Abstract
This study reports that hairy and enhancer of split homolog-1 (HES1), known to repress gene transcription in progenitor cells of several cell lineages, was strongly expressed in cells and tissues of T-cell lymphoma expressing the oncogenic chimeric tyrosine kinase nucleophosmin (NPM)–anaplastic lymphoma kinase [ALK; ALK+ T-cell lymphoma (TCL)]. The structural analysis of the Orange domain of HES1 indicated that HES1 formed a highly stable homodimer. Of note, repression of HES1 expression led to inhibition of ALK+ TCL cell growth in vivo. The expression of the HES1 gene was induced by NPM-ALK through activation of STAT3, which bound to the gene's promoter and induced the gene's transcription. NPM-ALK also directly phosphorylated HES1 protein. In turn, HES1 up-regulated and down-regulated in ALK+ TCL cells, the expression of numerous genes, protein products of which are involved in key cell functions, such as cell proliferation and viability. Among the genes inhibited by HES1 was thioredoxin-interacting protein (TXNIP), encoding a protein implicated in promotion of cell death in various types of cells. Accordingly, ALK+ TCL cells and tissues lacked expression of TXNIP, and its transcription was co-inhibited by HES1 and STAT3 in an NPM-ALK–dependent manner. Finally, the induced expression of TXNIP induced massive apoptotic cell death of ALK+ TCL cells. The results reveal a novel NPM-ALK–controlled pro-oncogenic regulatory network and document an important role of HES and TXNIP in the NPM-ALK–driven oncogenesis, with the former protein displaying oncogenic and the latter tumor suppressor properties.
Anaplastic lymphoma kinase (ALK), normally expressed only in immature neural cells,1 acts as a potent oncogene in diverse neoplasms, including distinct subsets of T- and B-cell lymphomas, inflammatory myofibroblastic tumors, lung adenocarcinoma, and familial and sporadic neuroblastomas.2, 3, 4 With the exception of neuroblastomas where the entire, yet mutated, ALK gene is expressed, expression of ALK in other malignancies stems from chromosomal rearrangements fusing the ALK gene and partner genes, largely specific for the given tumor type. In T-cell lymphomas (TCLs; ALK+ TCL), the nucleophosmin (NPM) gene is by far the most frequent fusion partner of the ALK gene.5 The resulting NPM-ALK chimeric protein is constitutively expressed and activated through autophosphorylation.6,7 NPM-ALK is highly oncogenic, both in vitro and in vivo.8, 9, 10, 11 Strikingly, it transforms normal CD4+ T lymphocytes into immortalized and tumor-forming cells morphologically and immunophenotypically indistinguishable from patient-derived ALK+ TCL cells and tissues.12 NPM-ALK executes its oncogenic function by high-jacking cell signaling pathways physiologically utilized by IL-2 and related cytokines,13 leading to the activation of cell signal transduction proteins, including STAT3.4,14 Persistent activation of these signal transmitters leads to chronic modulation of expression of genes, the protein products of which govern key cell functions, such as cell proliferation, apoptosis, evasion of the immune response,15, 16, 17 protection from the negative effects of hypoxia18,19 and preservation of NPM-ALK expression.4,20
Hairy and enhancer of split homolog-1 (HES1) belongs to the family of seven (HES 1 to 7) basic helix-loop-helix (bHLH) proteins that function as transcription factors.21,22 It plays a regulatory role in the control of cell cycle arrest, proliferation, cell differentiation, survival, and apoptosis in neuronal, endocrine, and T-lymphocyte progenitor cells and cancer cells. Of note, HES1 disruption leads to tumor regression without perturbing homeostasis of normal stem cells.23 HES1 is expressed at high levels in the hematopoietic stem cell–rich CD34+/[CD38/Lin]−/low subpopulation but at low levels in more mature progenitor cell populations. HES1 is highly expressed in the quiescent CD34+ hematopoietic precursor cells but becomes reduced in CD34+ cells that have divided,24 likely reflecting some degree of cell maturation. HES1 is also essential for the inhibition of hematopoietic stem cell differentiation toward the myeloid lineage, therefore supporting early T-lineage differentiation. It does so through repression of CCAAT/enhancer binding protein (C/EBP)-α, suggesting that HES1 is essential for T-cell lymphopoiesis.25 Signaling pathways involved in the up-regulation of HES1, Notch, Hedgehog, and Wnt, are frequently and aberrantly activated in cancer.23
Thioredoxin-interacting protein (TXNIP) (alias vitamin D3 up-regulating protein-1 or thioredoxin-binding protein-2) belongs to the superfamily of α-arrestin genes in humans. TXNIP is identified as a binding partner of thioredoxin (TRX), and is a key component of cellular oxidation-reduction regulation. Disruption of TXNIP by gene targeting in mice causes a predisposition to death with severe bleeding, hypoglycemia, and hyperinsulinemia, and to liver steatosis during fasting.26 TXNIP expression is regulated by nutrition status, feeding-fasting cycles, obesity, high glucose, and amino acids.27 TXNIP has been classified as a tumor suppressor because of its low expression observed in breast, lung, liver, gastric cancer, and glioma cells.28 Also, it is associated with tumorigenesis and metastasis. Interestingly, TXNIP is active in different compartments of the cell.29 In the cytoplasm, it binds to and inhibits TRX1 and thereby interferes with the ability of TRX1 to modulate the cellular oxidation-reduction state, resulting in oxidative stress and increased susceptibility to apoptosis.30 TXNIP can also enter the mitochondria, where it interacts with mitochondrial TRX2, releasing apoptosis signal-regulating kinase 1 (ASK1) from its inhibition by TRX2 and allowing for phosphorylation and activation of ASK1. This, in turn, leads to cytochrome c release from the mitochondria, cleavage of caspase-3, and apoptosis.31
The current study focused on the expression and function of HES1 and TXNIP in ALK+ TCL as well as the structure of the HES1 Orange domain. It found that HES1 is regulated by NPM-ALK on two levels through the induction of its gene expression via STAT3 and through direct phosphorylation of HES1 protein. In turn, HES1 together with STAT3 inhibited expression of the TXNIP gene to protect ALK+ TCL cells from apoptosis, indicating a key role of this novel network in the NPM-ALK–mediated malignant transformation of T lymphocytes.
Materials and Methods
Cells and Tissues
SUDHL-1, SUP-M2, JB6, Karpas 299, SR-786, and L-82 cell lines were derived from ALK+ TCL patients.12, 13, 14, 15, 16, 17, 18, 19, 20 Sez-4, SeAx, Myla2059, MyLa3675, (Mac)2A, and (Mac)2B were developed from primary skin lymphomas.13 The NA1 cell line was established by NPM-ALK gene transduction of CD4+ T cells.12 Peripheral blood mononuclear cells (PBMCs) harvested from healthy adults were isolated by Ficoll/Paque centrifugation and stimulated in vitro for 72 hours with a mitogen [phytchemagglutinin (PHA); Sigma, St. Louis, MO] to activate T cells (PBMC/PHA blasts). ALK+ TCL tissues were obtained from lymph nodes or extranodal tumors as biopsies performed for diagnostic purposes. The diagnosis was established by standard morphologic and immunohistochemical criteria, including expression of the CD30 and ALK proteins.
Mouse Xenograft Studies
All experiments were Institutional Animal Care and Use Committee approved. To examine the effect of HES1 inhibition on ALK+ TCL tumor growth in vivo, we used CRISPR-Cas9 genome editing technology. Briefly, to construct a CRISPR/Cas9 plasmid vector targeting HES1, genomic RNA sequence targeting the human HES1 gene was designed and inserted into the lenti-CRISPRv2 vector. The single-guide RNA/Cas9 expression vectors targeting HES1 or control empty vector were used to transfect SUDHL-1 cells. Both HES1-depleted and control SUDHL-1 cells were injected into immunodeficient NSG mice (Jackson Laboratories, Bar Harbor, ME), 10 mice per group (HES1– versus HES1+ construct), in two separate experiments. SUDHL-1 cells were also transfected with the Click-beetle green protein, permitting detection of cells by bioluminescence. Detection of the ALK+ TCL tumors was performed by bioluminescence imaging using a Xenogen Spectrum system and Living Image version 4.0 software (PerkinElmer, Waltham, MA) after i.p. injection of 150 mg/kg D-luciferin (Caliper Life Sciences, Waltham, MA). The P value was calculated using the Wilcoxon rank-sum test.
NMR Sample Preparation, Spectroscopy, and Structure Determination
Recombinant human HES1 Orange domain was expressed in Escherichia coli BL21 (DE3) cells and purified by nickel-affinity chromatography, followed by ion-exchange and size exclusion chromatography. The size and purity of the proteins were analyzed by SDS-PAGE and liquid chromatography–mass spectrometry. All nuclear magnetic resonance (NMR) experiments were performed on a Bruker Advance 800-MHz spectrometer (Bruker Corp., Billerica, MA). 1H, 13C, and 15N resonance assignments were conducted by measuring the three-dimensional spectra. NMR spectra were processed using the NMR Pipe program and analyzed using the Sparky program. The structure of the Orange domain was determined using the Xplor-NIH software version 2.24 (NIH, Bethesda, MD). For the dimer structure calculation, 30 interunit distance restraints, together with symmetry restraints and non-crystallographic symmetry restraints, were applied. Finally, 100 structures were calculated using 20,000 steps of simulated annealing, and a final ensemble of the 20 lowest energy structures was selected. The atomic coordinates and experimental data (code 7C4O) have been deposited in the Protein Data Bank (https://www.wwpdb.org, last accessed May 19, 2021).
Immunohistochemical Tissue Analysis
Immunohistochemical staining of formalin-fixed, paraffin-embedded ALK+ TCL tissues, 24 of which were assembled in tissue microarrays, was performed using the Bond Polymer Refine Detection System (Leica Biosystems, Richmond, IL) and epitope retrieval. In brief, the slides were heat treated for antigen retrieval in Epitope Retrieval Solution 1 (ER1) solution, and sections were incubated with the primary antibodies to ALK (Dako North America, Carpinteria, CA), CD30 (Dako North America), HES1 [HES1 (D6P2U) rabbit monoclonal antibody number 11988; Cell Signaling Technology, Danvers, MA] or TXNIP (TXNIP rabbit monoclonal antibody EPR14774; Abcam, Cambridge, UK). For interpretation, the immunostained slides were evaluated by light microscopy, and the images were photographed using the microscope-attached camera (Leica, Danvers, MA).
Treatment of ALK+ T Cells with ALK Inhibitor
Four ALK+ TCL cell lines and one NPM-ALK–transformed CD4+ T-cell line were treated with 100 nmol/L of ALK inhibitor (CEP-28122; Cephalon, Frazier, PA) and harvested for RNA and protein extraction and functional assays.
DNA Oligonucleotide Array
Total RNA from ALK+ TCL cells treated in triplicate cultures with ALK inhibitor (CEP-28122) for 6 hours was reverse transcribed, biotin labeled, hybridized to the U133 Plus 2.0 or Human Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA), and analyzed using the Partek GS (Partek Inc., Chesterfield, MO). Hybridization signal intensities were calculated after normalization based primarily on the signal intensity data of the provided RNA control, indicating fold difference of the experimental samples versus negative control. The expressed genes were analyzed using Kyoto Encyclopedia of Genes and Genomes, Ingenuity Pathway Analysis, and Gene Ontology databases.
RNA-Sequencing Analysis
RNA was adapter ligated and analyzed by high-throughput sequencing. Variants were called from the transcriptome using Partek Flow (Partek Inc., Chesterfield, MO), and expression calculations were performed using the Cufflinks package version 2.0 (http://cole-trapnell-lab.github.io/cufflinks). The expressed genes were identified using Partek algorithms and assigned to the cell pathways and programs using the above-mentioned databases. The experimental data (code GSE199658) have been deposited at Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo).
RT-PCR and RT-qPCR
For quantitative RT-PCR (RT-qPCR) experiments, total RNA was extracted (RNeasy kit; Qiagen, Germantown, MD) and reverse transcribed using ABI high-capacity RNA-to-cDNA kit (Life Technologies, Carlsbad, CA). Expression levels of HES1 mRNA and TXNIP mRNA were quantified on ABI PRISM 7700 sequence detection system with TaqMan gene expression assay kits (HES1, Hs00172878_m1; TXNIP, Hs00197750_m1; β-actin, Hs9999903; Life Technologies). All assays were performed in duplicate. The fold differences in RNA levels were calculated on the basis of the difference between CT values (ΔCT) obtained for the experimental and control mRNA.
Western Blot Analysis
Western blot analyses and, where applicable, immunoprecipitations were performed using antibodies against HES1 (D6P2U; Abcam), TXNIP (D5F3E), phosphorylated (p-) ALK and p-STAT3 (all from Cell Signaling Technology), total NPM-ALK (BD Pharmingen, San Diego, CA), and β-actin (Santa Cruz Biotechnology, Dallas, TX), according to standard protocols, as described.12,17,20
siRNA Assay
A mixture of four ALK-, STAT3-, or HES1-specific or control, nontargeting siRNAs (Dharmacon, Lafayette, CO) was introduced into cells for 72 hours by using Lipofectamine 2000 (Life Technologies), as previously described,17,20 or by using Nucleofector Solution T (Lonza Bioscience, Quakertown, PA) for CD4+ T-cell–derived NA1 cells.
Electrophoretic Mobility Shift Assay
Nuclear proteins were extracted and incubated with biotin-labeled DNA probe 5′-TCTTTTTCGTGAAGAACTCCAAA-3′ (forward), corresponding to the region within the 500-bp HES1 gene promoter upstream of the start codon ATG site that contains the STAT3 GAS binding site, located at position −51 to −58 upstream of the ATG site. In regard to the TXNIP gene promoter region of also 500-bp upstream of the ATG site, the labeled DNA probe 5′-CTTAATTCCTTAAAGTGAAATAATTTTTTGCAAAGGGG-3′ (forward) covered two STAT3 binding sites, located at positions −74 to −82 and −95 to −103. Proteins were separated by SDS-PAGE, transferred to membranes, and visualized using the HPR system (ThermoFisher, Waltham, MA).
HES1 Phosphorylation Assay
Recombinant HES1 (Origene, Rockville, MD) was incubated with recombinant active ALK (Life Technologies) for 30 minutes at 30°C in kinase buffer (25 mmol/L Tris, pH 7.5, 10 mmol/L MgCl2, 1 mmol/L EGTA, 0.5 mmol/L Na3VO4, 5 mmol/L β-glycerophosphate, 1 mmol/L dithiothreitol, and 500 μmol/L ATP) at the amounts of 200 and 100 ng. Reactions were stopped by adding 2× SDS-PAGE sample buffer, heated at 95°C for 5 minutes, and analyzed by immunoblotting using p-ALK and p-tyrosine antibodies with the antibody against total HES1 serving as a control.
Chromatin Immunoprecipitation Assay
Cell lysates of formaldehyde-fixed and sonicated cells were incubated with antibodies against STAT3 and IgG (R&D, Minneapolis, MN). DNA-protein immunocomplexes were obtained using protein A–agarose beads, extracted, and real-time quantitative PCR amplified using the primer set specific for the HES1 gene promoter [5′-CCCCGTCTACCTCTCTCCTT-3′ and 5′-CAGCTGGCATTTTCCTTTTT-3′ (forward)], to amplify the promoter region spanning from positions 10 to −195, counting from the start codon ATG site. For the TXNIP gene promoter, the primer pairs were 5′-TTGGTCGGGCTCCTGGTAAA-3′ and 5′-GTTTCAAGCAGGAGGCGGAA-3′ (forward) and 5′-CCCCTCTTTTTCTCCAAAGG-3′ and 5′-AGCTCCAAATCGAGGAAACC-3′ (forward) to cover the promoter regions from positions from −310 to −195 and from −53 to −306, respectively.
The results are presented as fold difference with binding of nontargeting IgG serving as the reference baseline value.
Cell Transfection with mRNA
PcDNA3 containing TXNIP DNA was linearized, and then a MEGAscript T7 RNA transcription kit (Ambion, ThermoFisher) was used to synthesize the TXNIP mRNA, according to the kit's protocol. The synthesized TXNIP mRNA was purified with RNeasy Mini Kit (Qiagen). After harvesting, cells were washed twice and resuspended in OptiMEM (Gibco/Invitrogen, ThermoFisher) at 30 × 106 cells/mL. Cell suspension (100 μL) was then mixed with 10 μg of TXNIP mRNA and electroporated in a 2-mm gap cuvette at 700 V for one to three pulses at 0.5 milliseconds/pulse, using a BTX ECM 830 square-wave electroporator (BTX Harvard, Holliston, MA). After the electroporation, cells were immediately suspended in culture medium and transferred to a culture incubator with 5% CO2 atmosphere and 37°C temperature, to minimize cell death.
MTT Enzymatic Conversion Assay
Cells were plated in 96-well plates at 3 to 5 × 103/well, transduced with TXNIP mRNA, labeled with MTT (Promega, Madison, WI) at 5 mg/mL for 4 hours, and solubilized with 10% SDS in 0.01 mol/L HCl. OD of the culture supernatant, corresponding to MTT conversion-mediated change in the supernatant color, was determined at 570 nm using a Titertek Multiskan reader (Titertek Instruments, Pforzheim, Germany).
Apoptosis Assays
Transfected cells were quantified by measuring green fluorescence in FL1 on the flow cytometer for transfection efficiency. Annexin-V staining was performed with the Annexin-V–FLUOS Staining Kit (Roche, Basel, Switzerland), according to the manufacturer's protocol. In brief, the cells were collected, washed, and resuspended in 100 μL binding buffer containing Annexin-V–FLUOS and/or propidium iodide. Samples were incubated for 15 minutes at room temperature and were analyzed with a Becton Dickinson (San Jose, CA) FACScan flow cytometer and CellQuest 5.1 software to acquire and analyze the data.
Results
ALK+ TCL Cells, Both Cultured and Primary, Express HES1
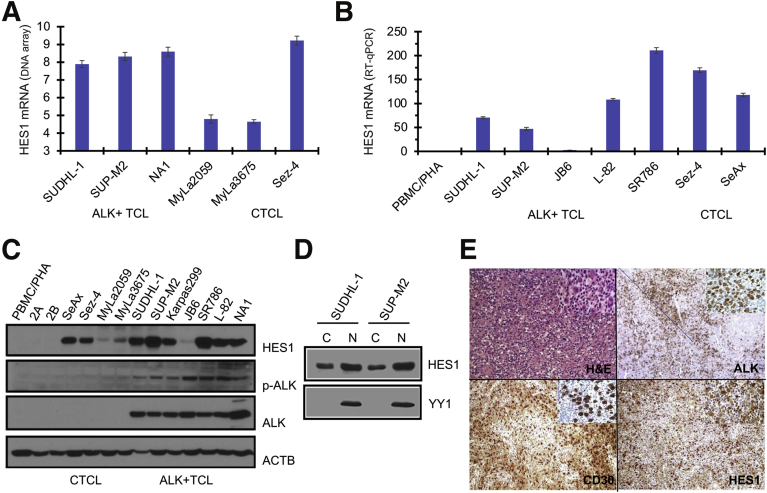
Oligonucleotide microarray-based gene expression profiling of ALK-positive cells revealed the strong expression of HES1 expression in the ALK+ TCL-derived cell lines SUDHL-1 and SUP-M2 as well as the NPM-ALK–transformed cell line NA1 (Figure 1A). Among ALK-negative cell lines derived from cutaneous T-cell lymphoma (CTCL), two cell lines representing the skin-based variant of the lymphoma (MyLa2059 and MyLa3675) showed limited HES1 mRNA expression, whereas one line (Sez-4) derived from a leukemic variant of CTCL displayed strong expression of the mRNA. To confirm and further evaluate HES1 expression in ALK+ TCL, RT-qPCR was performed on a larger panel of ALK+ TCL. Selected CTCL cell lines and normal PBMCs stimulated with PHA (PBMC/PHA blasts) comprising almost exclusively of activated T cells were used as controls (Figure 1B). Besides the index SUDH-L1 and SUP-M2 cell lines, two additional ALK+ TCL cell lines (L82 and SR786) strongly expressed HES1 mRNA. On the other hand, the mRNA was barely detectable in one (JB6) cell line. In addition to Sez-4, another leukemic CTCL cell line, SeAx, strongly expressed the mRNA, whereas normal PBMC/PHA blasts displayed no detectable HES1 mRNA, strongly suggesting that HES1 expression is restricted to malignant T cells.
Figure 1.
Hairy and enhancer of split homolog-1 (HES1) is expressed in anaplastic lymphoma kinase–positive (ALK+) T cells and tissues. A: mRNA expression of HES1 in ALK+ T-cell lymphoma (TCL)–derived cell lines (SUDHL-1 and SUP-M2), nucleophosmin (NPM)–ALK–transformed CD4+ T cells (NA1), and control cell populations [resting peripheral blood mononuclear cells (PBMCs) and activated PBMCs/PHA] detected by DNA oligonucleotide array analysis. The data are depicted as units of the normalized signal intensity. B: Expression of HES1 mRNA in the depicted ALK+ TCL cell lines, NA1, and control PBMC/PHA cells detected by quantitative RT-PCR (RT-qPCR). The results depict fold difference compared with control, after signal intensity normalization. C: Expression of HES1 protein in the depicted ALK+ T-cell and control populations, identified by Western blot analysis. D: Subcellular localization of HES1 in NPM-ALK–positive cells. Nuclear and cytoplasmic protein fractions isolated from SUDHL-1 and SUP-M2 cells were resolved by SDS-PAGE and analyzed by Western blot analysis. Blots were stripped and reprobed with anti-YY1 antibody to control the purity of fractions. E: Expression of HES1 protein in ALK+ TCL tissues detected by immunohistochemistry (representative images). Staining with hematoxylin and eosin (H&E) and antibodies against ALK and CD30 served as positive controls, highlighting the large anaplastic malignant ALK+ TCL cells within the tissue. Original magnification: ×40 (E, main images); ×100 (E, insets). ACTB, β-actin; C, cytoplasm; CTCL, cutaneous T-cell lymphoma; N, nucleus; p-ALK, phosphorylated ALK.
To determine the expression of the HES1 protein in ALK+ TCL, Western blot analysis was performed of ALK+ TCL and CTCL cell lines as well as normal PBMC/PHA blasts (Figure 1C). The results directly correlated with the mRNA expression data, showing HES1 protein expression in all NPM-ALK–expressing cell lines (six strong and one, JB6, weak) with nondetectable to weak expression in cell lines derived from skin-based types and strong in the leukemic variant of CTCL. To determine the cellular localization of the HES1 protein in ALK+ TCL cells, cytoplasmic and nuclear fractions were analyzed for the presence of the protein (Figure 1D). HES1 was detected in both the cytoplasm and nucleus, with the latter containing a higher concentration of the factor.
To confirm that HES1 is also expressed in primary ALK+ TCL cells, immunohistochemical staining for HES1 was performed of formalin-fixed, paraffin-embedded diagnostic tissue samples from 38 cases of ALK+ TCL (Figure 1E) (a representative image). In all the cases examined, most, if not all, malignant cells, identified by anaplastic-cell morphology and the expression of ALK and CD30, strongly expressed HES1, whereas the admixed much smaller, nonmalignant lymphocytes did not express this protein at a detectable concentration (cell morphology and staining pattern of large anaplastic malignant cells versus small nonmalignant lymphocytes are the easiest to appreciate in the ×100 insets located in the upper right corner of each ×40 image). These findings indicate that HES1 expression appears universal in ALK+ TCL and is absent in normal T lymphocytes.
Genetic Targeting of HES1 Suppresses Growth of ALK+ TCL in Vivo
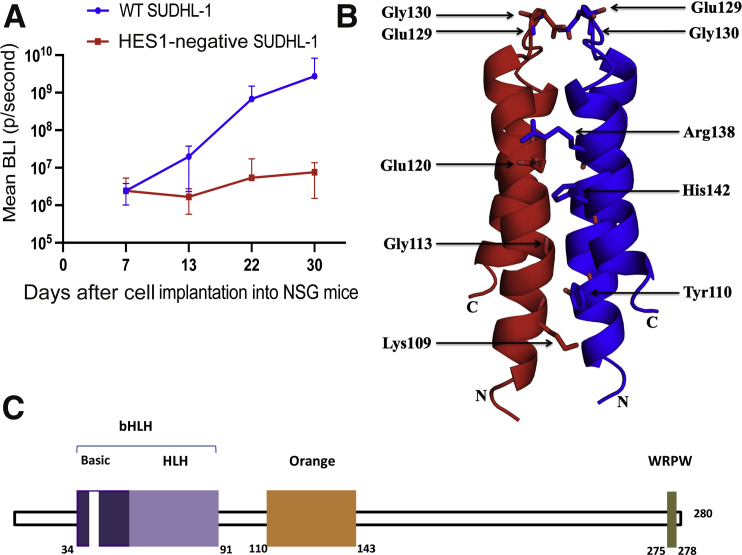
To determine the functional significance of HES1 expression, HES1 was depleted in SUDH-L1 ALK+ TCL cells using CRISPR-Cas9 genome editing technology (Supplemental Figure S1). These HES1-depleted as well as the control HES1-expressing SUDH-L1 cells were also simultaneously transfected with the Click-beetle green protein, permitting detection of cells by bioluminescence. Both of these cell populations were then injected into immunodeficient NSG mice. As shown in Figure 2A, loss of HES1 profoundly affected growth of the ALK+ TCL tumors, further supporting the important role of HES1 in NPM-ALK–induced lymphomagenesis.
Figure 2.
Hairy and enhancer of split homolog-1 (HES1) function and structure. A: Inhibition of HES1 suppresses growth of anaplastic lymphoma kinase–positive (ALK+) T-cell lymphoma (TCL) cells in vivo. HES1 gene expression was down-regulated in ALK+ TCL cells SUDHL-1 via CRISPR-Cas9 gene editing system, and the cells were injected into NSG mice via tail vain. ALK+ TCL cells transfected with empty plasmid were used as a control. ALK+ TCL tumor growth curves were evaluated by bioluminescence imaging (BLI), measured at the depicted days after cell injection, with P values of <0.43 (day 7), <0.02 (day 13), <0.0002 (day 22), and <0.0002 (day 30). The depicted results are representative of two independent experiments. B: Nuclear magnetic resonance structure of HES1 Orange domain. The structure of the HES1 Orange domain, which exists as a homodimer. The interacting residues in the dimer interface are shown. Eight of the residues, based on the structure, were selected for mutation to break the dimer. C: Schematic structure of HES1 protein. HES1 has a basic helix-loop-helix (bHLH) domain, an Orange domain, and the proline-rich C-terminus with the WRPW domain. WT, wild type.
Structure of the HES1 Orange Domain
There are three conserved domains in HES proteins: the bHLH domain, the Orange domain, and the WRPW motif. Although the structure of the bHLH domain has been solved,32 the structure of the Orange domain remained unknown. The structure of the human HES1 Orange domain (residues 103 to 150) has now been determined by NMR. The protein exists as a dimer in solution, and the monomer consists of two major helices (residues 105 to 127 and 134 to 149), separated by a small linker forming a U-shaped structure. The structure determination and refinement statistics are provided in Supplemental Table S1. The dimer had a buried surface area of 2200 Å2 with 6 hydrogen bonds and 12 salt bridges, mainly involving residues Lys109, Tyr110, Gly113, Glu120, Glu129, Gly130, Arg138, and His142 (Figure 2B). Such an extensive network of contacts potentially rendered the Orange domain, the middle of three conserved domains in HES proteins (Figure 2C), highly stable, and was responsible for the stability and dimerization of the full-length HES1 protein. Accordingly, mutational replacement of the predicted key interacting residues depicted in Figure 2B as single, double, or even triple mutations did not result in the breakdown of the dimer. This indicates that the dimer structure is highly stable, although three of the depicted mutations (Y110A, E120A, and E129A) markedly decreased the concentration of the HES1 protein (data not presented).
NPM-ALK Induces Expression of the HES1 Gene
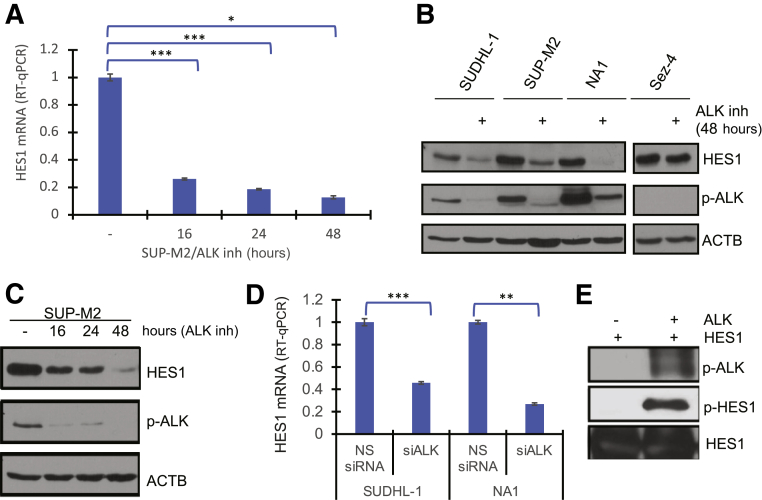
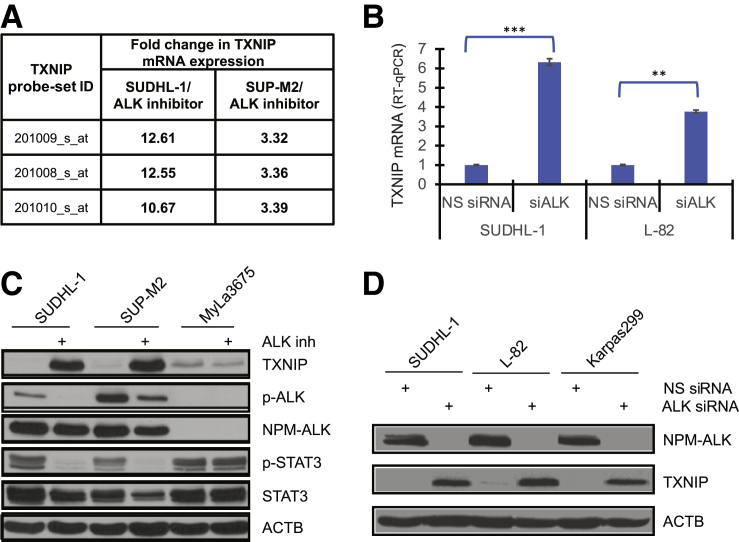
Given the ubiquitous expression of HES1 in ALK+ TCL cells and tissues (Figure 1), the potential role of NPM-ALK in activating the HES1 gene was explored. Exposure of NPM-ALK+ TCL SUP-M2 and SR786 cell lines to the highly specific ALK inhibitor CEP-28122 resulted in a profound decrease in HES1 mRNA within 16 hours after exposure to the inhibitor, which was maintained for at least 48 hours (Figure 3A). The decreased mRNA expression directly correlated with markedly diminished expression of the HES1 protein (Figure 3B), with delayed kinetics of the protein loss (Figure 3C) compared with the mRNA loss (Figure 3A). Similar inhibitory effects on HES1 expression at both mRNA (Figure 3D) and protein levels (Figure 4F) were observed following siRNA-mediated depletion of NPM-ALK expression, in support of the conclusion that NPM-ALK induces expression of the HES1 gene.
Figure 3.
Nucleophosmin–anaplastic lymphoma kinase (ALK) dependence of hairy and enhancer of split homolog-1 (HES1) expression. A: Loss of HES1 mRNA expression detected by quantitative RT-PCR (RT-qPCR) at 16, 24, and 48 hours in the depicted ALK+ T-cell lymphoma (TCL) cell lines exposed to 100 nmol/L of ALK inhibitor (inh) CEP-28122 or its vehicle alone. B: Western blot analysis–detected expression of HES1 and control proteins in ALK+ TCL and control ALK-negative Sez-4 cells cultured with the ALK inhibitor or its vehicle. C: Kinetics of HES1 protein loss induced by the ALK inhibitor. D: Inhibition of HES1 mRNA expression induced by ALK-specific siRNA (siALK) with non-specific (scrambled) siRNA (NS siRNA) serving as a negative control. E: ALK-mediated phosphorylation of HES1 detected using anti–phosphorylated tyrosine antibody. Detection of activated ALK [phosphorylated ALK (p-ALK)] using antibody against autophosphorylation site of ALK (Tyr 1604) served as a positive control. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. ACTB, β-actin; p-HES1, phosphorylated HES1.
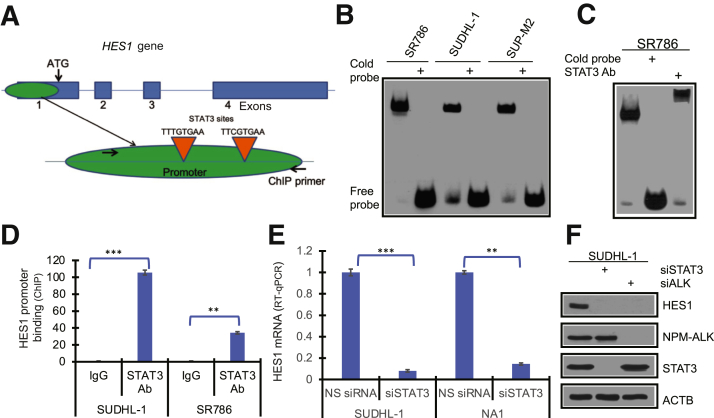
Figure 4.
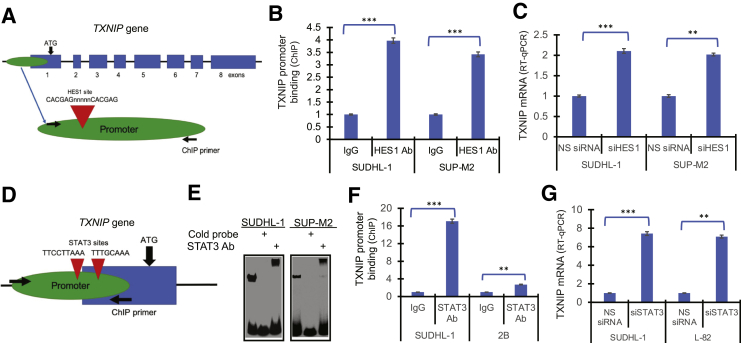
STAT3-mediated induction of hairy and enhancer of split homolog-1 (HES1) expression. A: Schematic depiction of two identified STAT3 binding sites within the promoter (marked with red triangles). B: Binding of the transcription factor STAT3 to HES1 gene promoter in vitro, detected by electrophoretic mobility shift assay (EMSA) in the depicted anaplastic lymphoma kinase–positive (ALK+) T-cell lymphoma (TCL) cell lines. C: In some experiments, the cell lysates were pre-incubated with unlabeled (cold) DNA oligonucleotide probe; and in other experiments, they were pre-incubated with a STAT3-specific antibody (supershift EMSA). D: Binding of STAT3 in ALK+ TCL cells to HES1 promoter in vivo, detected by chromatin immunoprecipitation (ChIP) assay. E: Inhibition of HES1 mRNA expression, induced by STAT3-specific siRNA (siSTAT3) with non-specific siRNA (NS siRNA) serving as control, detected by quantitative RT-PCR (RT-qPCR). F: Inhibition of HES1 protein expression induced by siSTAT3 and ALK siRNA (siALK) with the other depicted proteins serving as the siRNA specificity controls. ∗∗P < 0.01, ∗∗∗P < 0.001. Ab, antibody; ACTB, β-actin; NPM, nucleophosmin.
ALK Phosphorylates HES1 Protein
Because NPM-ALK is a constitutively activated tyrosine kinase, whether ALK was able to directly phosphorylate HES1 was examined next. To this end, recombinant HES1 protein was incubated with or without an active recombinant ALK protein (used instead of immunoprecipitated NPM-ALK, because HES1 has molecular weight similar to IgG) under in vitro kinase assay conditions. Immunoblotting using an antibody specific for p-Tyr revealed that HES1 was strongly phosphorylated by ALK (Figure 3E).
HES1 Gene Is Transcriptionally Activated by STAT3
Because STAT3 has emerged as the critical transcriptional gene modulator downstream of NPM-ALK,4 its potential role in the activation of the HES1 gene was examined. Sequence analysis of the promoter region of HES1 identified two putative STAT3 binding sites containing the canonical TT and AA dinucleotides (Figure 4A). Using electrophoretic mobility shift assays, binding of STAT3 was detected in cell extracts from three ALK+ T-cell lines to the 23-mer DNA oligonucleotide probe containing the proximal STAT3 binding site (Figure 4B). The binding was specific, as demonstrated by its abrogation following the addition of an unlabeled (cold) probe. The identity of STAT3 as the protein bound to the site was confirmed by using a supershift electrophoretic mobility shift assay, where an antibody against STAT3 further delayed migration of the probe containing the STAT3 binding site (Figure 4C). To demonstrate that STAT3 binds also to the HES1 gene promoter derived from the ALK+ TCL cells, chromatin immunoprecipitation assays were performed using anti-STAT3. anti-IgG antibody as well as a set of PCR primers that amplify the promoter segment containing both STAT3 binding sites were used as control (Figure 4A). Strong and specific binding of STAT3 to the promoter was identified (Figure 4D), further confirming that ALK+ TCL-derived STAT3 binds to the HES1 promoter. Finally, the depletion of STAT3 using a specific siRNA mix profoundly suppressed the expression of HES1 mRNA (Figure 4E) and protein (Figure 4F), documenting that STAT3 is the transcriptional activator of HES1.
HES1 and STAT3 Inhibit Gene Expression in an NPM-ALK–Dependent Manner
Given the ubiquitous expression of HES1 in ALK+ TCL cells (Figure 1), the suppressive effect of its loss on the in vivo growth of these cells (Figure 2A), and its reported function as a transcription factor, global scale RNA-sequencing analysis was performed in two ALK+ TCL cell lines treated with HES1-specific siRNA with scrambled siRNA serving as a control. This analysis identified 127 genes down-regulated and 71 genes up-regulated in common to both lines following HES1 depletion (Supplemental Tables S2 and S3). Many of the identified genes are involved in key cell functions, such as differentiation, signal transduction, adhesion, proliferation, and death (Supplemental Tables S4 and S5). Among the genes inhibited by HES1, TXNIP caught our particular attention because it alone was categorized as involved in a large number of cell functions key to malignant cells, including cell death (Supplemental Table S5).
To confirm that HES1 is a transcriptional regulator of TXNIP gene, the gene's promoter was analyzed in silico and a HES1 binding site was identified (Figure 5A). Accordingly, HES1 specifically bound to the TXNIP gene promoter, as determined by chromatin immunoprecipitation assay (Figure 5B). Furthermore, siRNA-mediated depletion of HES1 promoted transcriptional activity of the TXNIP gene (Figure 5C), as detected by RT-qPCR, in agreement with the genome-scale RNA-sequencing analysis (Supplemental Tables S2 and S3).
Figure 5.
Hairy and enhancer of split homolog-1 (HES1) and STAT3 inhibit thioredoxin-interacting protein (TXNIP) expression. A: Schematic diagram of TXNIP gene with depicted HES1 binding site in the gene's promoter. ATG marks transcription initiation site. Arrows indicate binding sites of primers used in chromatin immunoprecipitation (ChIP) assay. B: Binding of HES1 in anaplastic lymphoma kinase–positive (ALK+) T-cell lymphoma (TCL) cells to the proximal HES1 promoter detected by ChIP assay. C: Induction of TXNIP mRNA expression in ALK+ TCL cells caused by siRNA-mediated HES1 depletion with cells exposed to non-specific siRNA (NS siRNA) serving as controls. D: Schematic diagram of TXNIP gene promoter region with depicted STAT3 binding sites. ATG marks transcription initiation site. Arrows indicate binding sites of primers used in ChIP assay. E: Binding of the transcription factor STAT3 to the TXNIP gene promoter in ALK+ TCL cells detected by electrophoretic mobility shift assay (EMSA). Cell lysate pre-incubation with either unlabeled (cold) DNA oligonucleotide probe or STAT3-specific antibody (supershift EMSA) served as STAT3 binding specificity control. F: Binding of STAT3 to TXNIP promoter detected by ChIP assay. G: Induction of TXNIP mRNA expression triggered by STAT3 siRNA (siSTAT3) with NS siRNA serving as control. ∗∗P < 0.01, ∗∗∗P < 0.001. Ab, antibody; RT-qPCR, quantitative RT-PCR; siHES1, HES1 siRNA.
While scanning the TXNIP gene promoter, two canonical binding sites for STAT3 were also identified (Figure 5D). Subsequently, an electrophoretic mobility shift/supershift assay showed (Figure 5E) that STAT3 from ALK+ TCL cell lysates specifically bound to the DNA oligonucleotide probe corresponding to the identified site proximal to ATG start codon. Furthermore, STAT3 also bound to the TXNIP gene promoter, as determined by chromatin immunoprecipitation assay (Figure 5F). Finally, STAT3 depletion by siRNA promoted transcription of the TXNIP gene (Figure 5G), indicating that, similar to HES1, STAT3 was involved in the inhibition of TXNIP transcription.
Considering that NPM-ALK directly induces STAT3 activation and indirectly HES1 expression, and both act as suppressors of the TXNIP gene, the effect of NPM-ALK inhibition on TXNIP expression was examined, using both pharmacologic and molecular approaches. DNA oligonucleotide-array–based analysis of two different ALK+ TCL cell lines revealed marked up-regulation of TXNIP mRNA in response to pharmacologic ALK inhibition with a small-molecule ALK inhibitor (Figure 6A). Similar result of highly up-regulated TXNIP expression was obtained in two ALK+ TCL cell lines treated with ALK siRNA, using RT-qPCR analysis (Figure 6B). Pharmacologic ALK inhibition also resulted in profound up-regulation of TXNIP on the protein level, with ALK-negative CTCL cells (MyLA3675) serving as the specificity control of ALK inhibition (Figure 6C). Finally, siRNA-mediated depletion of NPM-ALK also led to profound up-regulation of TXNIP protein expression (Figure 6D), further supporting the conclusion that NPM-ALK induces suppression of the TXNIP gene.
Figure 6.
Nucleophosmin (NPM)–anaplastic lymphoma kinase (ALK) inhibits thioredoxin-interacting protein (TXNIP) expression. A: mRNA expression of TXNIP in ALK+ T-cell lymphoma (TCL)–derived cell lines (SUDHL-1 and SUP-M2) treated with ALK-specific inhibitor (ALK inh) or its vehicle detected by DNA oligonucleotide array analysis. The results are presented as fold change in TXNIP mRNA expression of ALK inhibitor– versus vehicle-treated cells detected by three different TXNIP-specific probes. B: Expression of TXNIP mRNA in the depicted ALK+ TCL cell lines transfected with siRNA, either NPM-ALK specific or non-specific (NS), detected by quantitative RT-PCR (RT-qPCR). C: Western blot analysis–detected expression of TXNIP protein in the depicted ALK+ TCL cells treated with ALK-specific inhibitor or its vehicle with ALK–TCL cells (MyLa3676) serving as a control. D: Impact of siRNA-mediated NPM-ALK depletion on expression of TXNIP protein in ALK+ T cells with NS siRNA serving as a negative control. ∗∗P < 0.01, ∗∗∗P < 0.001. ACTB, β-actin; ID, identifier; p-ALK, phosphorylated ALK; p-STAT3, phosphorylated STAT3; siALK, ALK siRNA.
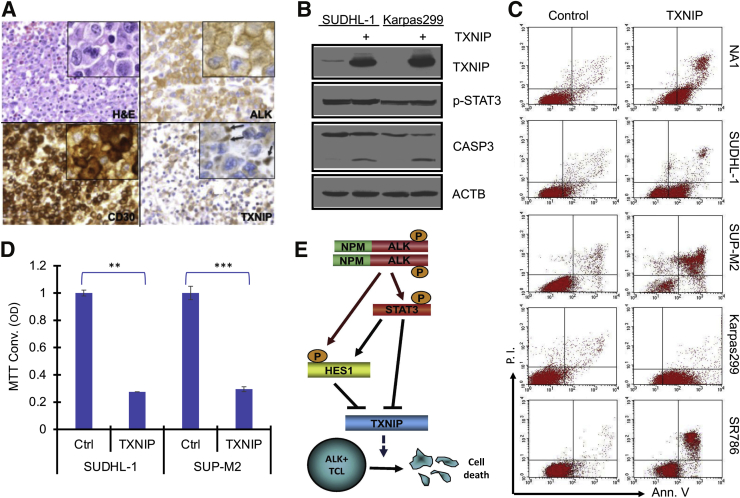
To also verify a lack of TXNIP expression in primary ALK+ TCL cells, immunohistochemical staining of the same set of diagnostic ALK+ TCL tissues used for HES1 staining was performed (Figure 1E). Indeed, the malignant cells identified by morphology and expression of ALK and CD30 exhibited negative or weak expression of TXNIP, whereas the admixed small, nonmalignant lymphocytes strongly expressed this protein (Figure 7A) (representative images are representative for the 38 evaluated ALK+ TCL cases).
Figure 7.
Expression of thioredoxin-interacting protein (TXNIP) inhibits growth of anaplastic lymphoma kinase–positive (ALK+) T-cell lymphoma (TCL) cells. A: Expression of TXNIP protein in ALK+ TCL tissues detected by immunohistochemistry (representative images). Staining with hematoxylin and eosin (H&E) and antibodies against ALK and CD30 served as positive controls (Ctrls), highlighting the malignant, highly anaplastic ALK+ TCL cells, particularly well visible in the insets. Arrows in the TXNIP staining inset point to strongly staining small reactive lymphocytes, representing an internal positive control. A: Representative for the 38 evaluated ALK+ TCL cases. B: TXNIP mRNA-mediated expression of TXNIP protein induced apoptotic cell death of ALK+ TCL cells detected by expression of cleaved caspase 3 (CASP3). C: TXNIP expression induced apoptotic cell death of ALK+ T cells seen in the annexin-V (Ann. V) assay. x Axis: cell-surface Ann. V expression; and y axis: propidium iodine (P.I.) uptake. D: Impact of TXNIP expression on ALK+ T-cell growth detected by MTT conversion (MTT Conv.) assay. MTT Conv. reflects the enzymatic activity of the ALK+ TCL cells, leading to change in the cell culture supernatant color, captured by measuring OD of the supernatants. B–D: Representative of three independent experiments. E: Schematic model of nucleophosmin (NPM)–ALK–induced protection from cell death of ALK+ TCL cells via STAT3–hairy and enhancer of split homolog-1 (HES1)–TXNIP network. ∗∗P < 0.01, ∗∗∗P < 0.001. Original magnification: ×40 (A, main images); ×100 (A, insets). ACTB, β-actin; p-STAT3, phosphorylated STAT3.
TXNIP Acts in ALK+ TCL Cells as an Inducer of Apoptotic Cell Death
To determine the effect of TXNIP expression, transfection of ALK+ TCL cell lines with TXNIP mRNA was performed. TXNIP expression induced cell-surface expression of annexin V, often combined with propidium iodine uptake (Figure 7B) and cleavage of caspase 3 (Figure 7C), all indicating apoptotic cell death. Accordingly, TXNIP expression profoundly suppressed cell growth, as determined by MTT conversion in the index ALK+ TCL cell lines SUDHL-1 and SUP-M2 (Figure 7D), indicating that NPM-ALK–induced, HES1- and STAT3–mediated inhibition of TXNIP expression protects ALK+ TCL from cell death.
Discussion
The role of the NPM-ALK in lymphomagenesis has been well established.4,12,33 In target T cells, NPM-ALK induces expression of pro-oncogenes promoting cell proliferation, survival, protection from hypoxia, and tumor immune evasion.2,14, 15, 16, 17,34,35 Furthermore, to preserve its own expression and function, NPM-ALK also inhibits expression of tumor suppressor genes by inducing their silencing epigenetically, primarily through DNA methylation of the gene promoters.4,17, 18, 19, 20,35
This study identified HES1 as a target of NPM-ALK–promoted up-regulation. Although associated in literature mainly with Notch signaling,36 HES1 expression is actually also induced by other signaling pathways, including bone morphogenic protein, fibroblast growth factor, leukemia inhibitory factor, NF-κB, Sonic hedgehog, and Wnt,37 and, now, NPM-ALK, suggesting that HES1 plays an important biological role in the whole spectrum of normal and malignant cells. The human HES protein family comprises seven members (HES 1 to 7), and they all contain three conserved domains: the bHLH domain, the Orange domain, and the WRPW domain (Figure 2C). The bHLH region of HES1 is flexible and unstructured in the absence of bound DNA, and the bHLH domain alone exists between an unfolded monomer and a folded dimer, which is only marginally stable.21 In contrast, the Orange domain exists as a more stable helical dimer,21 suggesting that it may be responsible for dimerization of HES1 as a whole. Our structural analysis of the Orange domain (Figure 2B) indicates that the domain is stable and, therefore, most likely provides a high degree of stability to the HES1 protein dimerization. The inability of several single, double, and triple mutations within the Orange domain to break the dimer formation (data not presented) further supported this conclusion.
Results of the current structural and mutational studies may lay the foundation of future studies aimed at targeted prevention of HES1 dimerization as a novel therapeutic approach to HES1-dependent malignancies, such as ALK+ TCL, as described in this report.
HES1 is essential for the self-renewal of neuronal stem cells and for repression of their maturation along the neuronal lineage.38 In addition, HES1 induces epithelial-mesenchymal transition and confers poor prognosis in most cancers. The epithelial-mesenchymal transition not only plays a key role in tumor metastasis, it is also associated with the acquisition of cancer stem cell characteristics.39,40 HES1 also influences the maintenance of hematopoietic stem cells by blocking their differentiation.41 Deletion of HES1 in hematopoietic progenitors impaired T-cell development by compromising the capacity of early lymphoid progenitors to seed and populate the thymus.42 However, the role of HES1 in T-cell lymphomagenesis, the exact mechanisms of its induction and activation, and, in more general terms, the mechanisms of HES1-mediated oncogenesis remained unknown.
Herein, the study found that HES1 mRNA and protein were highly expressed in ALK+ TCL cells (Figure 1) and that the HES1 gene expression clearly depended on the kinase activity of NPM-ALK (Figure 3). HES1 was also expressed in IL-2–dependent, NPM-ALK–negative CTCL, Sez-4, and SeAx cells, indicating that other, non–NPM-ALK cell-signaling mechanisms also induce HES1 expression in malignant T cells. NPM-ALK activated HES1 gene through a transcription factor STAT3 (Figure 4), the key mediator of the NPM-ALK–mediated cell transformation, as identified by us15,17 and others.14 Of note, NPM-ALK not only induced the expression of the HES1 gene, but also phosphorylated HES1 protein (Figure 3E). This indicates that NPM-ALK also modulated HES1 gene activity by identifying a novel, two-pronged strategy of an oncogenic kinase to regulate its key effector molecule, as schematically depicted in Figure 7E.
The study shows that HES1 is not only required to sustain growth of ALK+ TCL tumors (Figure 2A) but also regulates expression of almost 200 genes, both up-regulated and down-regulated, and is involved in several key cell functions (Supplemental Tables S2–S5), with the TXNIP gene involved in several of these functions, including cell death (Supplemental Table S5). Previous studies have shown that HES1 inhibits genes by either active or passive repression of their transcription.43,44 During active repression, the HES factors bind to the N box or to the class C site in the promoter region of target genes and repress their transcription.44 During passive repression, the HES factors form heterodimers with several bHLH activators and inhibit their binding to the E box in the promoter regions of target genes.44 The data indicate that HES1 actively inhibits transcription of the TXNIP gene, because it binds directly to its promoter (Figure 5, A and B).
Similar to HES1, STAT3 also inhibited the TXNIP gene (Figure 5G), directly by binding to the gene's promoter (Figure 5, D–F). Of note, interaction between STAT3 and HES1 has been previously reported,45 but in this case within the cell cytoplasm rather than the nucleus, where HES1 was found to facilitate cytoplasmic complex formation between Janus kinase 2 and STAT3. Given the role of NPM-ALK in regulation of both HES1 and STAT3, it was not surprising to find that ALK inhibition, either by suppression of its expression or activation, induced expression of TXNIP (Figure 6).
Inhibition of TXNIP expression is frequent in TCL and other malignancies, including acute myeloid leukemia and carcinomas of breast, lung, stomach, and liver. In regard to TCL, TXNIP expression is lost during the progression from the IL-2–dependent to IL-2–independent growth pattern in the human T-lymphotropic virus (HTLV1)–associated adult T-cell leukemia cells.46 Furthermore, transcriptional inhibition of the TXNIP gene by enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), the catalytic subunit of polycomb repressive complex 2, was found in the CTCL-type TCL,47 not only expanding the list of TCLs with suppressed TXNIP expression but also shedding additional light into the complex mechanisms of TXNIP gene regulation. The identification of NPM-ALK as the master regulator of TXNIP gene suppression implicates for the first time a bona fide oncogene of any kind and, more specifically, an oncogenic kinase in regulation of this important tumor suppressor.
Although TXNIP has been proposed previously to serve as a glucose48 and oxidative stress sensor,49 its postulated function as a regulator of apoptosis50 appears to be the most directly relevant to cancer, and best explains its expression loss in ALK+ TCL and other malignancies. Indeed, transfection of ALK+ TCL cells with TXNIP mRNA resulted in their apoptotic cell death (Figure 7, B–D), indicating that NPM-ALK–induced suppression is a critical component of malignant cell transformation mediated by this highly oncogenic kinase.
In summary, by combining microscopic, genetic, genomic, structural, biochemical, and functional analysis, an intricate regulatory network involving an oncogenic kinase and two transcription factors silencing a gene coding for a powerful pro-apoptotic protein was identified (Figure 7E). Acting through STAT3, the constitutively self-activated NPM-ALK induced expression of the HES1 gene, also phosphorylating HES1 protein. In turn, both HES1 and STAT3 suppressed the TXNIP gene protecting ALK+ TCL cells from apoptotic cell death, indicating that the TXNIP suppression is an essential component of the oncogenic program conferred by NPM-ALK on the target T cells. These findings may have translational implications and suggest that similarly complex networks exist in other types of malignancies, given the frequent loss of TXNIP expression in cancer.
Footnotes
Supported by National Cancer Institute grant R01CA228457 (M.A.W.), Abramson Cancer Center Translational Center of Excellence in Lymphoma (M.A.W.), Fox Chase Cancer Center Academic Research Fund (M.A.W.), and Singapore Ministry of Education grant R-154-000-632-112 (K.S.).
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2022.05.005.
Supplemental Data
CRISPR/Cas9-mediated depletion of hairy and enhancer of split homolog-1 (HES1). The depicted anaplastic lymphoma kinase–positive T-cell lymphoma SUDH-L1 and control HEK239 cells were transfected with either HES1 single-guide RNA or empty vector (EV) as control and examined for HES1 protein expression by Western blot analysis. The single-guide HES1 RNA and EV-transfected SUDHL-1 cells were injected into NSG mice and monitored for tumor growth, as shown in Figure 2A.
References
- 1.Yao S., Cheng M., Zhang Q., Wasik M., Kelsh R., Winkler C. Anaplastic lymphoma kinase is required for neurogenesis in the developing central nervous system of zebrafish. PLoS One. 2013;8:e63757. doi: 10.1371/journal.pone.0063757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boi M., Zucca E., Inghirami G., Bertoni F. Advances in understanding the pathogenesis of systemic anaplastic large cell lymphomas. Br J Haematol. 2015;168:771–783. doi: 10.1111/bjh.13265. [DOI] [PubMed] [Google Scholar]
- 3.Pall G. The next-generation ALK inhibitors. Curr Opin Oncol. 2015;27:118–124. doi: 10.1097/CCO.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 4.Werner M.T., Zhao C., Zhang Q., Wasik M.A. Nucleophosmin-anaplastic lymphoma kinase: the ultimate oncogene and therapeutic target. Blood. 2017;129:823–831. doi: 10.1182/blood-2016-05-717793. [DOI] [PubMed] [Google Scholar]
- 5.Morris S.W., Kirstein M.N., Valentine M.B., Dittmer K.G., Shapiro D.N., Saltman D.L., Look A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 6.Morris S.W., Naeve C., Mathew P., James P.L., Kirstein M.N., Cui X., Witte D.P. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 7.Shiota M., Fujimoto J., Semba T., Satoh H., Yamamoto T., Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9:1567–1574. [PubMed] [Google Scholar]
- 8.Bischof D., Pulford K., Mason D.Y., Morris S.W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarle R., Gong J.Z., Guasparri I., Pesci A., Cai J., Liu J., Simmons W.J., Dhall G., Howes J., Piva R., Inghirami G. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto J., Shiota M., Iwahara T., Seki N., Satoh H., Mori S., Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proc Natl Acad Sci U S A. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuefer M.U., Look A.T., Pulford K., Behm F.G., Pattengale P.K., Mason D.Y., Morris S.W. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997;90:2901–2910. [PubMed] [Google Scholar]
- 12.Zhang Q., Wei F., Wang H.Y., Liu X., Roy D., Xiong Q.B., Jiang S., Medvec A., Danet-Desnoyers G., Watt C., Tomczak E., Kalos M., Riley J.L., Wasik M.A. The potent oncogene NPM-ALK mediates malignant transformation of normal human CD4(+) T lymphocytes. Am J Pathol. 2013;183:1971–1980. doi: 10.1016/j.ajpath.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzec M., Halasa K., Liu X., Wang H.Y., Cheng M., Baldwin D., Tobias J.W., Schuster S.J., Woetmann A., Zhang Q., Turner S.D., Ødum N., Wasik M.A. Malignant transformation of CD4+ T lymphocytes mediated by oncogenic kinase NPM/ALK recapitulates IL-2-induced cell signaling and gene expression reprogramming. J Immunol. 2013;191:6200–6207. doi: 10.4049/jimmunol.1300744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiarle R., Simmons W.J., Cai H., Dhall G., Zamo A., Raz R., Karras J.G., Levy D.E., Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 15.Kasprzycka M., Marzec M., Liu X., Zhang Q., Wasik M.A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci U S A. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzec M., Zhang Q., Goradia A., Raghunath P.N., Liu X., Paessler M., Wang H.Y., Wysocka M., Cheng M., Ruggeri B.A., Wasik M.A. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q., Wang H., Kantekure K., Paterson J.C., Liu X., Schaffer A., Paulos C., Milone M.C., Odum N., Turner S., Marafioti T., Wasik M.A. Oncogenic tyrosine kinase NPM-ALK induces expression of the growth-promoting receptor ICOS. Blood. 2011;118:3062–3071. doi: 10.1182/blood-2011-01-332916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzec M., Liu X., Wong W., Yang Y., Pasha T., Kantekure K., Zhang P., Woetmann A., Cheng M., Odum N., Wasik M.A. Oncogenic kinase NPM/ALK induces expression of HIF1α mRNA. Oncogene. 2011;30:1372–1378. doi: 10.1038/onc.2010.505. [DOI] [PubMed] [Google Scholar]
- 19.Martinengo C., Poggio T., Menotti M., Scalzo M.S., Mastini C., Ambrogio C., Pellegrino E., Riera L., Piva R., Ribatti D., Pastorino F., Perri P., Ponzoni M., Wang Q., Voena C., Chiarle R. ALK-dependent control of hypoxia-inducible factors mediates tumor growth and metastasis. Cancer Res. 2014;74:6094–6106. doi: 10.1158/0008-5472.CAN-14-0268. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Wang H.Y., Liu X., Wasik M.A. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med. 2007;13:1341–1348. doi: 10.1038/nm1659. [DOI] [PubMed] [Google Scholar]
- 21.Coglievina M., Guarnaccia C., Pintar A., Pongor S. Different degrees of structural order in distinct regions of the transcriptional repressor HES-1. Biochim Biophys Acta. 2010;1804:2153–2161. doi: 10.1016/j.bbapap.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z.H., Dai X.M., Du B. Hes1: a key role in stemness, metastasis and multidrug resistance. Cancer Biol Ther. 2015;16:353–359. doi: 10.1080/15384047.2015.1016662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto N., Ueo T., Fukuda A., Kawada K., Sakai Y., Miyoshi H., Taketo M.M., Chiba T., Seno H. Distinct roles of HES1 in normal stem cells and tumor stem-like cells of the intestine. Cancer Res. 2017;77:3442–3454. doi: 10.1158/0008-5472.CAN-16-3192. [DOI] [PubMed] [Google Scholar]
- 24.Yu X., Alder J.K., Chun J.H., Friedman A.D., Heimfeld S., Cheng L., Civin C.I. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells. 2006;24:876–888. doi: 10.1634/stemcells.2005-0598. [DOI] [PubMed] [Google Scholar]
- 25.De Obaldia M.E., Bell J.J., Wang X., Harly C., Yashiro-Ohtani Y., DeLong J.H., Zlotoff D.A., Sultana D.A., Pear W.S., Bhandoola A. T cell development requires constraint of the myeloid regulator C/EBP-α by the Notch target and transcriptional repressor Hes1. Nat Immunol. 2013;14:1277–1284. doi: 10.1038/ni.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka S., Liu W., Masutani H., Hirata H., Shinkai Y., Yamada S., Yoshida T., Nakamura H., Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20:121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- 27.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P., Gao J., Wang X., Wen W., Yang H., Tian Y., Liu N., Wang Z., Liu H., Zhang Y., Tu Y. A novel indication of thioredoxin-interacting protein as a tumor suppressor gene in malignant glioma. Oncol Lett. 2017;14:2053–2058. doi: 10.3892/ol.2017.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalev A. Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic β-cell. Mol Endocrinol. 2014;28:1211–1220. doi: 10.1210/me.2014-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junn E., Han S.H., Im J.Y., Yang Y., Cho E.W., Um H.D., Kim D.K., Lee K.W., Han P.L., Rhee S.G., Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 31.Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popovic M., Wienk H., Coglievina M., Boelens R., Pongor S., Pintar A. The basic helix-loop-helix region of the transcriptional repressor hairy and enhancer of split 1 is preorganized to bind DNA. Proteins. 2014;82:537–545. doi: 10.1002/prot.24507. [DOI] [PubMed] [Google Scholar]
- 33.Wasik M.A., Zhang Q., Marzec M., Kasprzycka M., Wang H.Y., Liu X. Anaplastic lymphoma kinase (ALK)-induced malignancies: novel mechanisms of cell transformation and potential therapeutic approaches. Semin Oncol. 2009;36:S27–S35. doi: 10.1053/j.seminoncol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q., Raghunath P.N., Xue L., Majewski M., Carpentieri D.F., Odum N., Morris S., Skorski T., Wasik M.A. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q., Wang H.Y., Woetmann A., Raghunath P.N., Odum N., Wasik M.A. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108:1058–1064. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtsuka T., Ishibashi M., Gradwohl G., Nakanishi S., Guillemot F., Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi T., Kageyama R. Expression dynamics and functions of Hes factors in development and diseases. Curr Top Dev Biol. 2014;110:263–283. doi: 10.1016/B978-0-12-405943-6.00007-5. [DOI] [PubMed] [Google Scholar]
- 38.Sueda R., Imayoshi I., Harima Y., Kageyama R. High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes Dev. 2019;33:511–523. doi: 10.1101/gad.323196.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng S.Q., Alexandrou A.T., Li J.J. Breast cancer stem cells: multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7. doi: 10.1016/j.canlet.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao F., Zhang Y., Wang S., Liu Y., Zheng L., Yang J., Huang W., Ye Y., Luo W., Xiao D. Hes1 is involved in the self-renewal and tumourigenicity of stem-like cancer cells in colon cancer. Sci Rep. 2014;4:3963. doi: 10.1038/srep03963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guiu J., Shimizu R., D'Altri T., Fraser S.T., Hatakeyama J., Bresnick E.H., Kageyama R., Dzierzak E., Yamamoto M., Espinosa L., Bigas A. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J Exp Med. 2013;210:71–84. doi: 10.1084/jem.20120993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendorff A.A., Koch U., Wunderlich F.T., Wirth S., Dubey C., Brüning J.C., MacDonald H.R., Radtke F. Hes1 is a critical but context-dependent mediator of canonical Notch signaling in lymphocyte development and transformation. Immunity. 2010;33:671–684. doi: 10.1016/j.immuni.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Kageyama R., Ohtsuka T., Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi T., Kageyama R. Hes1 oscillations contribute to heterogeneous differentiation responses in embryonic stem cells. Genes (Basel) 2011;2:219–228. doi: 10.3390/genes2010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamakura S., Oishi K., Yoshimatsu T., Nakafuku M., Masuyama N., Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6:547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z., Lopez-Ramos D.A., Yoshihara E., Maeda Y., Masutani H., Sugie K., Maeda M., Yodoi J. Thioredoxin-binding protein-2 (TBP-2/VDUP1/TXNIP) regulates T-cell sensitivity to glucocorticoid during HTLV-I-induced transformation. Leukemia. 2011;25:440–448. doi: 10.1038/leu.2010.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi S., Sun J., Qiu L., Fu W., Wang A., Liu X., Yang Y., Kadin M.E., Tu P., Wang Y. Dual role of EZH2 in cutaneous anaplastic large cell lymphoma: promoting tumor cell survival and regulating tumor microenvironment. J Invest Dermatol. 2018;138:1126–1136. doi: 10.1016/j.jid.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 48.Hong S.Y., Hagen T. 2-Deoxyglucose induces the expression of thioredoxin interacting protein (TXNIP) by increasing O-GlcNAcylation - implications for targeting the Warburg effect in cancer cells. Biochem Biophys Res Commun. 2015;465:838–844. doi: 10.1016/j.bbrc.2015.08.097. [DOI] [PubMed] [Google Scholar]
- 49.Patwari P., Higgins L.J., Chutkow W.A., Yoshioka J., Lee R.T. The interaction of thioredoxin with Txnip: evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J., Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal. 2012;17:1738–1747. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CRISPR/Cas9-mediated depletion of hairy and enhancer of split homolog-1 (HES1). The depicted anaplastic lymphoma kinase–positive T-cell lymphoma SUDH-L1 and control HEK239 cells were transfected with either HES1 single-guide RNA or empty vector (EV) as control and examined for HES1 protein expression by Western blot analysis. The single-guide HES1 RNA and EV-transfected SUDHL-1 cells were injected into NSG mice and monitored for tumor growth, as shown in Figure 2A.