Abstract
Objective
Maternal hepatitis C virus (HCV) infection reported on birth certificates has been shown to underestimate HCV infection. We sought to determine the usefulness of HCV surveillance data for (1) quantifying the number of HCV-positive reproductive-aged women with a live birth, (2) comparing maternal HCV surveillance data with reported HCV infection status on birth certificates, and (3) delineating past versus current maternal infection to identify true perinatal exposures.
Methods
We extracted data from January 1, 2013, through December 31, 2017, on birth certificate indication of HCV exposure from the Tennessee Birth Statistical File, and we ascertained indication of HCV exposure by using laboratory data from the Tennessee National Electronic Disease Surveillance System (NEDSS) Base System (NBS). We conducted a sensitivity analysis comparing birth certificate indication of HCV exposure with HCV laboratory data to determine whether true perinatal exposure had occurred.
Results
During the study period, 6731 mothers with live births in Tennessee reported having HCV infection during pregnancy: 3295 (49.0%) had both laboratory and birth certificate indication of HCV infection, 2130 (31.6%) had indication of HCV infection on the laboratory report only, and 1306 (19.4%) had indication of HCV infection on the birth certificate only.
Conclusions
Using data from a public health HCV surveillance system with birth certificate data may improve the identification of HCV-infected pregnant women and perinatally exposed infants. Surveillance systems that include complete reporting of all HCV RNA results can be used to distinguish past from present maternal HCV infection to focus limited public health resources on currently infected mothers and their exposed infants.
Keywords: pregnancy, surveillance, data linkage, hepatitis C virus, maternal and child health, perinatal
Hepatitis C virus (HCV) infection is the most common bloodborne pathogen in the United States, affecting an estimated 4.1 million adults. 1 Reported cases of acute HCV infection nearly quadrupled from 2010 to 2017 in the United States; the most frequently reported risk factor was injection drug use, and the highest rates were among adults aged 20-49. 2,3 Because more than half of acutely infected people progress to chronic HCV infection, the rate of chronic HCV infection has also increased, including among women of reproductive age (15-49 years). 4,5 Of the nearly 65 000 newly reported cases of chronic HCV infection from January 1, 2013, through December 31, 2017, in Tennessee, 39 621 (61.6%) cases were among people of reproductive age, of whom 19 313 (48.7%) were female. Increasing prevalence of HCV infection among women increases the risk of vertical (mother-to-child) transmission, the most common route of pediatric HCV infection. A meta-analysis of perinatal HCV infection found an overall 5.8% vertical transmission rate and a 10.8% vertical transmission rate among mothers coinfected with HIV. 6 The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America recommended universal HCV screening of pregnant women in 2018, and the Centers for Disease Control and Prevention (CDC) followed suit in 2020. 7,8 No US Food and Drug Administration–approved HCV treatment regimens currently exist for pregnant women or children younger than age 3 years. 9,10 Children who are perinatally infected with HCV are not well studied.6
Most estimates of maternal HCV infection in pregnancy rely solely on birth certificate data. Analysis of US birth certificate data from 20092014 determined that the number of maternal HCV infections from reporting states had doubled and that the rate of HCV infection among women giving birth in Tennessee increased 163% (from 3.8 per 1000 live births in 2009 to 10.0 per 1000 live births in 2014). 11 Although maternal HCV infection reported on birth certificates has been used as a measure for perinatal HCV exposure, it has also been shown to underestimate the true prevalence. In addition, laboratory verification of HCV infection status before inclusion on the birth certificate is not routine practice. One study found that linking birth certificate data to HCV laboratory data resulted in a 62% increase above the birth certificate estimate alone, demonstrating incomplete or inaccurate capture of data on maternal HCV status on birth certificates. 12
Several states have begun developing chronic HCV surveillance registries. The Tennessee Department of Health (TDH) began processing all positive HCV laboratory reports on July 1, 2015. Negative HCV RNA laboratory reports became reportable in Tennessee on January 1, 2016, allowing Tennessee to begin capturing data on the prevalence of chronic HCV infection at that time; a negative HCV RNA test result indicates that a person has cleared his or her HCV infection either naturally or through curative treatment. Because of the high rates of newly diagnosed chronic HCV infection among women of reproductive age in Tennessee, 11 we sought to determine the usefulness of using HCV surveillance data to (1) quantify the number of HCV-positive reproductive-aged women with a live birth, (2) compare maternal HCV surveillance data with reported HCV infection status on birth certificates, (3) delineate past versus current maternal infection to identify true perinatal exposures, and (4) determine statewide and county-specific rates of perinatal HCV exposure among infants born to women with current HCV infection in Tennessee.
Methods
Data Sources
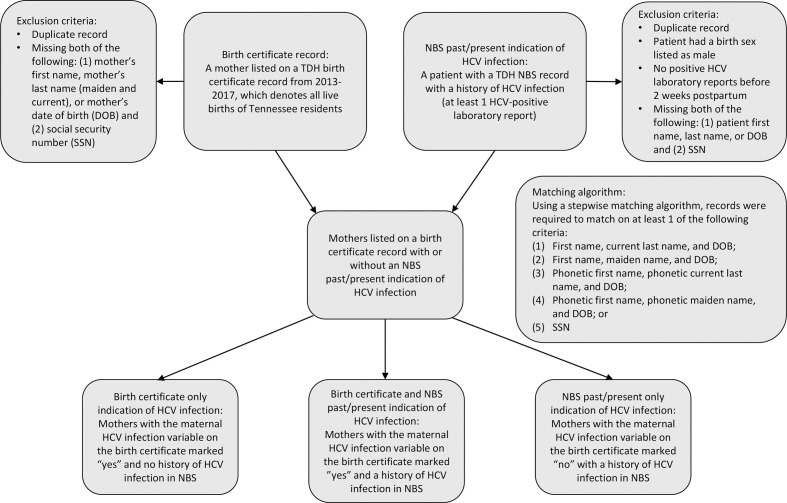
To determine the number of live births, we obtained birth certificate data from 2013-2017 from the TDH Birth Statistical File, which includes information on all births recorded in Tennessee and on births among Tennessee residents that occurred outside Tennessee. We excluded records if they did not contain either (1) the set of mother’s first name, mother’s last name (maiden and current), and mother’s date of birth (DOB) or (2) social security number (SSN) (Figure 1). To assess birth certificate reporting of HCV status, we extracted a variable from the birth certificate data that indicates whether a health care provider reported a mother as having HCV infection during pregnancy.
Figure 1.
Flow diagram depicting inclusion criteria and matching algorithm for birth certificate indication of maternal hepatitis C virus (HCV) infection and laboratory evidence of maternal HCV infection cohort from the National Electronic Disease Surveillance System Base System (NBS), Tennessee, 2013-2017. Abbreviation: TDH, Tennessee Department of Health.
We obtained HCV laboratory data from the TDH National Electronic Disease Surveillance System (NEDSS) Base System (NBS), an integrated information system used to manage laboratory and investigation data pertaining to all reportable conditions, including HCV. 13 We extracted an NBS patient demographic dataset on November 5, 2018, and de-duplicated data on patient first name, last name, and DOB. We excluded records if the patient had a birth sex listed as male or if the client was missing (1) first name, last name, or DOB and (2) SSN. We extracted an NBS laboratory dataset on November 5, 2018, to include all HCV laboratory reports for clients in the final dataset (Figure 1).
Data Matches
We matched maternal birth certificate and NBS demographic data using a stepwise matching algorithm, and we required records to match on ≥1 of the following criteria: (1) first name, current last name, and DOB; (2) first name, maiden name, and DOB; (3) phonetic first name, phonetic current last name, and DOB; (4) phonetic first name, phonetic maiden name, and DOB; or (5) SSN. We then joined the maternal birth certificate and demographic matched dataset to the laboratory dataset using a unique identifier for each patient record in NBS (Figure 1). Because records were linked using maternal birth certificate record, each live birth was uniquely counted and mothers appeared more than once in the analysis if they had >1 live birth, or a multiple-birth event, during 2013-2017.
Case Definitions
Surveillance case definitions are developed and agreed upon by CDC and the Council of State and Territorial Epidemiologists (CSTE). 14 No CDC/CSTE case definition for HCV-positive pregnant women currently exists. We used laboratory data to determine the perinatal HCV-exposure case status for each live birth as follows: (1) confirmed exposure: if a mother had ≥1 HCV RNA-positive laboratory report during pregnancy or, in the absence of an HCV RNA pregnancy laboratory report, ≥1 HCV RNA was conducted before pregnancy and the last HCV RNA test result before pregnancy was positive; (2) probable exposure: if a mother did not have an HCV RNA laboratory report but had an HCV antibody-positive laboratory report before or during pregnancy; or (3) no exposure: if a mother had a history of an HCV antibody-positive or HCV RNA-positive laboratory report and only HCV RNA-negative laboratory reports during pregnancy or, in the absence of an HCV RNA pregnancy laboratory report, ≥1 HCV RNA test was conducted before pregnancy and the last HCV RNA test result before pregnancy was negative. We defined a pregnancy laboratory report as any laboratory report collected 10 months before and up to 2 weeks after the child’s DOB. We excluded birth certificate–only cases from the perinatal HCV exposure case status, because no laboratory evidence was available to support whether the mother was infected with HCV.
Statistical Analysis
We assessed descriptive statistics, including frequencies and rates, by using SAS version 9.4 (SAS Institute, Inc), and we used the SAS SOUNDEX function to generate the phonetic name for each dataset. 15 We calculated HCV infant exposure rates using the number of probable or confirmed HCV perinatal exposures divided by the total number per 1000 live births. We determined geographic differences in HCV exposure rates by maternal county of residence listed in the birth certificate dataset, and we generated maps using Tableau version 9.4 (Tableau Software LLC). This study was approved by the TDH Internal Review Board.
Study Cohort
A total of 404 694 live births occurred among Tennessee residents from January 1, 2013, through December 31, 2017. As of November 5, 2018, a total of 328 950 unique female patient records and 205 879 female HCV laboratory reports were available in NBS. Matching of maternal birth certificate data from 2013 through 2017 with maternal HCV laboratory data in NBS yielded 9704 mothers with ≥1 HCV laboratory report (positive or negative) in NBS. Of these women, we excluded 3804 (39.2%) women because the earliest maternal HCV laboratory report was drawn >2 weeks after delivery and 475 (4.9%) women because the mother only had a history of HCV RNA-negative and/or HCV antibody-negative laboratory reports. The final maternal cohort in NBS with ≥1 positive HCV laboratory test result before 2 weeks postpartum was 5425. An additional 1306 women did not have any HCV laboratory reports in NBS but were reported as having HCV infection on the birth certificate.
Results
During the study period, 5751 mothers with 6731 live births in Tennessee reported having HCV infection during pregnancy: 49.0% (n = 3295) had both laboratory and birth certificate indication of HCV infection, 31.6% (n = 2130) had indication of HCV infection from the laboratory report only, and 19.4% (n = 1306) had indication of HCV infection on the birth certificate only (Table 1). Of 2481 mothers with an HCV RNA-positive laboratory report collected during pregnancy, 74.3% (n = 1844) had birth certificate indication of HCV infection compared with 56.1% (312 of 556) of mothers with an HCV RNA-positive laboratory result collected before pregnancy and 44.9% (841 of 1873) of mothers with a stand-alone antibody-positive laboratory report collected before or during pregnancy.
Table 1.
Hepatitis C virus (HCV) infection status among women with a live birth in Tennessee, 2013-2017 a
| Birth year | Live births, no. b | Mothers identified as having past or present HCV infection | |||
|---|---|---|---|---|---|
| Birth certificate only, c no. (%) | Birth certificate and NEDSS, d no. (%) | NEDSS only, e no. (%) | Total, no. | ||
| 2013 | 79 954 | 263 (28.2) | 361 (38.7) | 308 (33.0) | 932 |
| 2014 | 81 609 | 312 (26.9) | 508 (43.7) | 342 (29.4) | 1162 |
| 2015 | 81 374 | 333 (23.8) | 668 (47.7) | 400 (28.6) | 1401 |
| 2016 | 80 755 | 205 (12.7) | 904 (56.0) | 505 (31.3) | 1614 |
| 2017 | 81 002 | 193 (11.9) | 854 (52.7) | 575 (35.5) | 1622 |
| Total | 404 694 | 1306 (19.4) | 3295 (49.0) | 2130 (31.6) | 6731 |
aData sources: Tennessee Department of Health National Electronic Disease Surveillance System (NEDSS) Base System and the Tennessee Department of Health Birth Statistical File 2013-2017. 13
bLive births refers to the number of live births recorded in Tennessee and to births of Tennessee residents that occurred outside Tennessee.
cBirth certificate only refers to the number of mothers who had HCV infection denoted in the birth certificate but did not have laboratory evidence of HCV infection during pregnancy in NEDSS.
dBirth certificate and NEDSS refers to the number of mothers who had HCV infection denoted in the birth certificate and had laboratory evidence of HCV infection during pregnancy in NEDSS.
eNEDSS only refers to the number of mothers who did not have HCV infection denoted in the birth certificate but had laboratory evidence of HCV infection during pregnancy in NEDSS.
The proportion of maternal HCV infections reported exclusively on birth certificates decreased from 28.2% in 2013 to 11.9% in 2017; the proportion reported exclusively on HCV laboratory reports remained stable, ranging from 33.0% in 2013 to 35.5% in 2017; and the proportion reported by both methods improved from 38.7% in 2013 to 52.7% in 2017 (Table 1). Linking NBS laboratory data to birth certificate data (6731/4601) resulted in a 46.3% increase in identification of perinatally exposed infants during 2013-2017 and a 54.9% (1622/1047) increase in identification of perinatally exposed infants during 2017 alone.
Based on the timing and results of maternal laboratory reports in NBS, we determined that 9.5% (516 of 5425) of infants born to mothers with a history of HCV infection were not perinatally exposed to HCV infection (Table 2). The remaining 4909 perinatal HCV exposures (probable or confirmed) had an average exposure rate of 12.1 per 1000 live births, which increased by 93.7%, from 7.9 per 1000 live births in 2013 to 15.3 per 1000 live births in 2017. Using a 5.8% vertical transmission rate, 285 infants likely acquired HCV infection perinatally from 2013 through 2017 in Tennessee (data not shown).
Table 2.
Mothers identified as having hepatitis C virus (HCV) infection, by NEDSS maternal laboratory results, Tennessee, 2013-2017 a
| Birth year | NEDSS- identified mothers, b no. | Infants born to mothers with past or present HCV infection reported in NEDSS | ||||
|---|---|---|---|---|---|---|
| Probable exposure, c no. (%) | Confirmed exposure, d no. (%) | No exposure, e no. (%) | Total exposed, f no. (%) | HCV-exposed per 1000 live births, g % | ||
| 2013 | 669 | 304 (45.4) | 327 (48.9) | 38 (5.7) | 631 (94.3) | 7.9 |
| 2014 | 850 | 311 (36.6) | 501 (58.9) | 38 (4.5) | 812 (95.5) | 9.9 |
| 2015 | 1068 | 350 (32.8) | 631 (59.1) | 87 (8.1) | 981 (91.9) | 12.1 |
| 2016 | 1409 | 477 (33.9) | 770 (54.6) | 162 (11.5) | 1247 (88.5) | 15.4 |
| 2017 | 1429 | 430 (30.1) | 808 (56.5) | 191 (13.4) | 1238 (86.6) | 15.3 |
| Total | 5425 | 1872 (34.5) | 3037 (56.0) | 516 (9.5) | 4909 (90.5) | 12.1 |
Abbreviation: NEDSS, National Electronic Disease Surveillance System.
aData source: Tennessee Department of Health NEDSS Base System (NBS). 13
bThe number of mothers who had a history of HCV (past or present) indicated by an HCV antibody-positive or HCV RNA-positive laboratory test result before or during pregnancy.
cMothers who did not have an HCV RNA laboratory test result but had an HCV antibody-positive laboratory test result before or during pregnancy.
dMothers who had at least 1 HCV RNA-positive laboratory test result during pregnancy or, in the absence of an HCV RNA pregnancy laboratory test result, at least 1 HCV RNA test was conducted before pregnancy and the last HCV RNA test result before pregnancy was positive.
eMothers who had a history of an HCV antibody-positive or HCV RNA-positive laboratory test result and only HCV RNA-negative laboratory test results during pregnancy or, in the absence of an HCV RNA pregnancy laboratory test result, at least 1 HCV RNA test was conducted before pregnancy and the last HCV RNA test result before pregnancy was negative.
fTotal exposed = probable exposure + confirmed exposure.
gHCV exposed per 1000 live births = (total exposed/live births) x 1000.
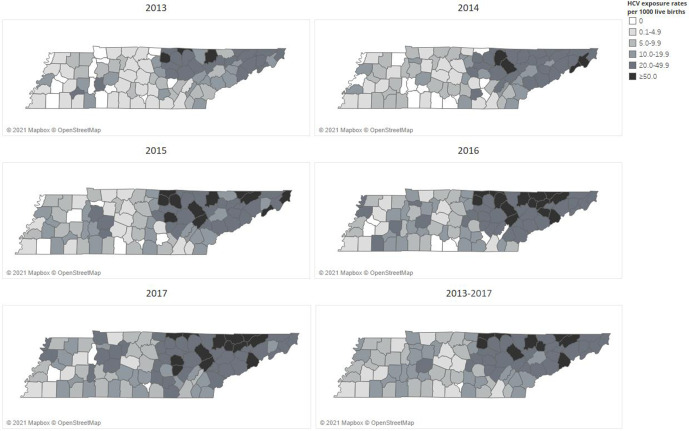
Rates of perinatal exposure per 1000 live births from 2013 to 2017 varied by county (Figure 2). Although the state rate of perinatal exposure from 2013 to 2017 was 12.1 per 1000 live births, temporal rates varied by year, from 7.9 in 2013 to 15.3 in 2017. In addition, county rates of perinatal exposure ranged from 0 to 106.2 per 1000 live births; the highest rates occurred in counties in northeastern Tennessee.
Figure 2.
Rates of perinatal hepatitis C virus (HCV) exposure per 1000 live births, by National Electronic Disease Surveillance System (NEDSS) laboratory results, Tennessee, 2013-2017. Data source: Tennessee Department of Health NEDSS Base System. 13
Discussion
Increasing rates of HCV infection among reproductive-aged women are mirrored by increasing rates of perinatal HCV exposure. 16 We found that linking HCV surveillance data with birth certificate data increased the identification of perinatally exposed infants in Tennessee by 46.3% from 2013 through 2017. The increase observed in 2017 (the first year that both positive and negative HCV RNA test results were reportable in Tennessee) was slightly lower but consistent with the 62% increase observed in another study and represents an important public health opportunity to improve the identification of affected mother–infant dyads. 12 A similar study found that using surveillance data increased identification of women with past or present HCV infection among women delivering live births in Ohio by 27.5% from 2012 to 2015. 17 By analyzing both positive and negative HCV RNA test results, we are better able to ascertain current versus past HCV infection, because a negative RNA test result would indicate a cleared or cured infection; thus, no perinatal HCV exposure would have occurred. In addition, three-quarters of women with an HCV RNA-positive specimen collected during pregnancy had indication of HCV infection on the birth certificate; however, indication of HCV infection on the birth certificate reduced to 56.1% when the HCV RNA-positive specimen was collected before pregnancy and to 44.9% when only an HCV antibody-positive specimen was collected before or during pregnancy. Furthermore, lack of birth certificate reporting of HCV infection among 25% of mothers who had a positive HCV RNA test result during pregnancy emphasizes the limitations of birth certificate–based HCV surveillance.
Our study used maternal laboratory results housed in a disease surveillance system to distinguish past from present maternal HCV infection during pregnancy. Ten percent of women originally identified as being HCV infected in our cohort turned out to have a past HCV infection rather than a current HCV infection, as evidenced by an HCV RNA-negative laboratory report during or directly before pregnancy. Using surveillance system laboratory reports, both positive and negative, to distinguish active from past maternal HCV infection allows limited public health resources to focus on mother–infant dyads at risk of vertical transmission. The ability to make this distinction will become increasingly important, because maternal HCV status should be evaluated in each pregnancy, and treatment should be initiated postpartum to avoid additional perinatal HCV exposures. 18
Nearly one-fifth of all mothers identified as having HCV infection in our cohort were identified by using data reported exclusively on birth certificates. Although this proportion decreased over time, we did not include this population of mothers in the tabulation of perinatal exposures or in geographic mapping because mothers with HCV infection denoted on the birth certificate had an 89.0% probability of having laboratory evidence of a confirmed or probable perinatal HCV exposure. HCV surveillance in Tennessee is a passive process that relies on laboratories to report HCV laboratory results; thus, laboratory indication of HCV infection can be missed if the report is not received. Conversely, birth certificate identification can also be misclassified if maternal HCV history is not verified by the health care provider and could rely on maternal self-report. Our results emphasize the importance of continuing to use birth certificate data in addition to surveillance data to identify affected mother–infant dyads.
Our study demonstrated large temporal and geographic differences in rates of reported perinatal HCV exposure across Tennessee, consistent with the distribution of newly reported chronic HCV infection and neonatal abstinence syndrome in the state, providing another opportunity to focus limited public health resources to have the greatest impact. 19 However, 44.4% of adults living with HCV infection in the United States are estimated to be unaware of their infection, and HCV testing practices among pregnant women vary widely among health care providers. 20 In the absence of universal HCV testing, reported perinatal exposure rates reflect, at least in part, local testing practices.
Limitations
This study had several limitations. First, chronic HCV surveillance in Tennessee was not routine until July 2015, negative HCV RNA laboratory test results were not reportable until January 2016, and chronic HCV infection was not a laboratory-reportable condition until January 2017. Second, with respect to data included in our study before July 2015, only electronic laboratory reports were used, which could have resulted in underreporting. Third, HCV status reported on birth certificates was likely not confirmed with an HCV laboratory test because women were not universally screened for HCV infection in pregnancy during the study period. Fourth, because pregnancy is not reportable in the context of HCV infection, we relied solely on birth certificate data and NBS record matching to identify exposure. Lastly, we only studied women of reproductive age in Tennessee; as such, our findings may not be generalizable to the rest of the United States.
Strengths
This study also had several strengths. First, we used 2 reliable data sources, NBS and Birth Statistical File data, to determine perinatal HCV exposure. Second, we analyzed data during a 5-year period, which allowed for a large sample size. Lastly, we analyzed laboratory data versus birth certificate data, which are reported by health care providers and have been shown to underestimate the prevalence of maternal HCV infection. 12
Conclusions
Combining data from a robust public health HCV surveillance system with birth certificate data may help improve the identification of HCV-infected pregnant women and perinatally exposed infants. Surveillance systems that include complete reporting of all HCV RNA test results (positive and negative) can be used to distinguish past from present maternal HCV infection, providing an opportunity to focus limited public health resources on currently infected mothers and their exposed infants. Strategies to accelerate the timely identification of affected mother–infant dyads include reporting pregnancy status on all viral hepatitis laboratory reports and matching of laboratory data to birth certificate data to better determine maternal HCV status. Implementation of such strategies may become more important if new tools, such as hepatitis C treatment during pregnancy or infant postexposure prophylaxis, become available to interrupt perinatal transmission.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Strengthening Surveillance in Jurisdictions With High Incidence of Hepatitis C Virus and Hepatitis B Virus Infections (CDC-RFA-PS17-1703).
ORCID iDs
Heather Wingate, MPH https://orcid.org/0000-0002-3244-2052
Lindsey Sizemore, MPH https://orcid.org/0000-0002-6187-7370
References
- 1. Hofmeister MG., Rosenthal EM., Barker LK. et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020-1031. 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Viral hepatitis surveillance—United States, 2017. 2019. Accessed May 14, 2021. https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm
- 3. Centers for Disease Control and Prevention . Viral hepatitis surveillance report 2019. 2019. Accessed May 21, 2021. https://www.cdc.gov/hepatitis/statistics/2019surveillance/HepC.htm
- 4. Seo S., Silverberg MJ., Hurley LB. et al. Prevalence of spontaneous clearance of hepatitis C virus infection doubled from 1998 to 2017. Clin Gastroenterol Hepatol. 2020;18(2):511-513. 10.1016/j.cgh.2019.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas DL., Astemborski J., Rai RM. et al. The natural history of hepatitis C virus infection. JAMA. 2000;284(4):450-456. 10.1001/jama.284.4.450 [DOI] [PubMed] [Google Scholar]
- 6. Benova L., Mohamoud YA., Calvert C., Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59(6):765-773. 10.1093/cid/ciu447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Association for the Study of Liver Diseases . HCV testing and linkage to care: recommendations for one-time hepatitis C testing. 2020. Accessed May 21, 2021. http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care
- 8. Centers for Disease Control and Prevention . Recommendations for hepatitis C screening among adults—2019; request for comment. Fed Regist. 2010;84(208):57733-57734. 10.1016/0196-335x(80)90058-8 [DOI] [Google Scholar]
- 9. US Preventive Services Task Force . Final recommendation statement: hepatitis C virus infection in adolescents and adults: screening. 2019. Accessed May 21, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/hepatitis-c-screening
- 10. American Association for the Study of Liver Diseases . HCV in children. 2020. Accessed May 21, 2021. https://www.hcvguidelines.org/unique-populations/children#:~:text=Direct%2Dacting%20antiviral%20(DAA),therapy%2C%20regardless%20of%20disease%20severity
- 11. Patrick SW., Bauer AM., Warren MD., Jones TF., Wester C. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009-2014. MMWR Morb Mortal Wkly Rep. 2017;66(18):470-473. 10.15585/mmwr.mm6618a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snodgrass SD., Poissant TM., Thomas AR. Notes from the field: underreporting of maternal hepatitis C virus infection status and the need for infant testing—Oregon, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(6):201-202. 10.15585/mmwr.mm6706a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . National Electronic Diseases Surveillance System (NEDSS): integrated surveillance information systems/NEDSS. Accessed May 21, 2021. http://wwwn.cdc.gov/nndss/nedss.html
- 14. Centers for Disease Control and Prevention . National Electronic Diseases Surveillance System (NEDSS): surveillance case definitions for current and historical conditions. 2020. Accessed May 21, 2021. https://wwwn.cdc.gov/nndss/conditions
- 15. Fan Z. Matching Character Variables by Sound: A Closer Look at SOUNDEX Function and Sounds-Like Operator (=*). Paper 072-29. Westat; 2000. Accessed May 21, 2021. https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/072-29.pdf
- 16. Koneru A., Nelson N., Hariri S. et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep. 2016;65(28):705-710. 10.15585/mmwr.mm6528a2 [DOI] [PubMed] [Google Scholar]
- 17. Gowda C., Kennedy S., Glover C., Prasad MR., Wang L., Honegger JR. Enhanced identification of maternal hepatitis C virus infection using existing public health surveillance systems. Paediatr Perinat Epidemiol. 2018;32(4):401-410. 10.1111/ppe.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . HCV challenges. 2019. Accessed May 21, 2021. https://www.cdc.gov/nchhstp/pregnancy/challenges/hcv.html
- 19. Wingate H., Sizemore L., Miller AM., Black J., Wester C. Hepatitis C virus infection among women of reproductive age as a primary prevention measure for neonatal abstinence syndrome infants; Tennessee, 2016. Abstract presented at the CSTE Annual Conference; June 2-6, 2019; Raleigh, NC.
- 20. Kim HS., Yang JD., El-Serag HB., Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. J Viral Hepat. 2019;26(5):596-602. 10.1111/jvh.13060 [DOI] [PubMed] [Google Scholar]