Key Points
Question
Can casirivimab and imdevimab effectively reduce the viral load of SARS-CoV-2 with lower doses and a subcutaneous route of administration?
Findings
In this phase 2 randomized clinical trial of outpatients with SARS-CoV-2, all casirivimab and imdevimab doses and routes of administration showed statistically significant and comparable reductions in viral load through day 7 vs placebo in those who were seronegative at baseline.
Meaning
These findings, combined with the results from additional studies examining clinical efficacy, justify lowering the dose of casirivimab and imdevimab from 2400 mg to 1200 mg and suggest that subcutaneous administration is a viable alternative to intravenous administration.
This randomized clinical trial assesses the virologic efficacy of casirivimab and imdevimab across different intravenous and subcutaneous doses compared with placebo.
Abstract
Importance
The monoclonal antibody combination of casirivimab and imdevimab reduced viral load, hospitalization, or death when administered as a 1200-mg or greater intravenous (IV) dose in a phase 3 COVID-19 outpatient study. Subcutaneous (SC) and/or lower IV doses should increase accessibility and/or drug supplies for patients.
Objective
To assess the virologic efficacy of casirivimab and imdevimab across different IV and SC doses compared with placebo.
Design, Setting, and Participants
This phase 2, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study included outpatients with SARS-CoV-2 infection at 47 sites across the United States. Participants could be symptomatic or asymptomatic; symptomatic patients with risk factors for severe COVID-19 were excluded. Data were collected from December 15, 2020, to March 4, 2021.
Interventions
Patients were randomized to a single IV dose (523 patients) of casirivimab and imdevimab at 300, 600, 1200, or 2400 mg or placebo; or a single SC dose (292 patients) of casirivimab and imdevimab at 600 or 1200 mg or placebo.
Main Outcomes and Measures
The primary end point was the time-weighted average daily change from baseline (TWACB) in viral load from day 1 (baseline) through day 7 in patients seronegative for SARS-CoV-2 at baseline.
Results
Among 815 randomized participants, 507 (282 randomized to IV treatment, 148 randomized to SC treatment, and 77 randomized to placebo) were seronegative at baseline and included in the primary efficacy analysis. Participants randomized to IV had a mean (SD) age of 34.6 (9.6) years (160 [44.6%] men; 14 [3.9%] Black; 121 [33.7%] Hispanic or Latino; 309 [86.1%] White); those randomized to SC had a mean age of 34.1 (10.0) years (102 [45.3%] men; 75 [34.7%] Hispanic or Latino; 6 [2.7%] Black; 190 [84.4%] White). All casirivimab and imdevimab treatments showed significant virologic reduction through day 7. Least-squares mean differences in TWACB viral load for casirivimab and imdevimab vs placebo ranged from –0.56 (95% CI; –0.89 to –0.24) log10 copies/mL for the 1200-mg IV dose to –0.71 (95% CI, –1.05 to –0.38) log10 copies/mL for the 2400-mg IV dose. There were no adverse safety signals or dose-related safety findings, grade 2 or greater infusion-related or hypersensitivity reactions, grade 3 or greater injection-site reactions, or fatalities. Two serious adverse events not related to COVID-19 or the study drug were reported.
Conclusions and Relevance
In this randomized clinical trial including outpatients with asymptomatic and low-risk symptomatic SARS-CoV-2, all IV and SC doses of casirivimab and imdevimab comparably reduced viral load.
Trial Registration
ClinicalTrials.gov Identifier: NCT04666441
Introduction
SARS-CoV-2 emerged in 2019 as the causal agent of COVID-19, responsible for a worldwide pandemic.1 While some infected individuals remain asymptomatic, others are at risk of developing a range of respiratory conditions, from mild symptoms to severe and often fatal illness.2,3 Recent studies among hospitalized patients found that high SARS-CoV-2 viral load is associated with increased mortality rates.4,5 Such findings suggest that a SARS-CoV-2 antiviral therapy given to outpatients may reduce the risk of hospitalization or death due to COVID-19.
Blockade of host cell entry using neutralizing antibodies against spike (S) proteins that bind to angiotensin-converting enzyme 2 (ACE2) is one mechanistic strategy to reduce SARS-CoV-2–related pathogenesis.6,7 Casirivimab and imdevimab are human, immunoglobulin G1 monoclonal antibodies (mAbs) that simultaneously bind the receptor-binding domain of the SARS-CoV-2 S protein to block interaction with ACE2. In vitro and preclinical studies have shown that these 2 antibodies, administered together, minimize viral escape due to multiple SARS-CoV-2 mutations.8,9,10 Preclinical data show that combination casirivimab and imdevimab retains neutralization potency against multiple variants of concern, including B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), B.1.429 (Epsilon), and P.1 (Gamma), but exhibits diminished neutralization activity against the B.1.1.529 and BA.1 (Omicron) variants.11 These results show a promising therapeutic approach of coadministering the antibodies 1:1 in combination (casirivimab and imdevimab) to reduce SARS-CoV-2 viral load and COVID-19 disease progression.
Results from the phase 3 portion of an adaptive study of outpatients with SARS-CoV-2 (NCT04425629) demonstrated the clinical efficacy of the 2400-mg intravenous (IV) and 1200-mg IV doses of casirivimab and imdevimab in patients at high risk of progressing to severe COVID-19.12 The phase 2 dose-ranging study reported here, with results available at the same time as the 1200-mg and greater IV efficacy results noted previously, assessed the virologic efficacy and safety of lower doses of IV and subcutaneous (SC) casirivimab and imdevimab in outpatients with asymptomatic or symptomatic SARS-CoV-2 infection, without risk factors for progressing to severe COVID-19.
Methods
Trial Design
This phase 2, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study was conducted at 47 sites across the United States. The first patient was enrolled on December 15, 2020, and the last day-7 visit was completed on March 4, 2021 (prior to the emergence of the B.1.1.529 Omicron variant).
The trial was conducted in accordance with the principles of the Declaration of Helsinki Good Clinical Practices and International Council for Harmonisation E-9 guidelines, as well as all applicable laws and regulations. The protocol and all amendments were reviewed and approved by an independent Institutional Review Board and Ethics Committee at each study center (eAppendix 1 in Supplement 1). The trial protocol and statistical analysis plan appear in Supplement 2. All participants provided written informed consent. This study is reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Nasopharyngeal (NP) swabs and blood samples were collected from patients every other day for the first week (eFigure 1 in Supplement 1). A telephone visit occurred during the fourth week to collect safety information. After the first month, patients had monthly visits for 4 additional months. The final visit (end of study, day 169) was a telephone call.
Patients
The study enrolled outpatients aged 18 years and older with a positive SARS-CoV-2 diagnostic test (using a local SARS-CoV-2 antigen, quantitative reverse transcription–polymerase chain reaction [RT-qPCR], or other molecular diagnostic assay) from a sample (such as NP, nasal, oropharyngeal, or saliva) collected within 72 hours prior to randomization. Eligible patients tested positive for SARS-CoV-2 but were either asymptomatic or symptomatic within 7 days before randomization. Eligible symptomatic patients were required to be at low risk for developing severe COVID-19, defined as meeting all the following criteria: body mass index (calculated as weight in kilograms divided by height in meters squared) less than 30; age 50 years or younger; not pregnant; and no cardiovascular disease or hypertension, chronic lung disease or asthma, type 1 or 2 diabetes, chronic kidney disease, or chronic liver disease. Eligible asymptomatic patients, with or without risk factors, could not have symptoms consistent with COVID-19 currently or at any time within 2 months and could not have received a COVID-19 vaccination prior to randomization. In assessing eligibility, the presence or absence of COVID-19 symptoms was determined at the discretion of investigators. Immunosuppressed patients were excluded. It was not feasible to wait for a patient’s serology status prior to randomization; thus enrolled patients with SARS-CoV-2 were randomized regardless of serostatus. Self-reported data on participants’ race and ethnicity (both based on fixed categories, ie, for race, American Indian or Alaska Native, Asian, Black, White, unknown, and not reported; for ethnicity, Hispanic or Latino, not Hispanic or Latino, or not reported) were collected to describe the demographic characteristics of the trial population.
Intervention and Assessments
Eligible patients were randomized according to a central randomization scheme using an interactive web response system. Patients received a single dose of 1 of the 6 doses of casirivimab and imdevimab, placebo IV, or placebo SC with a respective randomization ratio of 2:2:2:2:2:2:1:1 (eFigure 1 in Supplement 1). Baseline (day 1) was the day of study drug or placebo administration. The IV single-dose regimens were casirivimab and imdevimab at 300 mg (150 mg per mAb), at 600 mg (300 mg per mAb), at 1200 mg (600 mg per mAb), and at 2400 mg (1200 mg per mAb). The SC single-dose regimens were casirivimab and imdevimab at 600 mg (2 injections of 300 mg per mAb) and at 1200 mg (4 injections of 600 mg per mAb). Each injection volume was 2.5 mL. Health care professionals, patients, and caregivers were blinded to placebo and to the doses of casirivimab and imdevimab, but not to the route of administration.
End Points
The primary end point was the time-weighted average daily change from baseline (TWACB) in viral load (log10 copies per milliliter) to day 7, as measured by RT-qPCR of NP swab samples in patients who had a central laboratory–determined RT-qPCR positive test at baseline and were seronegative (ie, negative for anti-spike [S1] IgA, anti-spike [S1] IgG, and anti-nucleocapsid IgG) (eAppendix 2 and 3 in Supplement 1).
Secondary end points included the evaluation of additional indicators of virologic efficacy, safety, and tolerability, as well as casirivimab and imdevimab concentrations in serum over time (eAppendix 5 in Supplement 1). The key safety variables included the incidence of all treatment-emergent adverse events (TEAEs) up to day 29, grade 3 or greater TEAEs from day 30 to the end of the study (EOS), serious adverse events (SAEs) through EOS, and adverse events of special interest (AESIs). AESIs included all grade 2 or greater infusion-related reactions through day 4, grade 3 or greater injection-site reactions through day 4, grade 2 or greater hypersensitivity reactions through day 29, and any TEAE that led to a hospitalization or an emergency department visit throughout the study, regardless of relation to COVID-19.
Statistical Analysis
With the assumption that 50% of enrolled patients would be seronegative at baseline and to accommodate an early database lock due to regulatory updates, approximately 800 patients were randomized to enroll 400 seronegative patients for efficacy analyses (ie, 57 patients per active treatment group). It was estimated that a sample size of 57 patients per treatment group would provide 98% power to detect a difference of –0.73 log10 copies/mL (assuming an SD of 0.948) between any active treatment group and pooled placebo (IV and SC). The assumed SD and targeted difference used for powering were based on the observed variability and treatment difference of time-weighted average measurements in a companion phase 2 study of casirivimab and imdevimab in the outpatient treatment setting.13
The route of administration was not expected to alter pharmacodynamic response in patients who received placebo. The IV and SC placebo groups were therefore pooled for all virologic efficacy analyses. Active treatment groups were not pooled for any efficacy analysis.
The overall modified full analysis set (mFAS) included all randomized patients who received treatment and who had a positive central laboratory–determined SARS-CoV-2 RT-qPCR result from NP swab samples at randomization and was based on the treatment received. The primary analysis was conducted in the subset of patients in the overall mFAS who were seronegative at baseline for SARS-CoV-2 (seronegative mFAS). The efficacy analyses were based on observed data, with no imputation for missing data. The primary efficacy variable, TWACB in viral load, was calculated using the area under the curve (AUC) divided by the number of days (day 1 to day 7) (eAppendix 4 in Supplement 1). AUC was calculated using the linear trapezoidal rule. TWACB in viral load is a composite variable where multiple measurements are combined into a single variable using an algorithm that is defined in the protocol and elaborated on in the statistical analysis plan (Supplement 2). Consequently, the analysis of multiple visits does not require adjustment for the type I error (ICH E9)14 (eAppendix 4 in Supplement 1). Statistical analysis for TWACB in viral load was conducted using a scale of log10 copies per milliliter. An analysis of covariance model with treatment group as a fixed effect, and baseline viral load and treatment by baseline interaction as covariates, was fitted to the data for analysis of the primary end point. The overall type I error was controlled at the 5% level via a prespecified hierarchical testing procedure (ie, comparison between each active treatment group and placebo was formally tested). Tests were 2-tailed. Analyses were conducted in SAS version 9.4 (SAS Institute). Additional statistical analysis details are described in eAppendix 4 in Supplement 1. The safety assessments were summarized descriptively, and the safety analysis set (SAF) included all randomized patients who received any study drug.
Results
Participants
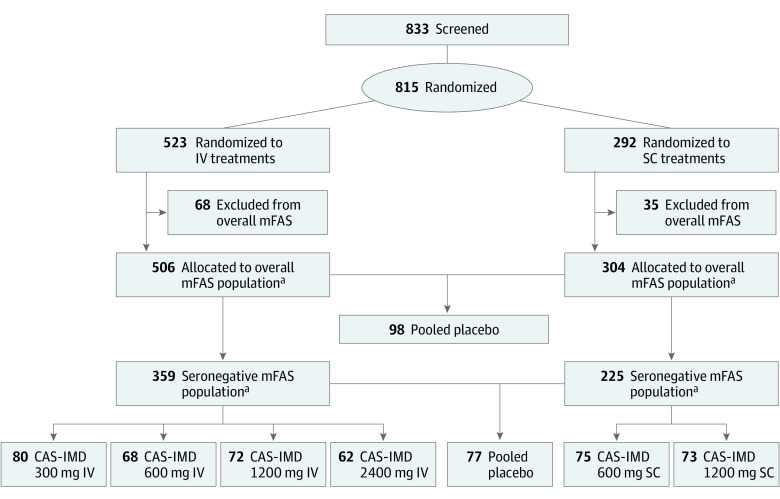
A total of 815 patients were randomized and assigned to IV administration (n = 523) or SC administration (n = 292), with 584 patients seronegative at baseline (Figure 1). Of those patients assigned to IV administration, 506 were SARS-CoV-2 positive via central laboratory–testing and therefore allocated to the overall mFAS; of those patients, 359 were seronegative at baseline for SARS-CoV2 antibodies and therefore allocated to the seronegative mFAS (mean [SD] age, 34.6 [9.6] years; 160 [44.6%] men; 14 [3.9%] Black; 121 [33.7%] Hispanic or Latino; 309 [86.1%] White). Of the patients assigned to SC administration, 304 were allocated to the overall mFAS, and 225 to the seronegative mFAS (mean [SD] age, 34.1 [10.0] years; 102 [45.3%] men; 78 [34.7%] Hispanic or Latino; 6 [2.7%] Black; 190 [84.4%] White). In the analysis sets for efficacy (overall mFAS and seronegative mFAS), placebo IV and placebo SC are pooled, and this is reflected in the numbers presented previously.
Figure 1. Study Flow Diagram.
Inclusion in the overall modified full analysis set (mFAS) population required a positive polymerase chain reaction test from a central laboratory at baseline; the placebo groups are pooled. The seronegative mFAS population is a proper subset of the overall mFAS, in which patients were also required to be seronegative at baseline. CAS indicates casirivimab; IMD, imdevimab.
aThese numbers include the pooled (intravenous [IV] and subcutaneous [SC]) placebo group members (98 in the overall mFAS and 77 in the seronegative mFAS).
Of the seronegative mFAS patients assigned to IV administration, 80 were treated with 300-mg casirivimab and imdevimab, 68 were treated with 600-mg casirivimab and imdevimab, 72 were treated with 1200-mg casirivimab and imdevimab, 62 were treated with 2400-mg casirivimab and imdevimab, and 77 were treated with placebo (pooled placebo group) (Table 1). In the IV group, the mean (SD) baseline viral load was 7.2 (1.5) log10 copies/mL, and 204 patients (56.8%) had a baseline viral load greater than 107 copies/mL.
Table 1. Demographic and Baseline Characteristics for Patients in the Seronegative Modified Full Analysis Set IV and SC Groups.
| Characteristic | Participants, No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled placebo | IV | SC | |||||||
| Casirivimab and imdevimab | Totala | Casirivimab and imdevimab | Totala | ||||||
| 300 mg | 600 mg | 1200 mg | 2400 mg | 600 mg | 1200 mg | ||||
| No. | 77 | 80 | 68 | 72 | 62 | 359 | 75 | 73 | 225 |
| Age, mean (SD), y | 35.1 (9.97) | 33.8 (8.90) | 33.9 (9.16) | 34.1 (10.51) | 36.3 (9.16) | 34.6 (9.55) | 33.5 (9.18) | 33.5 (10.88) | 34.1 (10.00) |
| Sex | |||||||||
| Male | 31 (40.3) | 33 (41.3) | 39 (57.4) | 29 (40.3) | 28 (45.2) | 160 (44.6) | 36 (48.0) | 35 (47.9) | 102 (45.3) |
| Female | 46 (59.7) | 47 (58.8) | 29 (42.6) | 43 (59.7) | 34 (54.8) | 199 (55.4) | 39 (52.0) | 38 (52.1) | 123 (54.7) |
| Ethnicity | |||||||||
| Hispanic or Latino | 27 (35.1) | 28 (35.0) | 16 (23.5) | 26 (36.1) | 24 (38.7) | 121 (33.7) | 30 (40.0) | 21 (28.8) | 78 (34.7) |
| Not Hispanic or Latino | 50 (64.9) | 52 (65.0) | 52 (76.5) | 43 (59.7) | 38 (61.3) | 235 (65.5) | 44 (58.7) | 50 (68.5) | 144 (64.0) |
| Not reported | 0 | 0 | 0 | 3 (4.2) | 0 | 3 (0.8) | 1 (1.3) | 2 (2.7) | 3 (1.3) |
| Race | |||||||||
| American Indian or Alaska Native | 0 | 2 (2.5) | 1 (1.5) | 0 | 0 | 3 (0.8) | 0 | 0 | 0 |
| Asian | 10 (13.0) | 2 (2.5) | 2 (2.9) | 7 (9.7) | 2 (3.2) | 23 (6.4) | 5 (6.7) | 9 (12.3) | 24 (10.7) |
| Black | 2 (2.6) | 3 (3.8) | 2 (2.9) | 3 (4.2) | 4 (6.5) | 14 (3.9) | 2 (2.7) | 2 (2.7) | 6 (2.7) |
| White | 64 (83.1) | 71 (88.8) | 63 (92.6) | 56 (77.8) | 55 (88.7) | 309 (86.1) | 67 (89.3) | 59 (80.8) | 190 (84.4) |
| Unknown | 0 | 0 | 0 | 1 (1.4) | 0 | 1 (0.3) | 0 | 1 (1.4) | 1 (0.4) |
| Not reported | 1 (1.3) | 2 (2.5) | 0 | 5 (6.9) | 1 (1.6) | 9 (2.5) | 1 (1.3) | 2 (2 .7) | 4 (1.8) |
| Weight, mean (SD), kg | 74.0 (16.0) | 73.1 (14.0) | 73.1 (13.5) | 73.1 (13.5) | 73.2 (12.3) | 73.3 (13.9) | 72.8 (12.5) | 74.5 (13.5) | 73.8 (14.1) |
| SARS-CoV-2 results from central laboratory, NP swab, mean (SD), log10 copies/mL | 7.0 (1.4) | 7.2 (1.6) | 7.4 (1.5) | 7.2 (1.5) | 7.3 (1.6) | 7.2 (1.5) | 7.4 (1.5) | 7.2 (1.6) | 7.2 (1.5) |
| Baseline viral load categories, copies/mL | |||||||||
| >103 | 77 (100) | 79 (98.8) | 68 (100) | 72 (100) | 62 (100) | 358 (99.7) | 73 (97.3) | 72 (98.6) | 222 (98.7) |
| >104 | 76 (98.7) | 78 (97.5) | 66 (97.1) | 70 (97.2) | 60 (96.8) | 350 (97.5) | 73 (97.3) | 71 (97.3) | 220 (97.8) |
| >105 | 72 (93.5) | 72 (90.0) | 63 (92.6) | 66 (91.7) | 55 (88.7) | 328 (91.4) | 70 (93.3) | 64 (87.7) | 206 (91.6) |
| >106 | 60 (77.9) | 61 (76.3) | 59 (86.8) | 54 (75.0) | 51 (82.3) | 285 (79.4) | 62 (82.7) | 58 (79.5) | 180 (80.0) |
| >107 | 38 (49.4) | 48 (60.0) | 42 (61.8) | 40 (55.6) | 36 (58.1) | 204 (56.8) | 49 (65.3) | 41 (56.2) | 128 (56.9) |
| Below lower limit of quantification | 0 | 1 (1.3) | 0 | 0 | 0 | 1 (0.3) | 2 (2.7) | 0 | 2 (0.9) |
Abbreviations: IV, intravenous; NP, nasopharyngeal; SC, subcutaneous.
Includes pooled placebo.
Of the seronegative mFAS patients assigned to SC administration, 75 were treated with 600-mg casirivimab and imdevimab, 73 were treated with 1200-mg casirivimab and imdevimab, and 77 were part of the pooled placebo group (Table 1). For the SC group, the mean (SD) baseline viral load was 7.2 (1.5) log10 copies/mL, and 128 patients (56.9%) had a baseline viral load greater than 107 copies/mL.
Patient demographics and baseline characteristics were generally balanced between the IV and SC groups and the pooled placebo group (Table 1). This was also true of the overall mFAS (n = 712) and SAF (n = 803; data not shown). Approximately 92.5% of the IV patients (478) and 91.3% of the SC patients (261) in the SAF were low-risk symptomatic patients, and the remaining were asymptomatic. All patients included in this analysis had the opportunity to complete the primary end point visit to day 7. Because of the earlier database lock, a total of 195 patients in the IV group and 106 patients in the SC group completed visits up to day 29 (SAF) as of the data cut-off of February 8, 2021. As such, future publications will include the full set of data for all patients enrolled in the study, regardless of randomization date.
Viral Load
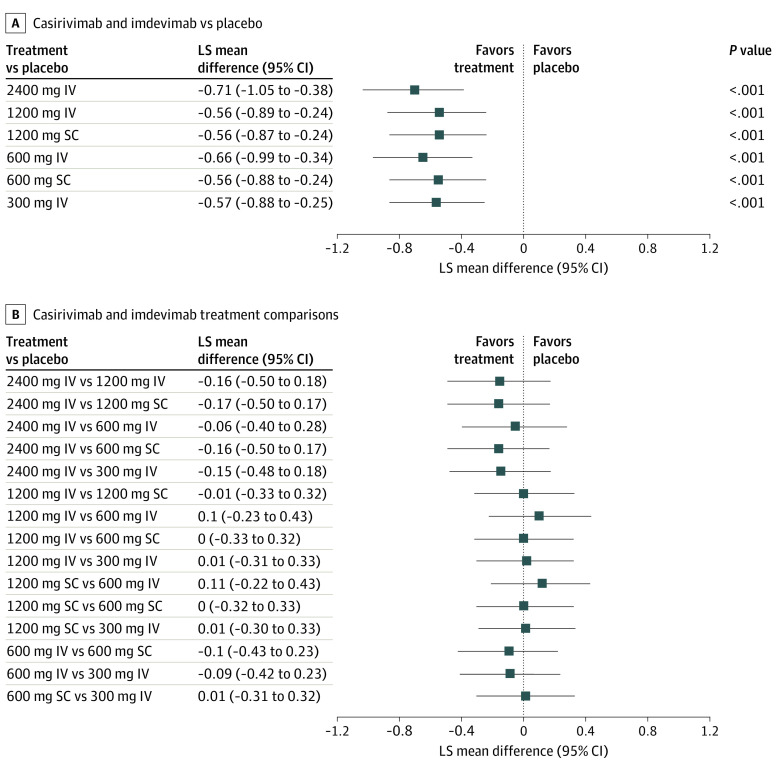
All casirivimab and imdevimab doses (SC and IV) significantly and similarly reduced viral load through day 7 vs pooled placebo (Figure 2A; eTable in Supplement 1). In the IV group, the least-squares mean (LSM) change in TWACB viral load between the pooled placebo group and casirivimab and imdevimab was –0.71 (95% CI, –1.05 to –0.38) log10 copies/mL for 2400-mg dose, –0.56 (95% CI, –0.89 to –0.24) log10 copies/mL for the 1200-mg dose, –0.66 (95% CI, –0.99 to –0.34) log10 copies/mL for the 600-mg dose, and –0.57 (95% CI, –0.88 to –0.25) log10 copies/mL for the 300-mg dose. All differences vs placebo were statistically significant (Figure 2A; eTable in Supplement 1). In the SC group, the LSM change in viral load between the pooled placebo group and casirivimab and imdevimab was –0.56 (95% CI, –0.89 to –0.24) log10 copies/mL for the 1200-mg dose and –0.56 (95% CI, –0.88 to –0.24) log10 copies/mL for the 600-mg dose. All differences vs the pooled placebo group were statistically significant (Figure 2A). All pairwise comparisons between active treatment groups, including different routes of administration, demonstrated similar treatment effects (Figure 2B).
Figure 2. Pairwise Comparisons of the Primary End Point.
A, Results for the seronegative modified full analysis set. B, Results for the seronegative per-protocol set. IV indicates intravenous; LS, least squares; and SC, subcutaneous.
All casirivimab and imdevimab doses (SC and IV) showed similar reductions in viral load from baseline at each visit (eFigure 2 in Supplement 1). A difference in viral load between casirivimab and imdevimab treatments and pooled placebo was apparent by day 3, 48 hours after administration of the study drug. In the seronegative mFAS (eFigure 2A in Supplement 1), the LSM differences from pooled placebo on day 3 in the IV treated groups were –0.44 log10 copies/mL for the 300-mg dose, –0.74 log10 copies/mL for the 600-mg dose, –0.66 log10 copies/mL for the 1200-mg dose, and –0.53 log10 copies/mL for the 2400-mg dose. The corresponding differences in the SC treated groups were –0.34 log10 copies/mL for the 600-mg dose and –0.49 log10 copies/mL for the 1200-mg dose. Similar results were seen in those regardless of baseline serology status (eFigure 2B in Supplement 1).
Pharmacokinetics
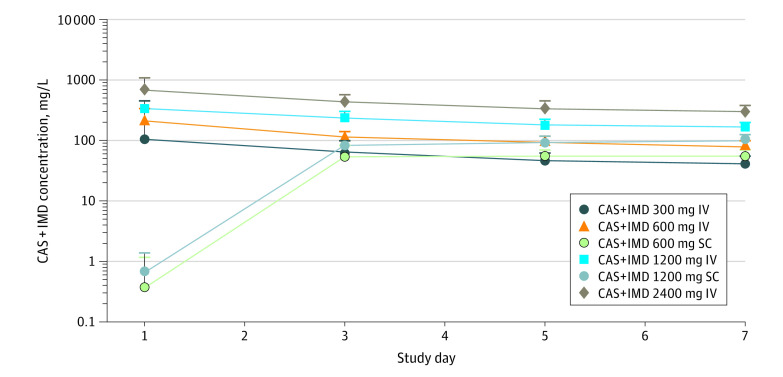
The concentration-time profiles for each antibody were essentially superimposable from day 1 to day 7 for any given dose; the mean concentrations of casirivimab and imdevimab in serum are therefore presented as the sum of total casirivimab and total imdevimab concentrations. After a single IV dose, antibody concentrations decreased linearly from day 1 to day 7 (Figure 3). For the SC doses, the mean concentrations of casirivimab and imdevimab reached near maximal values by day 3, with peak concentrations attained at day 7 (Figure 3). There was no association between TWACB in viral load vs total drug concentration through day 7 for the IV or SC casirivimab and imdevimab doses, suggesting that maximum effect on viral load was achieved at all dose levels (eFigure 3 in Supplement 1).
Figure 3. Mean Total Casirivimab (CAS) and Imdevimab (IMD) Concentrations in Serum in Nominal Time After a Single Dose.
The pharmacokinetic analysis set included all patients who received any study drug and who had 1 or more nonmissing drug concentration measurement following study drug administration. IV indicates intravenous; SC, subcutaneous.
Safety
Overall, in the SAF population (all randomized patients who received any study drug), all IV and SC casirivimab and imdevimab doses showed low rates of adverse events when compared with placebo. No serious safety concern was reported, and no dose-related safety findings were observed; no deaths occurred during the study (Table 2). One patient did not complete infusion of the study drug because of a TEAE of a mild, grade 1 infusion-related reaction from the 2400-mg IV dose, but the patient remained in the study. There were 2 SAEs reported in the 1200-mg IV and 2400-mg IV casirivimab and imdevimab groups, both of which were considered unrelated to the study drug or COVID-19. Both SAEs were miscarriages that occurred during the first trimester: 1 in primigravida and 1 in a patient with significant medical history of miscarriages. There were 5 additional pregnant women treated in the study without any adverse outcome. No participant experienced AESIs including grade 2 or greater infusion-related reaction or hypersensitivity reaction or grade 3 or greater injection-site reaction. The incidence of any TEAE that led to a hospitalization or an emergency department visit (regardless of relation to COVID-19) was low, and none of the events were related to study treatment.
Table 2. Adverse Events in the Safety Population in IV and SC Groupsa.
| Adverse Event | Participants, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| IV | SC | |||||||
| Placebo | Casirivimab and imdevimab | Placebo | Casirivimab and imdevimab | |||||
| 300 mg | 600 mg | 1200 mg | 2400 mg | 600 mg | 1200 mg | |||
| No. | 57 | 115 | 114 | 116 | 115 | 58 | 114 | 114 |
| Patients with | ||||||||
| Any TEAE | 10 (17.5) | 10 (8.7) | 16 (14.0) | 22 (19.0) | 9 (7.8) | 6 (10.3) | 5 (4.4) | 12 (10.5) |
| Any grade 3 or 4 TEAE | 1 (1.8) | 0 | 1 (0.9) | 1 (0.9) | 0 | 0 | 0 | 0 |
| Any SAE | 0 | 0 | 0 | 1 (0.9) | 1 (0.9) | 0 | 0 | 0 |
| Any AESI | 1 (1.8) | 0 | 1 (0.9) | 2 (1.7) | 0 | 0 | 0 | 1 (0.9) |
| Any serious AESI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infusion-related reaction (grade ≥2) through day 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection-site reactions (grade ≥3) through day 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity reactions (grade ≥2) through day 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any TEAE leading to death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any TEAE leading to withdrawal from the study medication | 0 | 0 | 0 | 0 | 1 (0.9) | 0 | 0 | 0 |
| Any TEAE leading to study infusion interruption | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AESI, adverse event of special interest; IV, intravenous; SAE, serious adverse event; SC, subcutaneous; TEAE, treatment-emergent adverse event.
The safety population includes all randomized patients who received any study drug.
Discussion
In this phase 2 dose-ranging study of asymptomatic or low-risk symptomatic outpatients who were SARS-CoV-2 RT-qPCR positive, casirivimab and imdevimab treatment resulted in a significant reduction in viral load (day 1 to day 7) vs placebo in those who were SARS-CoV-2 seronegative at baseline. Viral load showed similar reductions across all casirivimab and imdevimab treatments evaluated, including the lowest doses of 300-mg (150 mg of each mAb) IV or 600-mg (300 mg of each mAb) SC. Reduction in viral load occurred as early as 48 hours after the treatment administration (day 3 of measurement), decreasing (vs placebo) at all doses. Although maximal concentrations in serum were attained a few days later for SC vs IV dosing, casirivimab and imdevimab was rapidly absorbed into systemic circulation following SC administration and achieved concentrations that provided comparable maximal virologic efficacy to the IV doses at the earliest time point measured.
Incidences of TEAEs, SAEs, and AESIs were low and balanced across the casirivimab and imdevimab and placebo groups, suggesting no difference in the safety profile across all doses tested, whether SC or IV. The pharmacokinetics of each antibody were linear and dose proportional. Additional pharmacokinetic and pharmacodynamic modeling has further evaluated the relationship of drug concentration with changes in viral load to characterize the effect of casirivimab and imdevimab on SARS-CoV-2 viral dynamics; details are beyond the scope of the analyses presented here.15
Similar virologic efficacy and safety results were observed in a phase 1, 2, and 3 outpatient study of casirivimab and imdevimab administered as a 1200-mg, 2400-mg, or 8000-mg single IV infusion (NCT04425629).12 In November 2020, the 2400-mg IV dose of casirivimab and imdevimab received emergency use authorization (EUA) from the US Food and Drug Administration for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (aged 12 to 17 years; weighing 40 kg or greater) who had positive SARS-CoV-2 viral testing and who were at high risk of progressing to severe COVID-19.16 Moreover, the 1200-mg SC dose demonstrated efficacy in a COVID-19 prevention study of household contacts of SARS-CoV-2 positive index patients, and an early treatment study of SARS-CoV-2-infected patients (NCT04452318).17,18 As a result of these data, the EUA was updated for the treatment of mild-to-moderate COVID-19 and postexposure prophylaxis in individuals who are at high-risk of progressing to severe COVID-19 with a single 1200-mg IV or SC dose.19 However, data show that casirivimab and imdevimab are unlikely to be active against the currently circulating Omicron variant; therefore, as of January 24, 2022, casirivimab and imdevimab are not authorized for use in any US region.20
Despite the growing number of therapeutics with authorization or approval for the treatment and/or prevention of COVID-19, there remains a significant unmet need globally for effective COVID-19 therapies. Investigating different doses and methods of administration is key to establishing a lower dose with similar effectiveness, while providing convenience for patients. Identifying lower doses that reduce viral load while not compromising clinical efficacy in preventing hospitalization and death ensures the ability to provide therapeutics to as many individuals as possible.
Limitations
This study has a few limitations. First, the results of the present study suggest the minimum dose for virologic efficacy may be lower than those evaluated. Second, this study did not determine whether the equivalent effect on reduction in viral load in the nasopharynx will correlate to similar improvements in clinical outcomes. Third, while concentrations of each antibody on day 3 for the 600-mg SC dose were approximately 60 to several hundred times the concentrations required to neutralize wild-type SARS-CoV-2 virus and variants of concern in vitro, higher doses providing greater exposure margins may be advantageous against new variants. Fourth, in the United States, where all study sites were located, the dominant variants at baseline during the conduct of the study (participants randomized from December 15, 2020, to February 26, 2021) were B.1.427 and B.1.429 (Epsilon) or B.1.1.7 (Alpha).21 Enrollment was completed prior to the emergence of the B.1.1.529 (Omicron) variant and when the circulation of the B.1.617.2 (Delta) variant was very low in the United States.
Conclusions
In this randomized clinical trial including outpatients with asymptomatic and low-risk symptomatic SARS-CoV-2, conducted before the emergence of the Omicron variant, casirivimab and imdevimab significantly and comparably reduced viral load vs pooled placebo across all treatment groups, including doses as low as 300-mg IV or 600-mg SC, in patients seronegative for antibodies against SARS-CoV-2 at baseline. Similar results were also seen regardless of baseline serology status. Casirivimab and imdevimab showed low levels of adverse events across all doses evaluated. Further studies are required to evaluate whether lower doses of casirivimab and imdevimab achieve the same clinical outcomes as higher doses of casirivimab and imdevimab (ie, ≥1200 mg).
eAppendix 1. Study Oversight
eAppendix 2. Quantitative Virology Assay
eAppendix 3. SARS-CoV-2 Serology Testing
eAppendix 4. Additional Statistical Analysis Methods
eAppendix 5. Pharmacokinetic Analysis Methods
eTable. TWACB in Viral Load from Day 1 to Day 7 in Seronegative mFAS
eFigure 1. Study Design
eFigure 2. LS Mean Change From Baseline in Viral Load of IV and SC Doses at Each Visit in the Seronegative mFAS Population and Overall mFAS Population
eFigure 3. TWACB in Viral Load vs Total Casirivimab and Imdevimab Concentrations in Serum in Individual Patients at Days 3, 5, and 7 for IV and SC Treatment Groups
Trial Protocol and Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Ludwig S, Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth Analg. 2020;131(1):93-96. doi: 10.1213/ANE.0000000000004845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magleby R, Westblade LF, Trzebucki A, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2021;73(11):e4197-e4205. doi: 10.1093/cid/ciaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38(5):661-671.e2. doi: 10.1016/j.ccell.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355-359. doi: 10.1016/j.it.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copin R, Baum A, Wloga E, et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021;184(15):3949-3961.e11. doi: 10.1016/j.cell.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen J, Baum A, Pascal KE, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010-1014. doi: 10.1126/science.abd0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130-135. doi: 10.1038/s41586-021-03398-2 [DOI] [PubMed] [Google Scholar]
- 11.Regeneron Pharmaceuticals, Inc . Regeneron’s next generation monoclonal antibodies are active against all known variants of concern, including both Omicron and Delta. Accessed January 6, 2022. https://investor.regeneron.com/static-files/4aed42a1-3d26-48af-bd01-3f0c92938c11
- 12.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton T, Ali S, Sivapalasingam S, et al. REGEN-COV antibody combination in outpatients with COVID-19—phase 1/2 results. medRxiv. Preprint posted online March 18, 2022. doi: 10.1101/2021.06.09.21257915 [DOI]
- 14.European Medicines Agency . ICH E9 statistical principles for clinical trials. Accessed April 7, 2022. https://www.ema.europa.eu/en/ich-e9-statistical-principles-clinical-trials
- 15.Conrado DJ, Patel K, Lin K-J, et al. Viral kinetics in COVID-19 outpatients treated with casirivimab+imdevimab combination. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 18, 2022; Virtual. Accessed July 8, 2022. https://www.croiconference.org/wp-content/uploads/sites/2/resources/2022/croi2022-program-guide.pdf
- 16.Regeneron Pharmaceuticals, Inc . Regeneron's casirivimab and imdevimab antibody cocktail for COVID-19 is first combination therapy to receive FDA emgency use authorization. Accessed April 7, 2021. https://investor.regeneron.com/news-releases/news-release-details/regenerons-regen-cov2-first-antibody-cocktail-covid-19-receive
- 17.O’Brien MP, Forleo-Neto E, Musser BJ, et al. ; Covid-19 Phase 3 Prevention Trial Team . Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385(13):1184-1195. doi: 10.1056/NEJMoa2109682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien MP, Forleo-Neto E, Sarkar N, et al. ; COVID-19 Phase 3 Prevention Trial Team . Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327(5):432-441. doi: 10.1001/jama.2021.24939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration . Fact sheet for health care providers Emergency Use Authorization (EUA) of REGEN-COVTM (casirivimab and imdevimab). Accessed June 24, 2021. https://www.fda.gov/media/145611/download
- 20.US Food and Drug Administration . Emergency Use Authorization 091. Accessed February 10, 2022. https://www.fda.gov/media/145610/download
- 21.GISAID . Accessed April 8, 2022. https://www.gisaid.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Oversight
eAppendix 2. Quantitative Virology Assay
eAppendix 3. SARS-CoV-2 Serology Testing
eAppendix 4. Additional Statistical Analysis Methods
eAppendix 5. Pharmacokinetic Analysis Methods
eTable. TWACB in Viral Load from Day 1 to Day 7 in Seronegative mFAS
eFigure 1. Study Design
eFigure 2. LS Mean Change From Baseline in Viral Load of IV and SC Doses at Each Visit in the Seronegative mFAS Population and Overall mFAS Population
eFigure 3. TWACB in Viral Load vs Total Casirivimab and Imdevimab Concentrations in Serum in Individual Patients at Days 3, 5, and 7 for IV and SC Treatment Groups
Trial Protocol and Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement