Abstract
BACKGROUND
Vaccines against coronavirus disease 2019 have a high level of efficacy and safety across all populations. However, numerous case series have been published on neurological disorders, including Bell’s palsy, Guillain-Barre syndrome, transverse myelitis, and multiple sclerosis. The authors presented a case of trigeminal neuropathy after coronavirus vaccination in a patient who had undergone microvascular decompression (MVD) for trigeminal neuralgia (TN).
OBSERVATIONS
A 77-year-old woman presented with acute trigeminal neuropathy after receiving a Pfizer-BioNtech vaccination (tozinameran) against severe acute respiratory syndrome coronavirus 2. The patient had undergone MVD for TN and the facial pain completely disappeared. One month later, she received the first injection of the tozinameran vaccine. Twelve hours after vaccination, she presented with numbness and pain induced by touching any place on the entire right face. No eruption was observed on her face. The serum herpes zoster virus antibodies were confirmed within the normal range. Magnetic resonance imaging revealed no abnormalities. The authors suspected a right trigeminal neuropathy after vaccination. Administration of carbamazepine and pregabalin improved TN but facial numbness persisted, especially in the mandibular division.
LESSONS
The coronavirus is a possible etiology of secondary trigeminal neuropathy in the case of MVD for TN.
Keywords: case report, trigeminal neuropathy, COVID-19, vaccination, microvascular decompression
ABBREVIATIONS : COVID-19 = coronavirus disease 2019, MRI = magnetic resonance imaging, MVD = microvascular decompression, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, TN = trigeminal neuralgia
In March 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19) as a pandemic. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes serious morbidity and mortality. To prevent infection, scientists have developed vaccines and administered them to people worldwide.1,2 These vaccines often use mRNA, such as those developed by Pfizer-BioNtech or Moderna.1,2 They did not show serious adverse effects in ongoing phase III clinical trials,1,2 and there was a high level of efficacy and safety across all populations although many side effects of neurological involvements have been shown.1,2 The complications included Bell’s palsy,3 Guillain-Barre syndrome,4 transverse myelitis,5 and multiple sclerosis.6 However, the incidence is not well known. Herein, we describe a case of acute trigeminal neuropathy after receiving a Pfizer-BioNtech vaccination (tozinameran) against SARS-CoV-2 in a patient who had undergone microvascular decompression (MVD) for trigeminal neuralgia (TN).
Illustrative Case
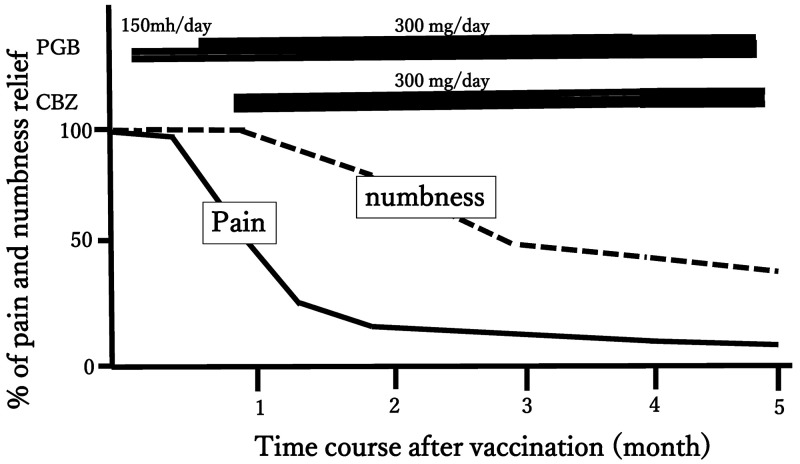
This case involves a 77-year-old woman with a medical history of hypertension and hyperlipidemia and no surgical history. Three years ago, she began to experience electric shock and pain on the right side of her face, especially in the mandibular area of the trigeminal nerve. Painful attacks were observed five to six times a day when the patient ate or touched her face. Medication was administered, but it was not effective enough for her. As a result, she decided to receive surgery, and MVD for TN was performed. In surgery, the superior cerebellar artery that compressed the right trigeminal nerve was moved and fixed to the cerebellar tentorium. Adequate decompression of the right trigeminal nerve was confirmed. After surgery, the facial pain completely disappeared and she was discharged 7 days after surgery without any drugs. One month after the surgery, she received the first injection of tozinameran vaccine in the right deltoid. Twelve hours after the injection, she presented with numbness and pain on the right side of her face, including the ophthalmic area through the mandibular divisions of the trigeminal nerve. She had no prior drug allergy or adverse reactions to previous vaccinations. Nonetheless, there was hypesthesia throughout the right side of her face to a greater degree over the mandibular division than in other regions. Severe electric facial pain was also noted over the ophthalmic area. There were no other systemic symptoms. No eruption was observed on the face or the auricular region over the clinical course. According to these observations, we assumed that results for herpes infection testing may be negative. Magnetic resonance imaging (MRI) of the brain revealed no abnormalities around the trigeminal nerve, excluding cerebral infarction, multiple sclerosis, and granulation tissue formation around the nerve. Later, we confirmed that the examination of serum varicella and herpes zoster virus antibodies titer on day 14 after vaccination was in the normal range. We suspected right trigeminal neuropathy after tozinameran vaccination. Figure 1 shows the clinical course of this case. Pregabalin was administered to reduce facial pain. The daily dose of pregabalin was increased from 150 to 300 mg because of no response within 2 weeks of starting the medication. Therefore, we added 300 mg of carbamazepine per day. To evaluate the response, the time course of pain and numbness was evaluated by the percentage of relief7 for 5 months (Fig. 1). As a result, the facial pain improved, but the facial numbness persisted despite treatment. Five months after vaccination, the numbness was gradually reduced except for the mandibular division. However, we will continue observing the patient in the outpatient unit.
FIG. 1.
Clinical course of the present case. The time course of pain and numbness was evaluated by the percentage of relief. CBZ = carbamazepine; PGB = pregabalin.
Discussion
Observations
The frequency of trigeminal neuropathy after coronavirus vaccination seems to be extremely rare.8,9 To date, only two cases of trigeminal neuropathy have been reported after vaccination against COVID-19, one with TN without facial sensory disturbance and the other with facial sensory disturbance (Table 1). Narasimhalu et al.8 reported the first case of trigeminal neuritis with cervical radiculitis that occurred 3 hours after vaccination (Pfizer-BioNtech). The symptoms included numbness, swelling, and pain over the left side of the face and neck. MRI findings of the left trigeminal nerve revealed abnormal asymmetrical thickening. In this study, MRI showed no abnormal findings, but contrast enhancement study should have been used. In this report, initially, pregabalin was administered but the pain persisted. As a result, the patient was treated with prednisolone and has shown improvement. Considering the clinical features, this reported case is similar to our case because of the facial numbness. Prednisolone should have been used in our case as well. Kaya and Kaya9 described the second case in which the patient presented with right TN without facial numbness 3 days after injection. The entire face was painful, and MRI did not show a remarkable change. However, the authors also chose steroids as a treatment. They speculated that the presence or absence of numbness may be due to the degree of nerve damage. In this study, the facial pain improved with carbamazepine and pregabalin but the numbness did not. However, we should have considered administering steroids. Three patients, including our case, had no significant medical history. In all three cases, the time between vaccination and onset of symptoms was short, which may indicate an immune-mediated inflammatory response mechanism because it needs less time compared to molecular mimicry.8,10
TABLE 1.
Summary of the cases presented with trigeminal neuropathy after the vaccination against COVID-19
| Authors | Age (yrs)/Sex | History | Time to Onset (hrs) | Facial Numbness | Facial Pain | Affected Area | Other Neurological Sx | Tx |
|---|---|---|---|---|---|---|---|---|
| Kaya & Kaya, 20219 |
45/F |
None |
72 |
– |
+ |
V1-V3 |
NP |
PGB, PSL |
| Narasimhalu et al., 20218 |
52/F |
DM HT HL, scoliosis |
3 |
+ |
+ |
V1-V3 |
Cervical, radiculitis |
PGB, PSL |
| Present case | 77/F | HT HL | 12 | + | + | V1-V3 | NP | PGB, CBZ |
CBZ = carbamazepine; DM = diabetes mellitus; HL = hyperlipidemia; HT = hypertension; NP = nothing particular; PGB = pregabalin; PSL = prednisolone; Sx = symptom; Tx = treatment; V1 = ophthalmic division of trigeminal nerve; V2 = maxillary division of trigeminal nerve; V3 = mandibular division of trigeminal nerve.
The first differential diagnosis was trigeminal neuropathy due to herpes infection. Facial eruption and serum antibody titer can differentiate herpes infection. The recurrence of TN due to postoperative adhesions around the nerve is also possible. Other causes include cerebral infarction and multiple sclerosis. MRI may be useful to rule out these conditions. However, based on our test results, we strongly suspected a vaccine-induced trigeminal neuropathy. The symptom happened suddenly, 12 hours after vaccination. The affected area was not only the mandibular division but the entire right side of the face, with the predominance of numbness rather than pain. It was determined that it was not a recurrence of TN. However, there have been no other reports of trigeminal neuropathy caused by vaccination after MVD for TN. Additionally, Molina-Gil et al.11 reported a case of COVID-19 infection presenting with TN. TN appeared earlier than respiratory symptoms due to COVID infection. The facial pain area was the right ophthalmic division without numbness. TN was improved by administrating abandon, a COVID treatment.
There are numerous reports about neurological complications of vaccinations against COVID-19.3–6,8,9,11 The mechanism behind these complications is not fully elucidated. Some researchers suggest that headache and facial pain occur when COVID-19 invades the central nervous system retrogradely by binding to angiotensin-converting enzyme type 2 receptors in the terminal branch of the trigeminal nerve that resides in the nasal mucosa.12 Others propose an indirect activation of the trigeminal-vascular system by a cytokine cascade.13 In this study, it is too early to say whether the patient had received MVD and an immunological reaction was involved or whether the patient was prone to trigeminal neuropathy due to damage to the surface of the trigeminal nerve caused by MVD. However, more studies are required to clarify the pathophysiology of trigeminal neuropathy after vaccination against COVID-19.8,9 There are currently no critical guidelines for vaccination in the postoperative situation. The vaccine decision will be made based on an individual’s condition. In this study, the surgery was completed without any problems, the general condition was stable, and the vaccination was approved. We recommend that vaccination should be given with great care, especially in postoperative cases of TN.
Lessons
Trigeminal neuropathy can be caused by the coronavirus vaccine. Furthermore, in patients who have undergone MVD for TN, the vaccination may cause trigeminal neuropathy, so careful observation is necessary. Steroids may be an option for treatment.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Onoda, Michiwaki, Suehiro, Kawashima. Acquisition of data: Onoda, Sashida, Wakamiya, Michiwaki. Analysis and interpretation of data: Onoda, Michiwaki, Shimoji, Suehiro. Drafting the article: Onoda, Sashida, Michiwaki, Kawashima. Critically revising the article: Fujiwara, Tanaka, Shimoji, Matsuno. Reviewed submitted version of manuscript: Onoda, Michiwaki, Shimoji, Suehiro, Yamane, Kawashima, Matsuno. Approved the final version of the manuscript on behalf of all authors: Onoda. Administrative/technical/material support: Onoda, Michiwaki, Suehiro. Study supervision: Onoda, Michiwaki, Yamane, Kawashima.
References
- 1. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2: preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2022;269(3):1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269(1):55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mureb M, Golub D, Benjamin C, et al. Earlier radiosurgery leads to better pain relief and less medication usage for trigeminal neuralgia patients: an international multicenter study. J Neurosurg. 2020;135(1):1–8. doi: 10.3171/2020.4.JNS192780. [DOI] [PubMed] [Google Scholar]
- 8. Narasimhalu K, Lee WC, Salkade PR, De Silva DA. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep. 2021;14(6):e242344. doi: 10.1136/bcr-2021-242344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaya A, Kaya SY. A case of trigeminal neuralgia developing after a COVID-19 vaccination. J Neurovirol. 2021 doi: 10.1007/s13365-021-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doser K, Hartmann F. Vermutete neurologische Nebenwirkungen der FSME-Impfung: Erfahrung der Schweizerischen Arzneimittel-Nebenwirkungs-Zentrale (SANZ) Praxis (Bern) 2002;91(5):159–162. doi: 10.1024/0369-8394.91.5.159. [DOI] [PubMed] [Google Scholar]
- 11. Molina-Gil J, González-Fernández L, García-Cabo C. Trigeminal neuralgia as the sole neurological manifestation of COVID-19: a case report. Headache. 2021;61(3):560–562. doi: 10.1111/head.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 13. Bobker SM, Robbins MS. COVID-19 and headache: a primer for trainees. Headache. 2020;60(8):1806–1811. doi: 10.1111/head.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]