Abstract
Bacterial antimicrobial resistance (AMR) continues to develop, with the horizontal transfer of antibiotic resistance genes (ARGs) through plasmids playing a major role. Recently, the antimicrobial resistance of R. anatipestifer has become increasingly severe, jeopardizing the development of the poultry industry. In this study, we used PromethION to determine the whole genome sequence of R. anatipestifer RCAD0416, a multidrug-resistant isolate from China. We detected a plasmid in the isolate. We named the plasmid pRCAD0416RA-1; the plasmid was 37356 bp in size with 36 putative open reading frames and included the blaOXA-347, floR, tet(X), ermF, ereD, and AadS resistance genes. Most resistance genes might be obtained from R. anatipestifer HXb2. Mobile elements and floR might be transmitted by plasmid pB18–2 from Acinetobacter indicus, and the ICEPg6Chn1 mobile elements can be transmitted from Proteus genomosp. The plasmid pRCAD0416RA-1 was transferred to Escherichia coli K-12 × 7232 via electroporation. Subsequent antimicrobial sensitivity tests (AST) showed a noticeable levels of antimicrobial resistance to β-lactams (4–8 fold), tigecycline (8 fold), and florfenicol (8 fold). These types of antibiotics are in common clinical use. The purpose of this article is to elucidate the basic characteristics of pRCAD0416RA-1 and the level of resistance mediated by blaOXA-347, floR, and tet(X).
Key words: blaOXA, floR, plasmid-mediated resistance, Riemerella anatipestifer, tigecycline, tet(X)
INTRODUCTION
Riemerella anatipestifer is the main pathogen of avian septicemic and exudative diseases. Commonly used antimicrobial agents against these pathogens are tetracycline, β-lactams, fluoroquinolones, aminoglycosides, and quinolones. Reports on R. anatipestifer have shown antimicrobial resistance (AMR) worldwide (Zhong et al., 2009; Nhung et al., 2017). The resistance spectrum of R. anatipestifer is becoming increasingly wider, and multidrug resistance is extremely severe. The occurrence of AMR (like resistance to erythromycin, florfenicol, tigecycline) is mostly mediated by antibiotic resistance genes (ARGs) (like ermF, floR, tet(X). Acquired ARGs can be carried by plasmids from the environment and other bacteria (Agyare et al., 2018). Existing R. anatipestifer plasmid on NCBI, the database shows that most plasmids have been isolated from strains in Taiwan (Weng et al., 1999; Chen et al., 2010; Guo et al., 2017).
Florfenicol is an animal-specific antibacterial drug that plays an important role in veterinary clinical medication. Chloramphenicol/florfenicol efflux MFS transporter floR causes bacterial resistance to florfenicol and chloramphenicol. Currently, tigecycline is one of the last-resort antibiotics for treating bacterial infection. The rampant utilization of tetracycline has resulted in an increase in the tetracycline-resistant strains (Zhu et al., 2018). Tetracyclines modifying enzyme Tet(X) degrades the following compounds: chlortetracycline, demeclocycline, doxycycline, minocycline, oxytetracycline, tetracycline, and tigecycline (Garcia-Echauri et al., 2015). The emergence and spread of tet(X) has hindered the treatment of bacterial diseases. Class D β-lactamase that encodes an amoxicillin resistance gene blaOXA-347 have been reported to cause changes in sensitivity to cefotaxime, imipenem, and penicillin (Zangenah et al., 2017). This gene has been found in the gut microbiomes of humans and wild and domestic animals, as well as in raw wastewater (Cheng et al., 2012; Bougnom et al., 2020; Loo et al., 2020).
Since 2012, there have been few reports on plasmids in R. anatipestifer (Chen et al., 2012; Li et al., 2022). In this study, we found a plasmid in R. anatipestifer isolate RCAD0416 from Sichuan, China. This plasmid mediates multidrug resistance and can spread among different bacteria. The emergence of multidrug-resistance plasmids enables the spread of resistance genes.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, Primers and Culture Sonditions
R. anatipestifer RCAD0416 was isolated from the respiratory tract of a healthy adult duck in Mianyang, Sichuan Province, China in 2017. Details of the bacterial strains and plasmids used in this study are listed in Table 1.
Table 1.
The strains and plasmids used in this study.
| Strain or plasmid | Descriptions | Resource or references |
|---|---|---|
| Strains R. anatipestifer ATCC 11845 |
Type strain | ATCC |
| R. anatipestifer RCAD0416 | Wild strain | Isolated from healthy adult duck. Sichuan, China |
|
E. coli DH5α E. coli DH5α (pET28a-D1J36_09695) E. coli DH5α (pET28a-D1J36_09740) E. coli DH5α (pET28a-D1J36_09870) E. coli DH5α (pBAD24) E. coli DH5α (pBAD24-blaOXA-347) |
Cloning host cell DH5α carrying pET28a-D1J36_09695 DH5α carrying pET28a-D1J36_09740) DH5α carrying pET28a-D1J36_09870) DH5α carrying pBAD24 DH5α carrying pBAD24-blaOXA-347 |
Laboratory collection This study This study This study This study This study |
|
E. coli K-12 × 7232 E. coli K-12 × 7232 (pRCAD0416RA-1) E. coli BL21(DE3) E. coli BL21(DE3) (pET28a) E. coli BL21(DE3) (pET28a-blaOXA-347) |
EndA1 hsdR17 (rK-mk+) glnV44 thi-1 recA1 gyrA relA1Δ(lacZYA-argF)U169λpir deoR (Φ80dlac Δ(lacZ)M15) E. coli K-12 × 7232 carrying pRCAD0416RA-1, FFCR expressing host cell E. coli BL21(DE3) carrying pET28a E. coli BL21(DE3) carrying pET28a-blaOXA-347 |
Roland et al. (1999) Laboratory collection Laboratory collection This study This study |
| Plasmids | ||
| pET28a pBAD24 |
E.coli protein expression plasmid, IPTG-inducible promoter, KanR E. coli expression vector containing the arabinose pBAD promoter. AMPR |
Laboratory collection Guzman et al. (1995) |
Abbreviations: ATCC, American type culture collection; AMP, ampicillin; FFC, florfenicol; KAN, kanamycin.
R. anatipestifer was cultured on tryptic soy agar (Oxoid Ltd., Basingstoke, UK) or in tryptic soy broth (Oxoid Ltd.) at 37°C. E. coli was cultured on Lysogeny Ager plates or Mueller–Hinton broth at 37°C. The following antibiotics were added, as required: 10 μg/mL florfenicol (FFC), 40 μg/mL kanamycin (KAN) or 100 μg/mL ampicillin (AMP).
Antibiotics Susceptibility Test
The following antibiotics were used in bacterial sensitivity tests: pipemidic acid (PIP), florfenicol (FFC), colistin (COL), azithromycin (AZI), erythromycin (ERM), norfloxacin (NOR), enrofloxacin (ENR), streptomycin (S); chloramphenicol (C), gentamicin (CN), lincomycin (MY), tetracycline (TET), tigecycline (TGC), doxycycline (DOX), ampicillin (AMP), cefoxitin (FOX), cephradine (CH), oxytetracycline (OTC), minocycline (MIN), chlortetracycline (CTC), demeclocycline (DMC), cephalothin (CE), amoxicillin (AMO), imipenem (IPM), and cefepime (FEP). The above mentioned antibiotics were stored at a concentration of 10,240 μg/mL. All antibiotics were obtained from Dalian Meilun Biotech Co., Ltd. (Dalian, China).
Minimum inhibitory concentrations (MICs) were determined according to the performance standard of the Clinical and Laboratory Standards Institute (CLSI) (VET01, 2018). Hundred microlitre of bacterial solution and 100 μL of antibiotics of different concentrations were added to the 96-well microtiter plate, the final concentrations of the antibiotics ranged from 0.25 to 512 μg/mL. The R. anatipestifer isolate was diluted during the logarithmic growth period with TSB to OD600 = 0.04, and the concentration of the bacterial inoculum was approximately 106 CFU/mL. The inoculated microplates were incubated at 37°C for 24 h (Zhu et al., 2018). All studies were carried out in triplicate.
Whole-Genome Sequencing and Analysis
To obtain genetic information for RCAD0416, high-quality genomic and plasmid DNA were extracted with a TIANamp Bacteria DNA Kit (Tiangen Biotech Co, Ltd., Beijing, China). Genome sequencing of R. anatipestifer RCAD0416 was conducted using PromethION (Oxford Nanopore Technologies, ONT) and Illumina PE150. Canu v1.5 (Koren et al., 2017) was used to assemble the filtered subreads. The assembled results were polished twice by Racon v3.4.3 (https://github.com/isovic/racon) (Vaser et al., 2017) using long reads. The output was further polished once by Pilon (v1.24, parameters, “—fix all”) (Walker et al., 2014) with the 100 × Illumina reads. Final assemblies were trimmed, oriented and circularized using Circlator (v1.5.5, default parameters, https://sanger-pathogens.github.io/circlator/) (Hunt et al., 2015). Gene prediction was performed using Prodigal v2.6.3 (Hyatt et al., 2010) and NCBI Prokaryotic Genome Annotation Pipeline (PGAP, https://www.ncbi.nlm.nih.gov/genome/annotation_prok/) (Li et al., 2021). The genome completeness of the strain was evaluated using the CheckM14 genome quality estimator (Parks et al., 2015). Acquired antimicrobial resistance genes were predicted using the Comprehensive Antibiotic Resistance Database (Alcock et al., 2020)(CARD) and ResFinder 4.1 (Zankari et al., 2017; Bortolaia et al., 2020) (https://cge.cbs.dtu.dk/services/ResFinder/). The whole-genome sequence of R. anatipestifer RCAD0416 and plasmid sequence of pRCAD0416RA-1 have been deposited in GenBank under the accession number CP073239.1 and CP073240.1, respectively.
Transformation of pRCAD0416RA-1
To explore the resistance level mediated by the resistance gene for pRCAD0416RA-1, we transferred pRCAD0416RA-1 into E. coli K-12 × 7232 via electroporation. The DH5α bacterial solution was inoculated into LB broth and cultured to OD600 = 0.8. To prepare competent cells, the culture was centrifuged at 4000 r/min at 4°C for 10 min, use 30 mL chilled ddH2O to resuspend cells, repeat the centrifugation and resuspension steps 2 times. Use 5 mL chilled 10% glycerol resuspend cells, centrifugal, 2 to 3 mL chilled 10% glycerol to resuspend again, dispense 100 μL of each tube into EP tubes. Store at −80°C. Then, pRCAD0416RA-1 was extracted by TIANprep Mini Plasmid Kit (Tiangen Biotech Co, Ltd., Beijing, China) and used nanodrop to measure the concentration. 0.3 μg, 0.5 μg, and 1 μg of plasmid were added to the competent cells. Electric shock conversion was performed under an electric field strength of 2.5 kV/cm. A volume of 200 μL of LB was rapidly added to the cells. The cells were incubated at 37°C for 30 min. Transformants were selected using LB agar plates containing florfenicol (10 μg/mL) (Zhao et al., 2018). The transformation efficiency was reported as the number of transformants/μg of DNA (Papagianni et al., 2007). Each experiment was repeated thrice.
Expression of blaOXA-347 and tet(X) Genes in E. coli
To confirm the function of blaOXA-347 in β-lactam resistance, blaOXA-347 was amplified with the primers pBAD24-blaOXA-347-F (5’-GGAATTCgttgtgcgtcatctttgtgcgacag-3’) and pBAD24-blaOXA-347-R (5’-CCCAAGCTTggcacgaaagccggcaattatcggc-3’). The amplicon was linked to pBAD24 by restriction enzyme ligation. Three tet(X) genes in pRCAD0416RA-1 were amplified using primers with EcoRI and HindIII by PCR. The genes were then cloned into plasmid pBAD24 to produce pBAD24-tet(X). The 4 new plasmids were transferred to DH5α and culture on Luria–Bertani agar (LB) medium containing 100 μg/mL ampicillin. The transformants were verified by PCR and subjected to susceptibility tests using the broth microdilution method (Sun et al., 2020).
Protein tet(X) Structure Homology Modeling Using SwissModel
The amino acid sequences of the three tet(X) proteins on the plasmid were uploaded to SwissModel (Waterhouse et al., 2018).
Overexpression of the blaOXA-347 gene in E. coli BL21
We used the plasmid ligation method described above to construct pET28a-blaOXA-347. Then, we transferred pET28a-blaOXA-347 to the E. coli BL21 strain. The successful transformants were grown overnight at 37°C in LB containing 50 µg/mL kanamycin under continuous shaking (Dey et al., 2021). The cells were diluted with fresh LB (1:100) and cultured to OD600 = 0.6, after which 0.5 mM/L isopropyl β-D-1-thiogalactopyranoside (IPTG) was added. The cells were incubated for 4 h at 30°C under shaking at 180 r/m.
RESULTS
A Resistance Plasmid, pRCAD0416RA-1, Carrying the tet(X), ereD, ermF, Aads, blaOXA-347, and floR Genes, was Found in R. anatipestifer
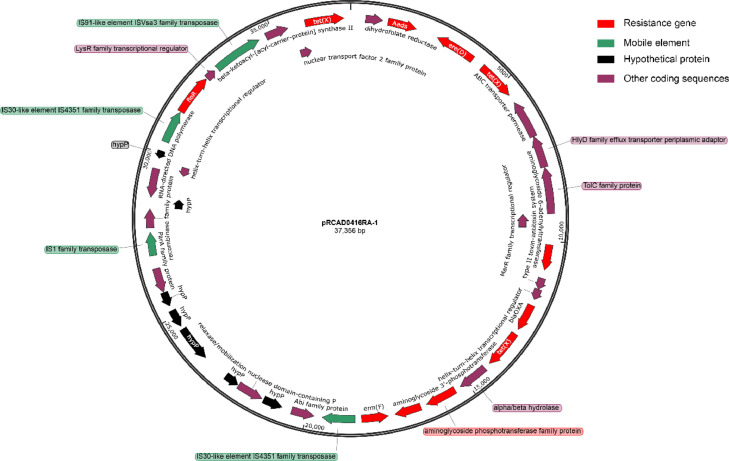
A multidrug-resistant RCAD0416 was isolated from ducks from Sichuan, China. The RCAD0416 strain was resistant to β-lactams, aminoglycosides, tetracyclines, macrolides, and florfenicol (Table 2). We used bacterial genome third-generation sequencing technology and a bioinformatics platform to construct and analyze the RCAD0416 genome completion map. We identified a plasmid in RCAD0416. We named the plasmid pRCAD0416RA-1. This circular plasmid consists of 37,356 nucleotides, has a GC content of 36.47%, and encodes 36 genes. The length of the gene coding region was 30,109 bp (80.6%). Seven of the predicted ORFs were hypothetical proteins; the other ORFs were assigned functions; where 8 ORFs were resistance genes. The genetic prediction results showed that pRCAD0416RA-1 is a resistance (R) plasmid. The genotype of the resistance gene was consistent with the antibiotic sensitivity (Figure 1). To determine the transferability of pRCAD0416RA-1, we used electroporation to transfer pRCAD0416RA-1 to E. coli K-12 × 7232. The transformation efficiency of electroporation was 8.82 × 102 CFU/μg DNA. The R plasmid caused a maximum 8-fold increase in the antibiotic sensitivity of the recipient. The most significant sensitivity changes were observed for β-lactams, florfenicol, chloramphenicol and tetracyclines. There was a 4- to 8-fold increase in the MIC of these antibiotics.
Table 2.
MICs values of antibiotics tested in this study.
| Strains | MIC (mg/L) |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pipemidic Acids | Chloramphenicol |
Colistins | Macrolides |
Aminoglycosides |
Lincomycins | Tetracyclines |
β-lactams |
|||||||||||||||||
| PIP | C | FFC | COL | CLA | ERM | NOR | ENR | S | CN | MY | TET | TGC | OTC | MIN | CTC | DOX | DMC | AMP | FOX | CE | AMO | IPM | CEF | |
| RCAD0416 | 128 | 32 | 32 | >1024 | 512 | 64 | 32 | 32 | 256 | 256 | 512 | 32 | 16 | 128 | 0.5 | 1 | ||||||||
| 7232 | 32 | 8 | 8 | 4 | 4 | 128 | 64 | <0.25 | 8 | 2 | 512 | 8 | 1 | 4 | 16 | 64 | ||||||||
| 7232-pRCAD0416RA-1 | 64 | 32 | 64 | 4 | 4 | 256 | 128 | <0.25 | 8 | 1 | 512 | 32 | 8 | 32 | 128 | 128 | ||||||||
| DH5α-pBAD24 | 4 | 0.5 | 32 | 4 | 16 | 8 | 2 | |||||||||||||||||
| DH5α-pBAD24-tetX1 | 8 | 4 | 64 | 4 | 32 | 8 | 4 | |||||||||||||||||
| DH5α-pBAD24-tetX2 | 8 | 4 | 32 | 4 | 32 | 8 | 4 | |||||||||||||||||
| DH5α-pBAD24-tetX3 | 8 | 1 | 32 | 4 | 32 | 8 | 4 | |||||||||||||||||
| BL21-pET28a | 4 | 4 | 4 | 2 | 2 | 4 | ||||||||||||||||||
| BL21-pET28a-blaOXA347 | 256 | 32 | 8 | 2 | 2 | 128 | ||||||||||||||||||
| DH5α-pBAD24 | >512 | 4 | 4 | 4 | 0.5 | 4 | ||||||||||||||||||
| DH5α-pBAD24-blaOXA347 | >512 | 32 | 8 | 4 | 0.5 | 4 | ||||||||||||||||||
Abbreviations: AMP, Ampicillin; AMO, Amoxicillin; AZI, Azithromycin; C, Chloramphenicol; CN, Gentamicin; COL, Colistin; CTC, Chlortetracycline; CH, Cephradine; CE, Cephalothin; DMC, Demeclocycline; DOX, Doxycycline; ENR, Enrofloxacin; ERM, Erythromycin; FEP, Cefepime; FFC, Florfenicol; FOX, Cefoxitin; IPM, Imipenem; MIN, Minocycline; MY, Lincomycin; NOR, Norfloxacin; PIP, Pipemidic Acid; S, Streptomycin; OTC, Oxytetracycline; TET, Tetracycline; TGC, Tigecycline.
Figure 1.
The plasmid profile of pRCAD0416RA-1 from R. anatipestifer strain RCAD0416. Genes with different functions are marked with different colors: red, antimicrobial resistance gene; green, mobile element; black, hypothetical protein; purple, other function.
AMR Genes tet(X), blaOXA-347, and floR Mediate R. anatipestifer Resistance to Tigecycline, β-lactams, Florfenicol and Chloramphenicol
To compare the activity of the 3 plasmid-carrying tet(X) (D1J36_09695, D1J36_09740 and D1J36_09870), we constructed the 3 plasmids and conducted a susceptibility analysis. At the genetic level, the similarity between D1J36_09695 and D1J36_09740 was very high, reaching 99.57%. The amino acid sequence similarity for these 2 genes reached 98.97%. D1J36_09870 was quite different from the other 2 genes. In particular, 10 amino acids from the sequence of the other 2 proteins are deleted in the D1J36_09870 sequence. Existing reports on tet(X) show that changes or deletions in front of the amino acids encoded by tet(X) will create new tet(X) variants (Sun et al., 2019). Therefore, we speculate that amino acid deletions or mutations in this region will cause changes in the function of the tet(X) gene. The susceptibility test results provided evidence for our conjecture. The gene functions showed that the D1J36_09695 and D1J36_09740 have the same ability to mediate bacterial resistance to tetracycline drugs. D1J36_09695 and D1J36_09740 induce a higher level of resistance in bacteria than D1J36_09870. D1J36_09695 has 93.70% sequence identity with tetracycline destructase tet(X7). D1J36_09740 and D1J36_09870 have 91.80% and 87.57% sequence identity, respectively, with the tet(X2) protein. The protein structure model (Figure 2) further supports that the 3 proteins belong to the tet(X) family. We also found that coexistence of the 3 genes in pRCAD0416RA-1 did not increase bacterial resistance to tetracycline. Multiple copies of the tet(X) gene are common in R. anatipestifer (Umar et al., 2021). This high carrying rate motivated us to explore the gene source. Most of the hits obtained for tet(X) using BLAST on NCBI were Flavobacteriales, Weeksellaceae, and Riemerella. Studies have identified Flavobacteriaceae (Zhang et al., 2020) or Riemerella as potential ancestral sources of the tigecycline resistance gene tet(X). The BLAST results provided evidence for these inferences.
Figure 2.
Structural modeling of (A) D1J36_09695, (B) D1J36_09740 and (C) D1J36_09870; cartoon representations of models of (D) tet(X7) (salmon) and D1J36_09695 (cyan), (E) tet(X2) (hot pink) and D1J36_09740 (yellow), and (F) tet(X2) (purple), and D1J36_09870 (deep blue). The arrow indicates a difference between models.
The gene blaOXA-347 is a class D beta-lactamase. In previous reports, blaOXA-347 was found to confer amoxicillin and meropenem resistance to bacteria (Zangenah et al., 2017). However, we did not find expression of this resistance gene in subsequent experiments. We found that this gene, especially after overexpression, mediates resistance to other β-lactam antibiotics, such as ampicillin, cephalothin, and cefoxitin (Table 1). blaOXA-347 can be transmitted between bacteria through transposons (Zangenah et al., 2017). Analysis of the upstream and downstream gene functions of blaOXA-347 revealed the IS30-like element IS4351 family transposase (Figure 1). This transposition pattern is also present in human gut flora (Cheng et al., 2012).
RCAD0416 is resistant to florfenicol and chloramphenicol. The MIC of florfenicol in transformants with pRCAD0416RA-1 increased 8-fold (from 8 mg/L to 64 mg/L), and the MIC of chloramphenicol increased 4-fold (from 8 mg/L to 32 mg/L). These results indicate that the resistance of the RCAD0416 strain to florfenicol may derive from this plasmid.
Fragments on Plasmids From Genome and Plasmids
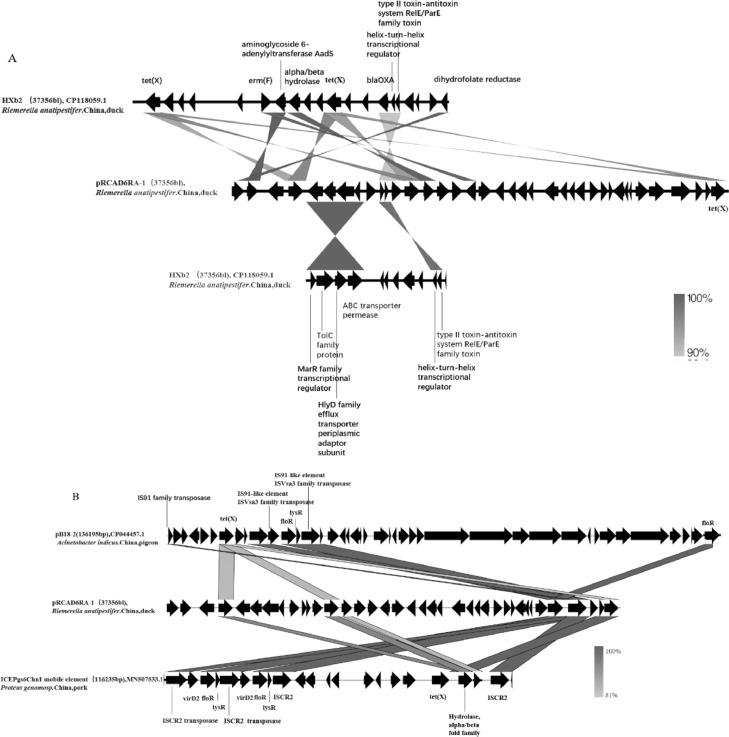
The BLAST results from NCBI showed that the plasmid with the highest similarity was R. anatipestifer HXb2, with plasmid sequence coverage reaching 39%. Figure 3A shows that most of the resistance genes, such as ermF, tet(X), blaOXA-347, and AadS, were derived from the genome.
Figure 3.
(A) Comparison of genomic fragment sequence of plasmid pRCAD0416RA-1 from R. anatipestifer strain RCAD0416 with the genome sequence of R. anatipestifer HXb2 (CP118059.1) (>90%) in the GenBank database, indicated by grey shading. (B) Comparison of genomic fragment sequence of plasmid pRCAD0416RA-1 from R. anatipestifer strain RCAD0416 with the sequence of plasmid pB18–2(CP044457.1) and ICEPg6Chn1 mobile element (MN507533.1) retrieved from GenBank, indicated by gray shading. The grey shadow indicates a genetic similarity above 81%.
We also observed two matches, the Acinetobacter indicus strain B18 plasmid pB18–2 (18% coverage) and Proteus genomosp. 6 strain T60 ICEPgs6Chn1 mobile element (22% coverage) (Figure 3B). The coverage of other matches was lower than 9%. The mobile elements on this plasmid are also the same as the above 2 plasmids.
DISCUSSION
The first plasmid in R. anatipestifer reported on NCBI (https://www.ncbi.nlm.nih.gov/) was found in Taiwan (Chang et al., 1998). We used the third-generation sequencing method to discover the plasmid from an R. anatipestifer strain in mainland China. Most of the fragments, especially the resistance genes, on the plasmid are from the HXb2 genome. However, there are no mobile elements related to transposition in these fragments. Mobile elements on this plasmid are from other plasmids and ICE. As the sources of the resistance genes and mobile elements are very different, it can be speculated that pRCAD0416RA-1 has recombined at least twice. Existing reports have demonstrated resistance genes of R. anatipestifer resulting from natural conversion. The emergence of multidrug-resistance plasmids is a means of disseminating resistance genes.
We tried to transfer the plasmid and found that its transfer ability was poor. We tried unsuccessfully to transfer the plasmid to ATCC 11845 and RA-CH-1 by natural transformation and to DH5α by CaCl2 transformation (data not shown). Only electroporation was successful for plasmid transfer. The conversion efficiency was low. As we mentioned earlier, the full length of pRCAD0416RA-1 is 37356 bp, which may affect plasmid transformability. Electroporation creates holes in a cell membrane through transient currents, allowing DNA from foreign macromolecules to enter the cell. Apart from the current study, few reports on plasmids in R. anatipestifer have appeared since 2012. The scarcity of plasmids may explain their poor transformability. According to the results of the plasmid sequence alignment, the plasmid may have undergone recombination. Mobile originals on plasmids provide opportunities for recombination. Under the influence of the transposon, floR can transfer this (Walker et al., 2014) plasmid from other bacilli. We found that concomitant existence of tet(X) in the sequenced R. anatipestifer genome with blaOXA (Li et al., 2022). This type of adjoining mode also exists in pRCAD0416RA-1.
We transferred the plasmid to E. coli K-12 × 7232 and found changes in the antibiotic sensitivity to β-lactams, tetracyclines, florfenicol, and chloramphenicol. An analysis of the sequencing results showed that the plasmid carries resistance genes for β-lactams, florfenicol, aminoglycosides, tetracyclines, and erythromycin. Class D β-lactamase blaOXA-347 has been found in the gut microbiomes of humans and wild and domestic animals, as well as in raw wastewater (Cheng et al., 2012; Bougnom et al., 2020; Loo et al., 2020). This type of β-lactamase confers carbapenem resistance to bacteria. The resistance gene blaOXA-347 has been reported to mediate bacterial resistance to amoxicillin. However, in this study, we did not find that blaOXA-347 conferred carbapenem and amoxicillin resistance to R. anatipestifer and E. coli. A gene function analysis showed that only overexpression of blaOXA-347 resulted in an increased level of resistance. We have found in previous studies that strains (Capnocytophga cynodegmi and Capnocytophga stomatis) carrying this gene are resistant to cefotaxime, imipenem, penicillin G, and amoxicillin. However, the resistance phenotype cannot represent the resistance level of blaOXA-347 because no single gene function has been verified. The resistance phenotype may also be affected by other factors, such as the existence of a nonspecific efflux mechanism and unknown resistance genes or resistance mechanisms. The blaOXA-347 protein has 88.85% identity and 98.35% coverage with blaOXA-347 in the strains mentioned above. Moreover, pRCAD0416RA-1 is a low-copy-number plasmid vector. The copy number of a plasmid affects the resistance level. An increase in the plasmid copy number has been found to contribute to elevated carbapenem resistance in K. pneumoniae carrying blaOXA (Shen et al., 2020). This result may explain the low resistance to β-lactam antibiotics.
There are three tet(X) genes in pRCAD0416RA-1. Two of these genes mediates bacterial resistance to tigecycline, and the resistance level is low (8-fold). These genes have been found to mediate low-level tigecycline resistance. This result was confirmed in a study on tet(X14) (identity and coverage >99%) (Cheng et al., 2020). The third gene does not have a clear resistance phenotype but can also change the sensitivity of E. coli to tetracycline. Multiple copies of tet(X) commonly exist in R. anatipestifer (Umar et al., 2021; Li et al., 2022). This phenomenon has also been reported in Gram-positive bacteria (Li et al., 2020). We are thus led to consider the significance of this coexistence relationship. A high-level structural analysis shows that all 3 proteins belong to the tet(X) family. However, there are differences among the structures and ligand binding sites of the 3 proteins. However, the resistance phenotypes of D1J36_09695 and D1J36_09740 are highly similar. The existence of multiple tet(X) has no superposition or synergistic effect on the resistance level. Moreover, when R. anatipestifer carries multiple tet(X), the level of resistance is not necessarily higher than that mediated by a single tet(X).
In summary, we report the discovery of wild plasmid pRCAD0416RA-1 in R. anatipestifer. This plasmid carries a gene that mediates resistance to β-lactams, erythromycin, florfenicol, chloramphenicol, tetracyclines, and aminoglycosides. We transformed the plasmid into other strains through experiments and verified the mobility of the plasmid. We explored the function of plasmid-mediated tet(X), floR, and blaOXA-347 and analyzed the origin of genes. We concluded that the resistance genes on the plasmid were transferred from the genome or plasmid of other strains through recombination.
Disclosures
The authors declare no competing interests.
REFERENCES
- Agyare C., Boamah V.E., Osei C.N.Z.a.B., et al. Antibiotic use in poultry production and its effects on bacterial resistance. Antimicrobial Resistance - A Global Threat. K. Yashwant, ed. IntechOpen, London. 2018:1–20. [Google Scholar]
- Alcock B.P., Raphenya A.R., Lau T.T.Y., et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–d525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolaia V., Kaas R.S., Ruppe E., Roberts M.C., Schwarz S., Cattoir V., Philippon A., Allesoe R.L., Rebelo A.R., Florensa A.F., Fagelhauer L., Chakraborty T., Neumann B., Werner G., Bender J.K., Stingl K., Nguyen M., Coppens J., Xavier B.B., Malhotra-Kumar S., Westh H., Pinholt M., Anjum M.F., Duggett N.A., Kempf I., Nykasenoja S., Olkkola S., Wieczorek K., Amaro A., Clemente L., Mossong J., Losch S., Ragimbeau C., Lund O., Aarestrup F.M. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnom B.P., Thiele-Bruhn S., Ricci V., et al. Raw wastewater irrigation for urban agriculture in three African cities increases the abundance of transferable antibiotic resistance genes in soil, including those encoding extended spectrum β-lactamases (ESBLs) Sci.Total Environ. 2020;698 doi: 10.1016/j.scitotenv.2019.134201. [DOI] [PubMed] [Google Scholar]
- Chang C.F., Hung P.E., Chang Y.F. Molecular characterization of a plasmid isolated from Riemerella anatipestifer. Avian Pathol. 1998;27:339–345. doi: 10.1080/03079459808419349. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Lee S.H., Chou C.H., et al. Detection of florfenicol resistance genes in Riemerella anatipestifer isolated from ducks and geese. Vet. Microbiol. 2012;154:325–331. doi: 10.1016/j.vetmic.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Tsao M.Y., Lee S.H., et al. Prevalence and molecular characterization of chloramphenicol resistance in Riemerella anatipestifer isolated from ducks and geese in Taiwan. Avian Pathol. 2010;39:333–338. doi: 10.1080/03079457.2010.507761. [DOI] [PubMed] [Google Scholar]
- Cheng G., Hu Y., Yin Y., et al. Functional screening of antibiotic resistance genes from human gut microbiota reveals a novel gene fusion. FEMS Microbiol. Lett. 2012;336:11–16. doi: 10.1111/j.1574-6968.2012.02647.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen Y., Liu Y., et al. Identification of novel tetracycline resistance gene tet(X14) and its co-occurrence with tet(X2) in a tigecycline-resistant and colistin-resistant Empedobacter stercoris. Emerg. Microbes Infect. 2020;9:1843–1852. doi: 10.1080/22221751.2020.1803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey C., Thool M., Bhattacharyya S., et al. Generation of biologically active recombinant human OCT4 protein from E. coli 3. Biotech. 2021;11:207. doi: 10.1007/s13205-021-02758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Echauri S.A., Cardineau G.A. TETX: a novel nuclear selection marker for Chlamydomonas reinhardtii transformation. Plant Methods. 2015;11:27. doi: 10.1186/s13007-015-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Hu D., Guo J., et al. The role of the regulator fur in gene regulation and virulence of Riemerella anatipestifer assessed using an unmarked gene deletion system. Front. Cell Infect. Microbiol. 2017;7:382. doi: 10.3389/fcimb.2017.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M., De Silva N., Otto T.D., et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015:16. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt D., Chen G.L., Locascio P.F., et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformat. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S., Walenz B.P., Berlin K., et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Jiang Y., Peng K., et al. Phenotypic and genomic analysis reveals Riemerella anatipestifer as the potential reservoir of tet(X) variants. J. Antimicrob. Chemother. 2022;77:374–380. doi: 10.1093/jac/dkab409. [DOI] [PubMed] [Google Scholar]
- Li R., Lu X., Peng K., et al. Deciphering the structural diversity and classification of the mobile tigecycline resistance gene tet(X)-bearing plasmidome among bacteria. mSystems. 2020;5 doi: 10.1128/mSystems.00134-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., O’Neill K.R., Haft D.H., DiCuccio M., Chetvernin V., Badretdin A., Coulouris G., Chitsaz F., Derbyshire M.K., Durkin A.S., Gonzales N.R., Gwadz M., Lanczycki C.J., Song J.S., Thanki N., Wang J., Yamashita R.A., Yang M., Zheng C., Marchler-Bauer A., Thibaud-Nissen F. RefSeq: expanding the Prokaryotic Genome Annotation Pipeline reach with protein family model curation. Nucleic Acids Res. 2021;49:D1020–D1028. doi: 10.1093/nar/gkaa1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo E.X.L., Zain A., Yap G.C., et al. Longitudinal assessment of antibiotic resistance gene profiles in gut microbiomes of infants at risk of eczema. BMC Infect. Dis. 2020;20:312. doi: 10.1186/s12879-020-05000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens: a review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianni M., Avramidis N., Filioussis G. High efficiency electrotransformation of Lactococcus lactis spp. lactis cells pretreated with lithium acetate and dithiothreitol. BMC Biotechnol. 2007;7:15. doi: 10.1186/1472-6750-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D.H., Imelfort M., Skennerton C.T., et al. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Zhang H., Gao Q., et al. Increased plasmid copy number contributes to the elevated carbapenem resistance in OXA-232-producing Klebsiella pneumoniae. Microb. Drug Resistance. 2020;26:561–568. doi: 10.1089/mdr.2018.0407. [DOI] [PubMed] [Google Scholar]
- Sun J., Chen C., Cui C.Y., et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019;4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Gao H., Liu Y., et al. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg. Microbes Infect. 2020;9:1102–1113. doi: 10.1080/22221751.2020.1768805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar Z., Chen Q., Tang B., et al. The poultry pathogen Riemerella anatipestifer appears as a reservoir for Tet(X) tigecycline resistance. Environ. Microbiol. 2021;23:7465–7482. doi: 10.1111/1462-2920.15632. [DOI] [PubMed] [Google Scholar]
- Vaser R., Sovic I., Nagarajan N., et al. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.J., Abeel T., Shea T., et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A., Bertoni M., Bienert S., et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–w303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S., Lin W., Chang Y., et al. Identification of a virulence-associated protein homolog gene and ISRa1 in a plasmid of Riemerella anatipestifer. FEMS Microbiol. Lett. 1999;179:11–19. doi: 10.1111/j.1574-6968.1999.tb08701.x. [DOI] [PubMed] [Google Scholar]
- Zangenah S., Andersson A.F., Özenci V., et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:657–662. doi: 10.1007/s10096-016-2842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E., Allesøe R., Joensen K.G., et al. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017;72:2764–2768. doi: 10.1093/jac/dkx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Dong N., Shen Z., et al. Epidemiological and phylogenetic analysis reveals Flavobacteriaceae as potential ancestral source of tigecycline resistance gene tet(X) Nat. Commun. 2020;11:4648. doi: 10.1038/s41467-020-18475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liang S., Dai Q., et al. Regulated delayed attenuation enhances the immunogenicity and protection provided by recombinant Salmonella enterica serovar Typhimurium vaccines expressing serovar Choleraesuis O-polysaccharides. Vaccine. 2018;36:5010–5019. doi: 10.1016/j.vaccine.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Zhong C.Y., Cheng A.C., Wang M.S., et al. Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Dis. 2009;53:601–607. doi: 10.1637/8552-120408-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Zhu D.K., Luo H.Y., Liu M.F., et al. Various profiles of tet genes addition to tet(X) in Riemerella anatipestifer isolates from ducks in China. Front. Microbiol. 2018;9:585. doi: 10.3389/fmicb.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]