe and effective typhoid vaccines have been available for individuals aged 2 years and older since the early 1990s but have not been used on a large scale in high-burden countries partly due to low immunogenicity in children under 2 years of age and short duration of protection [1], [6], [16], [17], [18], [19], [20]. Typhoid conjugate vaccines (TCVs) are recently available and have improved properties, such as suitability for use in children from 6 months of age, a longer estimated duration of protection, and similar safety profiles to the previous vaccines [1], [20], [21], [22]. TCV efficacy was demonstrated at 82 % among children 9 months to 16 years of age after 1 year in Nepal, and at 84 % among children 9 months to 12 years of age after 18 months of follow-up in Malawi [23], [24]. Longer-term duration of protection still needs to be assessed, however TCV is associated with long-term immunogenicity findings [25]. TCVs have become a sustainable solution for use as a public health measure thanks to 1) the World Health Organization (WHO) prequalification of Typbar-TCV (Bharat Biotech International Limited, India) in December 2017, 2) WHO recommendation to use TCV for the control of typhoid fever in endemic as well as epidemic settings, and 3) the decision by Gavi, the Vaccine Alliance (Gavi) to fund TCV introduction in eligible countries [1], [26], [27].
In Zimbabwe, inadequate water and sanitation infrastructure have led to recurring outbreaks of waterborne diseases, including over 16,398 suspected and 619 confirmed cases (unspecified laboratory methods) of typhoid fever notified between 2009─2017 [28]. In Harare, the capital city with an estimated population of 1.8 million, typhoid fever is endemic with cases recorded all year-round; seasonal outbreaks during the rainy period (typically October-April) have occurred annually since 2010, including a large outbreak from October 2017 to June 2018 with over 4,300 suspected and confirmed reported cases [29], [30], [31], [32], [33]. The high-density southwestern suburbs of Harare, characterized by low socioeconomic status, intermittent water supply, frequent sewer line breaks, and low elevation, have historically been affected by typhoid fever, especially during the rainy season [29], [30], [31], [32], [33]. There are also reports of increasing antimicrobial resistance in S. Typhi to ciprofloxacin, the first-line drug in Zimbabwe [28], [34]. Given typhoid endemicity in Zimbabwe, the MOHCC applied in 2018 to Gavi for TCV introduction into its routine immunization program, a 3 year process before TCV could be successfully rolled out in May 2021 [20]. In the meantime, Gavi approved in September 2018, a separated MOHCC request for 340,000 TCV doses for rapid implementation in response to a new seasonal typhoid fever outbreak in Harare. In collaboration with partners, the MOHCC conducted a reactive vaccination campaign, using Typbar-TCV in early 2019, complementing WaSH efforts for outbreak control and prevention. Here, we describe the context, the implementation and the results in term of coverage rates and vaccination delivery costs of this campaign in a high-risk densely populated urban area in Harare, which was the first TCV campaign in Africa and the first Gavi-supported outbreak response TCV campaign.
2. Methods
2.1. Typhoid surveillance data review and targeted areas for vaccination
To select areas for the TCV campaign, the MOHCC reviewed typhoid surveillance data from the Harare City Health Department (HCHD) surveillance system from January 2016 to June 2018. The main selection criteria were the attack rates by age group and suburb, as well as the trend in the number of cases by suburb. From January 2016 to June 2018, 6,556 suspected and confirmed cases were reported from 21 suburbs of Harare. Further details of these cases and area of residence were not available. The largest seasonal outbreak took place between October 2017 and June 2018 with a total of 4,330 reported cases, including 250 blood and stool culture-confirmed cases (HCHD, personal communication). Overall, 24 % of the cases (1,049) in that outbreak were among the 0–4 years old, while the 5–14 years old group, and the ≥ 15 years old group accounted for 23 % (1,004) and 53 % (2,277) of the cases, respectively. The attack rates per 1,000 individuals by age group were respectively 4.32 (0–4 years), 2.83 (5–14 years) and 1.89 (≥15 years). The overall attack rate for Harare was 2.40 per 1,000 residents and was unusually high in Mbare (18.9 per 1,000). Seventy-eight percent of cases (3,384) came from four densely populated suburbs: Mbare, Kuwadzana, Glen View, and Budiriro.
Given the size of the affected population in Harare and the limited number of TCV doses available for the campaign, the MOHCC selected nine suburbs including the most affected ones during the period 2016–2018 and three informal settlements located within the capital city and that were not adequately reached by the city’s water and sanitation services.
2.2. Campaign planning and implementation
We collected and reviewed written records regarding the planning, implementation (including social mobilization), coordination, and monitoring of the campaign from the main stakeholders (i.e., the MOHCC, the HCHD, WHO, and UNICEF).
2.3. Campaign monitoring and administrative coverage
During the campaign, each individual vaccinated was tallied by sex and age group (six months–4 years, 5–15 years, and 16–45 years where relevant). Tally sheets were collected and entered into an Excel spreadsheet at the end of every day to monitor the performance of the campaign by suburb, using 2019 population projections estimates calculated from the 2012 national census [35].
We calculated vaccine wastage as the percentage of the number of doses issued by the central storage facility that were lost based on the number of vaccinated individuals from the tally sheets.
2.4. Vaccine delivery costing
Incremental financial costs were estimated from the provider perspective, inclusive of monetary expenditures by the Zimbabwean government and external partners, for the period from November 2018 until March 2019, not including the coverage survey or the cost of external evaluations. Cost data were collected retrospectively from the administrative and financial records and consultations with personnel involved in procurement, account management, and program implementation. Costs were summed by program activity, with any shared costs attributed to the TCV campaign based on the proportion of time or space used. The cost per dose administered was estimated by dividing the total cost by the number of doses administered. Costs were collected in U.S. dollars and reported in nominal 2018–19 values. We did not adjust for inflation because the timeframe was less than one year. Operational costs were supported by funding from Gavi, WHO AFRO, the MOHCC, and Higherlife Foundation, with in-kind support provided from the MOHCC, the HCHD and were managed by the WHO Zimbabwe country office.
2.5. Adverse events following immunization (AEFI)
To capture serious and non-serious AEFI, we relied on the routine passive surveillance system operated by the MOHCC and the Medicine and Control Authority of Zimbabwe (MCAZ) as per national guidelines [36]. Additionally, short-term active hospital-based surveillance was implemented for 10 adverse events of special interest (such as anaphylaxis, thrombocytopenia, seizures, meningitis, etc.) through 42 days following the end of the TCV campaign.
2.6. Ethical approval
The vaccination campaign was conducted as part of the public health response to the typhoid outbreak and was approved by the MOHCC of Zimbabwe. Vaccination was done on a voluntarily basis and no informed consent was collected by the vaccination team.
3. Results
3.1. Typhoid surveillance data review and targeted areas for vaccination
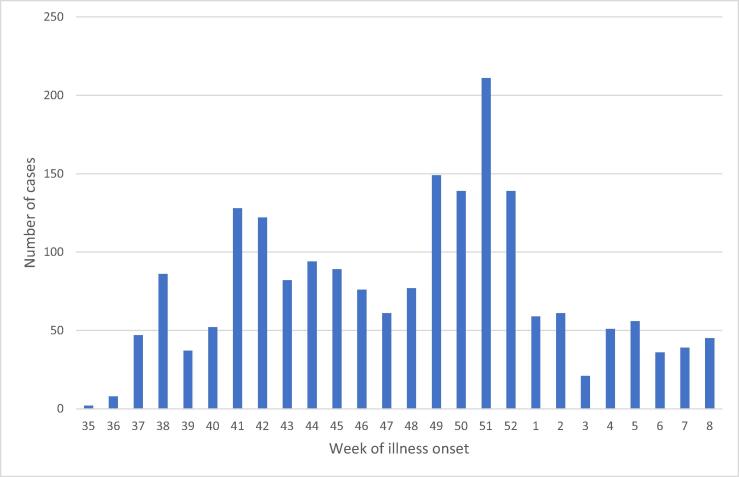
In August 2018, another seasonal outbreak began. From 27 August 2018 to 24 February 2019, the MOHCC reported 1,967 suspected and confirmed typhoid cases (Fig. 1) and only one typhoid-related death. The number of cases peaked in the epidemiological week 51 of 2018 with 211 cases (Fig. 1). The median age of cases was 14 years old (range 0–91 years), and 45 % (895) of cases were female. 0–4 age group accounted for 27 % (531) of the cases, while 5–15 years old group and greater than 15 years old group accounted for 24 % (472) and 49 % (964) of all cases, respectively.
Fig. 1.
Number of confirmed and/or suspected cases of typhoid by week of onset—Harare, Zimbabwe, August 2018–February 2019.
Of the cases, 75 % (1,476) were reported from the nine suburbs selected by the MOHCC for the campaign. The nine suburbs accounted for 52 % of Harare’s population. The target age group for vaccination included all children aged 6 months–15 years. In one suburb, Mbare, the age group was extended up to 45 years. While many cases were reported among adults in other areas, they could only be targeted in one suburb due to limited vaccine availability. The target population was 373,027 individuals of whom 88,856 (24 %) lived in informal settlements.
3.2. Campaign planning and implementation
The campaign took place from 25 February to 04 March 2019. Before the start of the campaign, the MOHCC established a coordination committee with representatives from all partners involved. They met weekly to review implementation plans, budgets, logistics, monitoring tools, and communication materials. The campaign was planned and implemented by the HCHD and was coordinated by the MOHCC. Two consultants were deployed by WHO to assist with the process. Because Typbar-TCV was not registered in Zimbabwe, the MCAZ approved its use in an emergency setting and facilitated the importation process.
Several trainings were held as follows: 1) training of trainers for supervisors and vaccination team leads, 2) training for all vaccinators and school health coordinators, 3) training for social mobilisers, 4) training on AEFI surveillance and management for supervisors and vaccination team leads, and 5) refresher training on causality assessments for the National AEFI Committee.
Over 190 social mobilizers and health promotion officers from HCHD conducted various sensitization and mobilization activities such as 1) launch of the campaign at a press conference leading to mass media coverage, 2) roadshows conducted in the targeted suburbs throughout the campaign 3) local TV and radio channels carried out relevant messaging, 4) use of flyers and posters describing the characteristics of typhoid and TCV, as well as dates and locations of vaccination sites, developed in both English and the local language, Shona, and 5) mobilisers wore hats and t-shirts printed with campaign-specific messages. These activities began 11 days before the start of the campaign and continued throughout the campaign in each targeted suburb. We collaborated with social mobilisers from various churches to minimize possible vaccine hesitancy among religious communities.
To reach the targeted population in eight days, the MOHCC used a combination of vaccination strategies, including fixed and mobile sites, creche and school-based strategies, and door-to-door activities. A total of 416 nurses were grouped into 52 teams. Each team consisted of 10 people, eight nurses and two social mobilisers from the targeted areas, allowing them to separate into smaller groups when attendance was low to reach a broader population. In addition, 49 supervisors were mobilised and 100 school health coordinators supported the school-based vaccination strategy. Vaccination sites remained open all day and activities also took place during the weekend to reach people at churches, trading centres, and markets. All teams were provided with emergency trays to manage anaphylactic reactions. Sick people, pregnant women, and lactating women with children under 6 months were excluded.
Upon arrival in January 2019, the MOHCC stored the vaccines at the Central Vaccine Store in cold rooms between 2 °C and 8 °C. During the campaign, vaccines and syringes were distributed to each suburb every three days, while coolant packs were provided daily. Vaccinators were instructed to check the vaccine vial monitor before administering each dose, and an open vial policy was followed by discarding open vials at the end of each day. Waste was collected daily to be incinerated at Harare Central Hospital.
3.3. Campaign monitoring and administrative coverage
Vaccination teams administered TCV to a mean of 766 people/day/team (range 444–1,085). The daily number of doses administered was almost twice as high on weekdays (873 doses/day) than on weekends (446 doses/day). Vaccine wastage was less than 0.01 % (392 of 319,090 used doses). There were no reports of doses being discarded due to invalid vaccine vial monitors.
A total of 318,698 people were vaccinated with TCV, resulting in overall administrative coverage of 85.4 % across all targeted age groups and suburbs, including adults in Mbare (Table 1); 60,327 (50.3 %) were female. Among all children, TCV coverage was 88.4 % with the highest coverage in Mufakose (104.9 %) and the lowest in Hopley (77.8 %). The coverage was 72.4 % (range 57.5–89.4 %) among children aged 6 months–4 years, and 97.1 % (range 74.3–125.2 %) among children aged 5–15 years, with 89.9 % of them being vaccinated in schools. In Mbare, coverage among adults aged 16–45 years was 66.7 %, and slightly more males than females were vaccinated (51.0 % vs 49.0 %).
Table 1.
Number of people vaccinated and administrative coverage by age group and suburb during the TCV campaign—Harare, Zimbabwe, 2019.
| Target population | Number vaccinated | Administrative coverage (%) | |
|---|---|---|---|
| Age group | |||
| 6 mo.–4 yrs. | 114,388 | 82,768 | 72.4 |
| 5–15 yrs. | 208,429 | 202,457 | 97.1 |
| 16–45 yrs.a | 50,210 | 33,473 | 66.7 |
| All | 373,027 | 318,698 | 85.4 |
| 6 mo-15 yrs. by suburb | |||
| Budiriro | 46,876 | 37,322 | 79.6 |
| Dzivarasekwa | 27,399 | 27,944 | 102.0 |
| Glen Norah | 28,251 | 28,436 | 100.7 |
| Glen View | 43,961 | 38,756 | 88.2 |
| Hatcliffe | 17,533 | 14,879 | 84.9 |
| Hopley | 43,924 | 34,182 | 77.8 |
| Kuwadzana | 62,268 | 56,453 | 90.7 |
| Mbare | 31,159 | 24,760 | 79.5 |
| Mufakose | 21,446 | 22,493 | 104.9 |
| All Suburbs | 322,817 | 285,225 | 88.4 |
Vaccination among this age group took place in Mbare suburb only.
Details of the safety evaluation have been published separately. However the results from enhanced monitoring, including review of national passive safety surveillance data, hospital-based surveillance for 10 conditions of special interest, and adverse events reported during the coverage survey, supported the safety profile of the TCV vaccine [37].
3.4. Vaccine delivery costing
The total estimated financial cost of the TCV campaign in Harare was US$ 761,864, or US$ 2.39 per dose administered (including the cost of vaccine and vaccination supplies) (Table 2). The cost of vaccine per dose was US $1.50 not including freight and insurance per dose (US $0.02) and vaccination supplies per dose (U.S. $0.08). The largest share of the financial costs (67 %; US$ 510,470) was the procurement of vaccine and vaccination supplies, followed by service delivery (15 %; US$ 114,421), which included per diem and transportation for vaccinators to deliver vaccinations, and social mobilization (8.8 %; US$ 67,241). Other program activities were less than 5 % each of the total financial costs.
Table 2.
Estimated financial costs of TCV campaign by program activity—Harare, Zimbabwe, 2019 (2018–19 nominal U.S. dollars).
| Program Activity | Financial cost (US$) | Share of total financial cost |
|---|---|---|
| Procurement of vaccine and vaccination supplies* | 510,470 | 67.0 % |
| Service Delivery** | 114,421 | 15.0 % |
| Social mobilization | 67,241 | 8.8 % |
| Supervision and monitoring | 26,666 | 3.5 % |
| Training | 27,759 | 3.6 % |
| Other planning and preparation | 13,083 | 1.7 % |
| Vaccine logistics and cold chain | 1,160 | 0.2 % |
| AEFI monitoring | 1,064 | 0.1 % |
| Total | 761,864 | 100.0 % |
| Average cost per dose administered*** including vaccine and vaccination supplies | 2.39 | |
| Average cost per dose administered*** not including vaccine and vaccination supplies | 0.79 |
Procurement of vaccine and vaccination supplies includes vaccine at US $1.50 per dose, syringes, and safety boxes as well as freight, insurance, and transportation of these commodities.
Service delivery includes the costs of directly delivering the vaccine to the individual and includes personnel time, non-vaccine supplies, per diem, transport, and contracted services.
Using administrative coverage of 318,698 vaccine doses administered, not including 392 doses wasted.
4. Discussion
This report documents the first public sector use of TCV in Africa. It is as well as the first Gavi-approved use of TCV in response to an outbreak globally. It reached many people within a short time. The particularly high coverage among school-aged children indicates a good community acceptance for this vaccine. Previous TCV campaigns conducted in India and Pakistan in 2018 reached 113,420 children from 9 months to 14 years of age (71 % administrative coverage), and 207,000 children from 6 months to 10 years of age respectively [38], [39]. The campaign in India used a different delivery strategy, with vaccination taking place during the weekends over six weeks to avoid disruption of routine immunization services. Another campaign in Pakistan in 2019 reached 87,993 children from 6 months to 15 years of age, including a 48 % coverage in school [40].
Overall, administrative coverage reached over 85 % of the target population and 88.4 % of the children aged 6 months–15 years. This is higher than the coverages in the Indian and Pakistani campaigns among children aged 9 months to 14 years [38], [40]. This is likely due to the success of the school-based strategy with a high proportion of children aged 5–15 years vaccinated in schools. As well daily review of data allowed for efficient planning, monitoring, and reallocation of resources during the campaign to maximize campaign performance and improve coverage in low-performing areas.
The coverage was lower among children in the youngest age group, highlighting the need for targeted vaccination strategies that consider children who are too young to attend school and those who do not attend creches. Differences in coverage between suburbs may be partly due to inaccurate population estimates. In addition, some children may have attended school in a suburb other than the one they reside in, resulting in potential coverage discrepancies.
Based on local typhoid epidemiology data, the MOHCC made the decision to vaccinate adults aged 16–45 years in one suburb. Coverage was lower among adults compared to that of children, still reaching two-thirds of them, as it is more challenging to gather together this active and mobile population to a vaccination site, in the short time frame of a reactive campaign. This is in line with findings from other campaigns, such as reactive oral cholera vaccine campaigns conducted in Harare in late 2018 (Personal communication MOHCC) and elsewhere [41]. Vaccination during the weekends was effective in reaching adults, and younger children, with similar numbers of adults vaccinated on weekdays and weekends. However globally the average number of people vaccinated on weekdays was higher compared to that of on the weekends.
This report provides the first estimate of the financial costs of TCV when used for outbreak response in an urban setting in Africa, which can aid in planning such campaigns in Zimbabwe and elsewhere. Along with information on averted health impacts and cost of illness, these results can help inform consideration of the cost-effectiveness of the use of TCV in typhoid prevention and control interventions. The estimated financial cost, including vaccine and vaccination supplies of US $2.39 per dose in the Zimbabwe campaign was less than the estimated cost for a 2018 TCV introduction campaign in India (US $4.31 per dose). However, the operational costs per dose without vaccine and vaccination supplies was higher in Zimbabwe (US $0.79) than that of in India (US $0.45) because the Zimbabwe cost analysis (from the provider perspective) included costs incurred by partners, such as WHO in its financial costs whereas the India cost analysis (from the local government perspective) did not [38]. In addition, immunization delivery costs are typically expected to differ across settings due to other contextual and programmatic differences (e.g. underlying price levels, personnel composition of vaccination delivery teams, social mobilization efforts, geographic dispersion of vaccination locations).
Several factors contributed to the successful planning and implementation of the campaign. First, knowledge of the local typhoid epidemiology and collaboration between the MOHCC and its partners allowed for the vaccine request to Gavi, the selection of a vaccination strategy, and the regulatory approval by MCAZ which was needed for the importation of the vaccine. Second, establishing a coordination committee with all the partners was instrumental in the timely and adequate planning, implementation, and monitoring of the campaign. Third, good collaboration between the health and education personnel allowed for an effective school-based vaccination strategy. Fourth, Harare being an urban setting with a Central Vaccine Store, short distances made it easier to organize the cold-chain and vaccine distribution. This also allowed for the simultaneous deployment of more than 50 vaccination teams, resulting in the completion of the campaign within a short time. Finally, the campaign required robust preparedness and operational capacity and benefited from the experience of the recent oral cholera vaccine campaign which was organized by the same teams and targeted the same suburbs [42].
Several challenges were encountered during the campaign. First, the vaccination campaign took place two months after the number of typhoid cases peaked (Fig. 1). While the campaign was initially planned for implementation before the next anticipated seasonal peak (likely in October–November 2018), the campaign was delayed until February 2019 due to late vaccine arrival, a concurrent cholera outbreak and political unrest in Harare in January 2019 [42], [43]. To mitigate the effect of the recent instability in the affected suburbs, a comprehensive mobilization strategy was implemented 10 days before the campaign started. The delay may have resulted in a lower impact on reducing the transmission. Similarly, additional data are needed to understand whether restricting the targeted age groups to children under 16 years, except for one suburb, might have lowered the impact of the campaign in controlling the outbreak, given that people aged 16 years and above accounted for 49 % of typhoid cases between 27 August 2018 and 24 February 2019. The MOHCC restricted vaccination to children under 16 years of age in eight out of nine suburbs because of the limited number of TCV doses available for the campaign.
5. Conclusion
A mass vaccination campaign implemented by the local health system in Harare was feasible in a short period of time to respond to an outbreak of typhoid fever and was well accepted within the community. Documentation of the campaign will help stakeholders in considering the TCV mass vaccination campaign as a useful tool in disrupting typhoid transmission in high burden countries, especially considering the rapidly increasing threat of antimicrobial resistance. Countries should consider using a school-based vaccination strategy in future campaigns, particularly in settings with high school enrollment. Lessons learned from this campaign will also inform the implementation of future campaigns in similar contexts either preemptively in identified typhoid fever hotspots or reactively in response to an ongoing outbreak.
Data statement
Data associated with this manuscript is unsuitable to post and is country owned.
Disclaimer
The findings represent the personal views of the authors and not the official position of the institutions with which the authors are affiliated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Tito Rwamushaija, Adam Soble, Anderson Chimusoro, Sujeet Jain, David Olson, Sarah Bennett, Tapfumanei Mashe, Tanatsiwa Mandoreba, Ida-Marie Ameda, Adwoa D. Bentsi-Enchill and Cathy Ndiaye for their support around the vaccination campaign and Sarah W. Pallas and Taiwo O. Abimbola for their input in vaccine delivery costing.
Funding
This work was supported by Gavi, the Vaccine Alliance (Grant number: 1923-ZWE-11f-A/ 19-ZWE-15a-B) and WHO-AFRO.
References
- 1.WHO | Weekly Epidemiological Record, 30 March 2018, vol. 93, 13 - Typhoid vaccines: WHO position paper [Internet]. [cited 2022 Apr 30]. Available from: https://www.who.int/publications-detail-redirect/typhoid-vaccines-who-position-paper-march-2018.
- 2.Stanaway J.D., Reiner R.C., Blacker B.F., Goldberg E.M., Khalil I.A., Troeger C.E., et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019 Apr 1;19(4):369–381. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhutta Z.A. Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child. 1996 Sep;75(3):214–217. doi: 10.1136/adc.75.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks F., von Kalckreuth V., Aaby P., Adu-Sarkodie Y., El Tayeb M.A., Ali M., et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health. 2017;5(3):e310–e323. doi: 10.1016/S2214-109X(17)30022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine MM. Chapter 61: Typhoid fever vaccines. In: Plotkin’s Vaccines, 2017; Seventh Edition:1114–1144.
- 6.Steele A.D., Hay Burgess D.C., Diaz Z., Carey M.E., Zaidi A.K.M. Challenges and Opportunities for Typhoid Fever Control: A Call for Coordinated Action. Clin Infect Dis. 2016 Mar 15;62(Suppl 1):S4–S8. doi: 10.1093/cid/civ976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appiah GD, Chung A, Bentsi-Enchill AD, Kim S, Crump JA, Mogasale V, et al. Typhoid Outbreaks, 1989–2018: Implications for Prevention and Control. 2020 Mar 30;tpmd190624. [DOI] [PMC free article] [PubMed]
- 8.Parry C.M., Hien T.T., Dougan G., White N.J., Farrar J.J. Typhoid Fever. N Engl J Med. 2002 Nov 28;347(22):1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 9.Kariuki S., Gordon M.A., Feasey N., Parry C.M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015 Jun;19(33 Suppl 3):C21–C29. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wain J., Hendriksen R.S., Mikoleit M.L., Keddy K.H., Ochiai R.L. Typhoid fever. The Lancet. 2015 Mar 21;385(9973):1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britto C.D., Wong V.K., Dougan G., Pollard A.J., Kang G. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS NeglTrop Dis. 2018 Oct 11;12(10):e0006779. doi: 10.1371/journal.pntd.0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klemm E.J., Shakoor S., Page A.J., Qamar F.N., Judge K., Saeed D.K., et al. Emergence of an Extensively Drug-Resistant Salmonella enterica Serovar Typhi Clone Harboring a Promiscuous Plasmid Encoding Resistance to Fluoroquinolones and Third-Generation Cephalosporins. mBio. 2018;9(1) doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghurnee O., Ghosh A.K., Abony M., Akhter Aurin S., Fatema A.N., Banik A., et al. Isolation of Multi-Drug Resistant (MDR) and Extensively Drug Resistant (XDR) Salmonella typhi from Blood Samples of Patients Attending Tertiary Medical Centre in Dhaka City. Bangladesh AiM. 2021;11(09):488–498. [Google Scholar]
- 14.Bentsi-Enchill A.D., Hombach J. Revised Global Typhoid Vaccination Policy. Clin Infect Dis. 2019;68(Supplement_1):S31–S33. doi: 10.1093/cid/ciy927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross A.G., Rahman M., Alam M., Zaman K., Qadri F. Can we ‘WaSH’ infectious diseases out of slums? Int J Infect Dis. 2020 Mar;1(92):130–132. doi: 10.1016/j.ijid.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Khan M.I., Pach A., Khan G.M., Bajracharya D., Sahastrabuddhe S., Bhutta W., et al. Typhoid vaccine introduction: An evidence-based pilot implementation project in Nepal and Pakistan. Vaccine. 2015;33:C62–C67. doi: 10.1016/j.vaccine.2015.03.087. [DOI] [PubMed] [Google Scholar]
- 17.WHO | Typhoid fever surveillance and vaccine use, South-East Asia and Western Pacific Regions, 2009-2013. Weekly Epidemiological Record. 2014;89(40):429–39. [PubMed]
- 18.Scobie H.M., Nilles E., Kama M., Kool J.L., Mintz E., Wannemuelher K.A:, et al. Impact of a targeted typhoid vaccination campaign following cyclone Tomas, Republic of Fiji, 2010. Am J Trop Med Hyg. 2014;90(6):1031–1038. doi: 10.4269/ajtmh.13-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milligan R, Paul M, Richardson M, Neuberger A. Vaccines for preventing typhoid fever. Cochrane Database of Systematic Reviews [Internet]. 2018 [cited 2022 May 7];(5). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001261.pub4/full. [DOI] [PMC free article] [PubMed]
- 20.Birkhold M., Mwisongo A., Pollard A.J., Neuzil K.M. Typhoid Conjugate Vaccines: Advancing the Research and Public Health Agendas. J Infect Dis. 2021;224(Supplement_7):S781–S787. doi: 10.1093/infdis/jiab449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO | Global Advisory Committee on Vaccine Safety, 30 November – 1 December 2016. Weekly Epidemiological Record. 2017 Jan 13;92(2):13–20. [PubMed]
- 22.Weekly Epidemiological Record, 25 January 2019, vol. 94, 4 - Global Vaccine Safety meeting: Safety of typhoid conjugate vaccine [Internet]. [cited 2022 May 7]. Available from: https://www.who.int/publications-detail-redirect/10665279829.
- 23.Shakya M., Colin-Jones R., Theiss-Nyland K., Voysey M., Pant D., Smith N., et al. Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. N Engl J Med. 2019;381(23):2209–2218. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel P.D., Patel P., Liang Y., Meiring J.E., Misiri T., Mwakiseghile F., et al. Safety and Efficacy of a Typhoid Conjugate Vaccine in Malawian Children. N Engl J Med. 2021;385(12):1104–1115. doi: 10.1056/NEJMoa2035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadrevu K.M., Raju D., Rani S., Reddy S., Sarangi V., Ella R., et al. Persisting antibody responses to Vi polysaccharide–tetanus toxoid conjugate (Typbar TCV®) vaccine up to 7 years following primary vaccination of children < 2 years of age with, or without, a booster vaccination. Vaccine. 2021 Oct 29;39(45):6682–6690. doi: 10.1016/j.vaccine.2021.07.073. [DOI] [PubMed] [Google Scholar]
- 26.WHO | Typhoid vaccine prequalified [Internet]. WHO. World Health Organization; [cited 2022 May 7]. Available from: https://www.who.int/news/item/03-01-2018-typhoid-vaccine-prequalified.
- 27.Gavi tVA. Gavi New Vaccine Support -Typhoid vaccine support [Internet]. [cited 2020 Oct 17]. Available from: https://www.gavi.org/types-support/vaccine-support/typhoid.
- 28.Mashe T., Gudza-Mugabe M., Tarupiwa A., Munemo E., Mtapuri-Zinyowera S., Smouse S.L., et al. Laboratory characterisation of Salmonella enterica serotype Typhi isolates from Zimbabwe, 2009–2017. BMC Infect Dis. 2019;19(1) doi: 10.1186/s12879-019-4114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavi proposal for mass vaccination campaign in response to typhoid outbreak in high-risk Harare suburbs, July 2018. Zimbabwe Ministry of Health and Child Care, MOHCC Personal Communication.
- 30.Polonsky J.A., Martínez-Pino I., Nackers F., Chonzi P., Manangazira P., Van Herp M., et al. Descriptive Epidemiology of Typhoid Fever during an Epidemic in Harare, Zimbabwe, 2012. PLoS ONE. 2014 Dec 8;9(12):e114702. doi: 10.1371/journal.pone.0114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PCMA Report - Domestic water supply, sanitation and hygiene products in Harare, Zimbabwe [Internet]. EMMA Toolkit. 2017 [cited 2020 Oct 17]. Available from: https://www.emma-toolkit.org/report/pcma-report-domestic-water-supply-sanitation-and-hygiene-products-harare-zimbabwe-0.
- 32.Davis WW. Notes from the Field: Typhoid Fever Outbreak — Harare, Zimbabwe, October 2016–March 2017. MMWR Morb Mortal Wkly Rep [Internet]. 2018 [cited 2022 May 7];67. Available from: https://www.cdc.gov/mmwr/volumes/67/wr/mm6711a7.htm. [DOI] [PMC free article] [PubMed]
- 33.N’cho HS. Notes from the Field: Typhoid Fever Outbreak — Harare, Zimbabwe, October 2017–February 2018. MMWR Morb Mortal Wkly Rep [Internet]. 2019 [cited 2022 May 7];68. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6802a5.htm. [DOI] [PubMed]
- 34.Guideline for the Management of Typhoid Fever 2011 [Internet]. Zimbabwe Ministry of Health and Child Care; 2011 [cited 2022 May 7]. Available from: https://library.adhl.africa/handle/123456789/3782.
- 35.2012 Census Thematic Report on Population Projections [Internet]. UNFPA Zimbabwe. 2016 [cited 2022 May 7]. Available from: https://zimbabwe.unfpa.org/en/publications/2012-census-thematic-report-population-projections.
- 36.MCAZ. Adverse Events Following Immunization Surveillance Guidelines 3rd Edition – MCAZ [Internet]. [cited 2022 May 7]. Available from: https://www.mcaz.co.zw/sdm_downloads/adverse-events-following-immunization-surveillance-guidelines-3rd-edition/.
- 37.Shaum A., Mujuru H., A., Takamiya M., Nathoo K., Sreenivasan N., Nyambayo P. Enhanced surveillance for adverse events following immunization during the 2019 typhoid conjugate vaccine campaign in Harare, Zimbabwe. Vaccine. 2022;40(26):3573–3580. doi: 10.1016/j.vaccine.2022.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Date K, Shimpi R, Luby S, N R, Haldar P, Katkar A, et al. Decision Making and Implementation of the First Public Sector Introduction of Typhoid Conjugate Vaccine-Navi Mumbai, India, 2018. Clin Infect Dis. 2020 Jul 29;71(Supplement_2):S172–8. [DOI] [PMC free article] [PubMed]
- 39.Qamar F.N., Yousafzai M.T., Khaliq A., Karim S., memon H., Junejo A., et al. Adverse events following immunization with typhoid conjugate vaccine in an outbreak setting in Hyderabad, Pakistan. Vaccine. 2020;38(19):3518–3523. doi: 10.1016/j.vaccine.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qamar F.N., Batool R., Qureshi S., Ali M., Sadaf T., Mehmood J., et al. Strategies to Improve Coverage of Typhoid Conjugate Vaccine (TCV) Immunization Campaign in Karachi, Pakistan. Vaccines. 2020;8(4):697. doi: 10.3390/vaccines8040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poncin M., Zulu G., Voute C., Ferreras E., Muleya C.M., Malama K., et al. Implementation research: reactive mass vaccination with single-dose oral cholera vaccine, Zambia. Bull World Health Organ. 2018;96(2):86–93. doi: 10.2471/BLT.16.189241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winstead A. Notes from the Field: Cholera Outbreak — Zimbabwe, September 2018–March 2019. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2022 May 7];69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6917a3.htm. [DOI] [PMC free article] [PubMed]
- 43.Revolt and Repression in Zimbabwe [Internet]. Crisis Group. 2019 [cited 2022 May 7]. Available from: https://www.crisisgroup.org/africa/southern-africa/zimbabwe/revolt-and-repression-zimbabwe.