Abstract
Tickborne diseases are an increasing public health threat in the United States. Prevention and diagnosis of tickborne diseases are improved by access to current and accurate information on where medically important ticks and their associated human and veterinary pathogens are present, their local abundance or prevalence, and when ticks are actively seeking hosts. The true extent of tick and tickborne pathogen expansion is poorly defined, in part because of a lack of nationally standardized tick surveillance. We surveyed 140 vector-borne disease professionals working in state, county, and local public health and vector control agencies to assess their 1) tick surveillance program objectives, 2) pathogen testing methods, 3) tick control practices, 4) data communication strategies, and 5) barriers to program development and operation. Fewer than half of respondents reported that their jurisdiction was engaged in routine, active tick surveillance, but nearly twothirds reported engaging in passive tick surveillance. Detection of tick presence was the most commonly stated current surveillance objective (76.2%). Most of the programs currently supporting tick pathogen testing were in the Northeast (70.8%), Upper and Central Midwest (64.3%), and the West (71.4%) regions. The most common pathogens screened for were Rickettsia spp. (Rickettsiales: Rickettsiaceae) and bacterial and viral agents transmitted by Ixodes (Acari: Ixodidae) ticks. Only 12% of respondents indicated their jurisdiction directly conducts or otherwise financially supports tick control. Responses indicated that their ability to expand the capacity of tick surveillance and control programs was impeded by inconsistent funding, limited infrastructure, guidance on best practices, and institutional capacity to perform these functions.
Keywords: tick, surveillance, One Health, tickborne disease, online survey
Tickborne diseases are an increasing concern in the United States, with more than 75% of vector-borne diseases of humans transmitted by ticks (Eisen et al. 2017, Rosenberg et al. 2018). Moreover, several newly recognized pathogens have been described, bringing the number of human pathogens in the United States to at least 16 (Eisen et al. 2017). The geographic distributions of ticks of medical and veterinary importance and their associated pathogens have continued to expand, putting an increasing number of communities at risk (Kugeler et al. 2015; Eisen et al. 2016a, 2017; Eisen and Eisen 2018). Changes in host diversity, abundance and distribution, human movement of animals, changes in land use, shifts in habitat, and weather patterns all contribute to range expansion, changes in tick population dynamics, and future risks of invasion by exotic tick species or exotic tickborne pathogens (Pérez de León et al. 2012, Sanders et al. 2013). Prominent examples of tick range expansion include the introduction and ongoing spread of Haemaphysalis longicornis (Neumann) (Acari: Ixodidae) (Beard et al. 2018, Raghavan et al. 2019) and the tropical lineage of Rhipicephalus sanguineus (Latreille) (Acari: Ixodidae) (Villarreal et al. 2018), which impact both public and animal (livestock, wildlife, and companion animals) health. The global spread of African swine fever virus has raised concerns about our knowledge of the distribution of putative Ornithodoros (Ixodida: Argasidae) tick vectors and the possibility that a sylvatic cycle could become established in the United States (Golnar et al. 2019, Wormington et al. 2019).
A national strategy to combat the threats posed by ticks and tickborne diseases should include the development of a network of collaborators able to share surveillance data and aid practitioners in responding to critical needs (Tick-Borne Disease Working Group 2018, Petersen et al. 2019, Wisely and Glass 2019, Junker 2020). However, anecdotal information from across the U.S. suggests that tick surveillance and reporting protocols vary widely across the country, limiting availability and usefulness of information to the public and human and animal healthcare providers at useful scales of time and space.
In 2018, the U.S. Centers for Disease Control and Prevention (CDC) issued guidance and funding to states to implement tick surveillance programs and they established a tick surveillance data collection module within an existing arthropod-borne disease surveillance system, ArboNET (Centers for Disease Control 2019a). However, states, counties, and tribes are not in full coordination, particularly in tick surveillance methodology. As a further step in building communities of practice in the field of vector-borne diseases, the CDC funded a cooperative agreement to support five regional Centers of Excellence in Vector-Borne Diseases (COEs) (Centers for Disease Control 2019b).
The goals of the present study were to describe the development and implementation of an online survey to gauge nationwide involvement in tick surveillance and control activities, and to analyze data obtained from the survey. As part of the CDC cooperative agreement, prior to CDC’s 2019 tick surveillance initiative, the five regional COEs developed and distributed a survey on tick programs and barriers to the development of programs across various agencies and jurisdictions. Here we examined survey data focused on five particular target activities of tickborne disease surveillance and control: 1) tick surveillance program objectives, 2) pathogen testing methods, 3) tick control, 4) data communication, and 5) barriers to program development and operation, including communication. We evaluated the range in capacity to conduct targeted activities across programs according to jurisdiction level and climate regions throughout the United States. A survey of this nature to gather information on national tick surveillance and control practices has not previously been conducted and has the capacity to highlight surveillance and control disparities between regions and provide support for centralized guidance on tick surveillance and control program development.
Materials and Methods
Survey Design and Development
The online survey consisted of 45 multiple choice, Likert scale, and free text response questions using the online software program Qualtrics (Qualtrics, Provo, UT). The survey was divided into question sets addressing the above five targets of interest. The COEs each recruited five key informants from their respective catchment areas to review and beta-test the initial survey questionnaire. Responses from preliminary testing were used to optimize the survey instrument. The following definitions were provided to respondents in the survey introduction and periodically within the body of the questionnaire:
Tick Surveillance: the collection of tick specimens and other relevant environmental samples to identify tick species in a given area and/or test for the presence of tickborne pathogens; this does not include the monitoring and reporting of clinical cases of tickborne disease in human patients.
Tick Control: the implementation of practices to reduce or eliminate the presence of ticks in the environment.
Active Surveillance: focused collection of tick samples from the field for identification, testing, or analysis.
Passive Surveillance: accepting tick samples submitted by the public, veterinarians, physicians, etc., for identification, testing, or analysis.
Respondents were asked to indicate their personal or program involvement in the above target activities of tickborne disease surveillance and control. Respondents indicating involvement in these activities were then asked to complete the corresponding question sets; respondents not directly involved in a listed target activity were forwarded to the next portion of the survey questionnaire. All survey respondents were asked to complete the question sets targeting information and data sharing, communication of program results, and barriers to program development and enhancement. ‘Communication’ encompassed any form of disseminating information to the public or other stakeholders. The full survey questionnaire is available in Supp File 1 (online only).
Survey Dissemination
Distribution of the survey questionnaire followed a chain referral, or snowball, sampling approach. Chain referral sampling is a technique used to include hard-to-reach populations or sample responses for sensitive issues. This nonrandom sampling approach generates a pool of participants through referrals, wherein subjects from the initial sample group are asked to recommend individuals to act as future participants (Biernacki and Waldorf 1981, Crouse and Lowe 2018, Siegel and Jones 2018). We chose this approach because tick surveillance and control activities are not well-coordinated in the United States and, therefore, we lacked a common reference list to contact surveillance and control professionals.
The survey was directly distributed to 147 individuals working in state, county, and local public health and vector control agencies, identified through the networks of the COEs. Survey participants were encouraged to disseminate the survey through their professional networks to those involved in tick surveillance or control activities, and who could respond appropriately. The survey was open for the period of July to September 2018. The Institutional Review Board of Cornell University determined that this quality improvement project did not meet the definition of human subjects research.
Analysis of Survey Content
Respondents were grouped into sub-state (county, local) and state (state, federal) jurisdiction categories and into climate regions according to the National Oceanic and Atmospheric Administration (NOAA) categorization 1 (National Oceanic and Atmospheric Administration National Centers for Environmental Information 2019). NOAA’s regional climate classification system was chosen because it divides states across the contiguous United States into nine climatically consistent regions based on current and historical conditions (Karl and Koss 1984): Northeast (region I), Southeast (region II), Upper Midwest (region III), Ohio Valley (region IV), South (region V), Northern Rockies (region VI), Southwest (region VII), Northwest (region VIII), West (region IX). These regions roughly align with the distributions of medically important ticks (Eisen et al. 2017). Respondents from territories outside the contiguous United States, including Hawaii and Mariana Islands, were grouped with the West (region IX).
Jurisdictional groups were compared using contingency table analyses. Due to limited sample sizes across several NOAA regions, analysis primarily consisted of the calculation of descriptive statistics for these categories. Analyses for the survey question sets addressing tick surveillance program operations, and objectives, pathogen testing, and tick control were restricted to those respondents who indicated they could comment on the given activity. For this reason, the sample size (n) varied for these question sets.
Respondents completing the question set regarding tick surveillance program objectives were asked to indicate the local importance of multiple tick species found within the United States, with options being: 0 = not important, 1 = low, 2 = medium, and 3 = high. Respondents were also asked to rank barriers to communication and dissemination of program information across three categories, with options being: 0 = not a barrier, 1 = minor barrier, and 2 = major barrier. Mean composite ratings for these ranking questions were compared across state and substate level respondents via independent samples t-tests. Text responses to open-ended questions were reviewed and coded based on content. Analyses were conducted using SPSS Version 25 (SPSS Inc 2017) (IBM Corp. Released 2017), ArcMap 10.6.1 (ESRI 2018), Rstudio 1.2.5001 (Rstudio Team 2019), and R version 3.2.1 (R Core Team 2015).
Results
Respondent Demographics
In total, 140 individuals responded to the survey. Of these, 122 (87.1%) completed all portions of the survey. Respondents represented organizations operating at local municipal (n = 15, 10.7%), county (n = 58, 41.4%), state (n = 65, 46.4%), and federal (n = 2, 1.4%) jurisdiction levels. The majority of respondents (n = 74, 52.9%) worked in states in the eastern United States. The most commonly identified employment sectors were public health (n = 68, 48.6%) and mosquito control (n = 48, 34.3%). Additional respondent employment data are presented in Supp Table 1 (online only).
Tick Surveillance Program Objectives
Eighty-four respondents (60.0%) reported that they could comment on tick surveillance operations in their jurisdiction. Among respondents qualified to comment on tick surveillance, 97.6% (n = 82) indicated that their jurisdiction was engaged in at least one form of tick surveillance; 71.4% (n = 60) of these respondent jurisdictions were involved in multiple types of tick surveillance. The majority reported that at least one program in their jurisdiction currently operates an ad hoc active (n = 52, 61.9%) or passive tick surveillance program (n = 55, 65.5%, Table 1). More state-level respondents reported ongoing tick surveillance activities of any form than sub-state respondents, though this difference was not statistically significant. Many of the respondents (n = 48, 57.1%) indicated that programs in their jurisdiction work with academic partners to conduct tick surveillance, with a significantly higher proportion of state-level respondents indicating these partnerships compared with sub-state level respondents (34 state, 14 sub-state, χ2 = 6.588, P = 0.037). The employment sectors most commonly identified by respondents as involved in tick surveillance were public health (n = 80, 95.2%), mosquito control (n = 44, 52.4%), cooperative extension (n = 26, 31.0%), and agriculture (n = 22, 26.2%).
Table 1.
Currently conducted and desired but not yet implemented forms for tick surveillance (routine active, Ad hoc, passive) by respondent jurisdiction
| Forms of tick surveillance | Respondent jurisdictiona | Total respondents (n = 84) | |
|---|---|---|---|
|
| |||
| Sub-state (n = 33) | State (n = 51) | ||
|
| |||
| Current conducted forms of Tick Surveillance | |||
| Routine active | 17 (51.5%) | 22 (43.1%) | 39 (46.4%) |
| Ad hoc active | 17 (51.5%) | 35 (68.6%) | 52 (61.9%) |
| Passive | 21 (63.6%) | 34 (66.7%) | 55 (65.5%) |
| Desired forms of Tick Surveillance | |||
| Routine active | 11 (33.3%) | 19 (37.3%) | 30 (35.7%) |
| Ad hoc active | 2 (6.1%) | 7 (13.7%) | 9 (10.7%) |
| Passive | 3 (9.1%) | 9 (17.7%) | 12 (14.3%) |
Sub-state respondents include those working at either a local or county agency. State respondents include those working at either a state or federal agency.
Objectives of tick surveillance programs fell into two categories, either focusing directly on the ticks, or on the pathogens they transmit (Table 2). Detection of tick presence was the most-commonly identified current objective (n = 64, 76.2%), followed by monitoring tick distribution and geographic spread (n = 48, 57.1%), monitoring for the emergence of new species (n = 46, 54.8%), monitoring abundance of ticks of public health importance (n = 43, 51.2%), and evaluation of tick abundance by species (n = 38, 45.2%). Jurisdiction-level differences in currently implemented surveillance objectives for tick vectors were overall minimal.
Table 2.
Objectives of tick and tickborne pathogen surveillance by jurisdiction. Respondents rated objectives as either currently implemented, desired but not currently feasible or not a current objective of their program
| Objectives | Current | Desired | Not a program objective | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Sub-state (n = 33) | State (n = 51) | Total (n = 84) | Sub-state (n = 33) | State (n = 51) | Total (n = 84) | Sub-state (n = 33) | State (n = 51) | Total (n = 84) | |
|
| |||||||||
| Ticks | |||||||||
| Detect presence by species | 27 (81.8%) | 37 (72.5%) | 64 (76.2%) | 4 (12.1%) | 7 (13.7%) | 11 (13.1%) | 3 (9.1%) | 0 (0.0%) | 3 (3.6%) |
| Evaluate abundance by species | 15 (45.5%) | 23 (45.1%) | 38 (45.2%) | 10 (30.3%) | 12 (23.5%) | 22 (26.2%) | 9 (27.3%) | 5 (9.8%) | 14 (16.7%) |
| Monitor abundance of ticks that are of public health concern | 17 (51.5%) | 26 (51.0%) | 43 (51.2%) | 10 (30.3%) | 13 (25.5%) | 23 (27.4%) | 7 (21.2%) | 4 (7.8%) | 11 (13.1%) |
| Monitor distribution of species | 19 (57.6%) | 29 (56.9%) | 48 (57.1%) | 5 (15.2%) | 11 (21.6%) | 16 (19.0%) | 10 (30.3%) | 3 (5.9%) | 13 (15.5%) |
| Monitor geographic spread | 16 (48.5) | 25 (49.0%) | 41 (48.8%) | 8 (24.2%) | 10 (19.6%) | 18 (21.4%) | 9 (27.3%) | 6 (11.8%) | 15 (17.9% |
| Monitor emergence of new species | 15 (45.5%) | 31 (60.8%) | 46 (54.8%) | 11 (33.3%) | 8 (15.7%) | 19 (22.6%) | 8 (24.2%) | 3 (5.9%) | 11 (13.1%) |
| Pathogens | |||||||||
| Detect presence in ticks | 17 (51.5%) | 32 (62.7%) | 49 (58.3%) | 12 (36.4%) | 9 (17.6%) | 21 (25.0%) | 5 (15.2%) | 2 (3.9%) | 7 (8.3%) |
| Evaluate prevalence in ticks | 14 (42.4%) | 26 (51.0% | 40 (47.6%) | 12 (36.4%) | 11 (21.6%) | 23 (27.4%) | 7 (21.2%) | 4 (7.8%) | 11 (13.1%) |
| Evaluate prevalence in reservoir hosts | 2 (6.1%) | 4 (7.8%) | 6 (7.1%) | 12 (36.4%) | 24 (47.1%) | 36 (42.9%) | 17 (51.5%) | 12 (23.5%) | 29 (34.5%) |
| Calculate public health risk | 12 (36.4%) | 20 (39.2%) | 32 (38.1%) | 13 (39.4%) | 15 (29.4%) | 28 (33.3%) | 13 (39.4%) | 15 (29.4%) | 28 (33.3%) |
Of those respondents reporting desired but not currently implemented activities for their tick surveillance program, over one-third (n = 30, 35.7%) indicated a desire to implement routine active surveillance (Table 1). At a more detailed level, the most frequently identified desired objectives were monitoring abundance of ticks of public health importance (n = 23, 26.2%), evaluating tick abundance by species (n = 22, 27.4%), and monitoring emergence of new tick species (n = 19, 22.6%; Table 2). In general, agreement between sub-state and state-level respondents was high for desired objectives.
With respect to assessing tickborne pathogens, currently implemented objectives included detection of pathogen presence in ticks (n = 49, 58.3%), evaluation of pathogen prevalence in ticks (n = 40, 47.6%), evaluation of pathogen prevalence in reservoir hosts (n = 6, 7.1%), and calculating public health risk (n = 32, 38.1%; Table 2). The most frequently desired objectives that were not currently implemented were evaluation of pathogen prevalence in reservoir hosts (n = 36, 42.9%) and calculation of the risk of tickborne illness to humans (n = 28, 33.3%).
NOAA regions with the largest proportion of programs currently conducting routine active tick surveillance were the Northeast (n = 19, 65.5%), Upper Midwest (n = 5, 83.3%), and the Northwest and West (n = 8, 88.9%). Detection of ticks was emphasized as a current objective in the Northern Rockies, Northeast, and Ohio Valley regions. Monitoring the distribution of ticks by species was less emphasized in the Southwest, and monitoring the abundance of ticks was less emphasized in the South. Evaluation of pathogen prevalence in reservoir hosts was most highly desired in the Upper Midwest (n = 5, 71.4%), the Northwest (n = 2, 66.7%), and the Ohio Valley (n = 7, 63.6%). Additional detail on current and desired objectives by NOAA region are included in Supp Table 2 [online only].
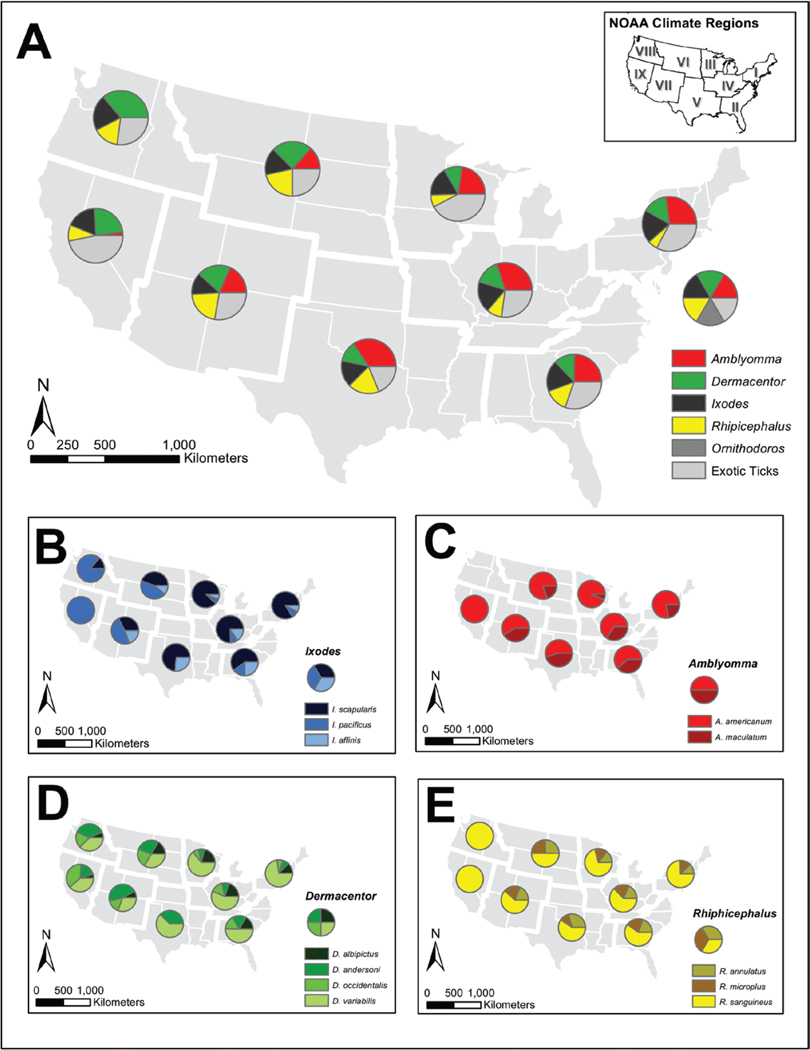
The tick species with the overall highest average importance ratings were Ixodes scapularis (Say) (Acari: Ixodidae), Dermacentor variabilis (Say) (Acari: Ixodidae), and Amblyomma americanum (Linnaeus) (Acari: Ixodidae) (Fig. 1). When viewed by NOAA region, the most highly rated ticks of importance were I. scapularis in the Northeast, Southeast, Upper Midwest, and Ohio Valley; A. americanum in the South; D. variabilis in the Northern Rockies and Northwest; R. sanguineus in the Northern Rockies and Southwest; and Ixodes pacificus (Cooley and Kohls) (Acari: Ixodidae) in the West. While D. variabilis and A. americanum did not have the highest average rating in the majority of regions, these tick species maintained a high average rating broadly across the United States, and in the eastern and midwest climate regions, respectively. Dermacentor andersoni (Stiles) (Acari: Ixodidae) and Dermacentor occidentalis (Marx) (Acari: Ixodidae) received higher average importance ratings in the Northern Rockies and Southwest. Of note, concern about the introduction of new species was highly rated in four out of the nine NOAA regions, with a concentration in eastern states. Additional detail on average ratings of importance by NOAA region are included in Supp Table 3 (online only).
Fig. 1.
Map of composite scores for importance of tick species by NOAA regions. (A) Importance of ticks by genera (Ixodes, Amblyomma, Dermacentor, Rhipicephalus, Ornithodoros) and new tick species (including Haemaphysalis longicornis). (B) Ixodes spp. (I. scapularis, I. pacificus, I. affinis (Neumann) [Acari: Ixodidae]). (C) Amblyomma spp. (A. americanum, A. maculatum (Koch) [Acari: Ixodidae]). (C) Dermacentor spp. (D. albipictus (Packard) [Acari: Ixodidae], D. andersoni, D. occidentalis, D. variabilis). (D) Rhipicephalus spp. (R. annulatus (Say) [Acari: Ixodidae], R. microplus (Canestrini) [Acari: Ixodidae], R. sanguineus)
Pathogen Testing Methods
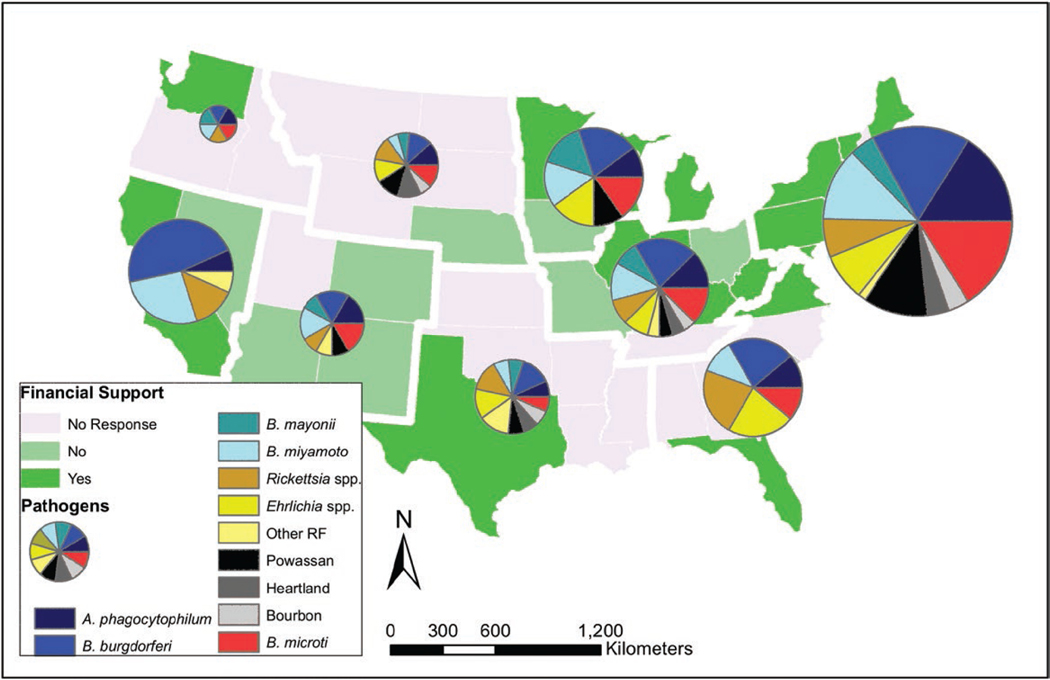
Sixty-six respondents (47.1%) reported that they could comment on tick pathogen testing operations in their jurisdiction. Of these respondents, 36 (25.7% of total respondents) stated that their jurisdiction directly conducts or otherwise financially supports the testing of ticks or other samples for zoonotic pathogens, 80.6% (n = 29) of whom worked at the state level. In the context of regional response rates, NOAA regions with the largest proportion of programs currently financially supporting tick pathogen testing were the Northeast (n = 17, 70.8%), Upper Midwest and Ohio Valley (n = 9, 64.3%), and the West (n = 5, 71.4%). Across all pathogens tested, the majority of laboratories performing tests were state-owned and academic laboratories (Table 3). The most common pathogens tested across NOAA regions were Rickettsia spp. (Rickettsiales: Rickettsiaceae) and bacterial and viral agents transmitted by Ixodes ticks (Fig. 2).
Table 3.
Respondent pathogen testing (bacterial, arboviral, protozoan) and laboratory conducting pathogen testing for responding entities
| Pathogens tested | Total respondents testing for pathogena | Laboratory conducting testing (n) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Bacterial, Ixodes vector | State | Academic | Private | CDC | ||
|
| ||||||
| B. burgdorferi | 47 (71.2%) | 19 (28.8%) | 19 (28.8%) | 3 (4.5%) | 9 (13.6%) | |
| A. phagocytophilum | 33 (50.0%) | 13 (19.7%) | 16 (24.2%) | 2 (3.0%) | 9 (13.6%) | |
| B. miyamotoi | 31 (47.0%) | 13 (19.7%) | 12 (18.2%) | 2 (3.0%) | 9 (13.6%) | |
| B. mayonii | 15 (22.7%) | 7 (10.6%) | 5 (7.6%) | 0 (0.0%) | 6 (9.1%) | |
|
| ||||||
| Bacterial, Other vector | State | Academic | Private | CDC | ||
|
| ||||||
| Rickettsia spp. | 21 (31.8%) | 11 (16.7%) | 8 (12.1%) | 1 (1.5%) | 4 (6.1%) | |
| Other relapsing fever spirochetes | 8 (12.1%) | 7 (10.6%) | 1 (1.5%) | 0 (0.0%) | 1 (1.5%) | |
| Ehrlichia spp. | 22 (33.3%) | 11 (16.7%) | 9 (13.6%) | 1 (1.5%) | 3 (4.5%) | |
|
| ||||||
| Arboviral | State | Academic | Private | CDC | ||
|
| ||||||
| Powassan | 22 (33.3%) | 9 (13.6%) | 10 (15.2%) | 2 (3.0%) | 3 (4.5%) | |
| Heartland | 9 (13.6%) | 4 (6.1%) | 5 (7.6%) | 0 (0.0%) | 1 (1.5%) | |
| Bourbon | 8 (12.1%) | 4 (6.1%) | 4 (6.1%) | 0 (0.0%) | 1 (1.5%) | |
|
| ||||||
| Protozoan, Ixodes vector | State | Academic | Private | CDC | ||
|
| ||||||
| B. microti | 33 (50.0%) | 14 (21.2%) | 15 (22.7%) | 2 (3.0%) | 9 (13.6%) | |
Not all respondents indicated the laboratory through which their samples were tested for pathogens.
Fig. 2.
Map indicates financial support (yes/no/no response) by state and pathogens tested by NOAA region. The background color indicates states with financial support. Pie charts are sized by number of responses and indicate the pathogens tested by each region: Bacterial pathogens vectored by Ixodes spp. (A. phagocytophilum, B. burgdorferi, B. mayonii, B. miyamotoi). Bacterial pathogens vectored by other spp. (other RF, Rickettsia, Ehrlichia), arboviral pathogens (Powassan, Heartland, Bourbon), and protozoan pathogens (B. microti). The size of each slice in the pie chart represents a pathogen and is representative of the number of responses given within NOAA regions.
Tick Control Programs
Forty-nine respondents (35.0%) reported that they could comment on tick control operations in their jurisdiction. Only 17 (12.1%) respondents indicated that their jurisdiction directly conducts or otherwise financially supports tick control, the majority of whom (n = 10, 58.8%) worked at the state level and in the Northeast (n = 11, 64.7%). Tick control activities were conducted by mosquito control agencies (n = 16, 32.7%), departments of environment or natural resources (n = 14, 28.6%), departments of public works (n = 11, 22.4%), and departments of health (n = 9, 18.4%). Several respondents indicated that control programs included academic and private partnerships (n = 12, 24.5%), the majority of which were with academic units (n = 10, 83.3%). The predominant method for tick control was host-targeted treatments, such as deer 4-poster systems and rodent-targeted bait boxes (n = 11, 22.5%), followed by vegetation modification (n = 10, 20.4%).
Program Communication and Data Sharing Practices
Respondents were asked a series of questions related to how information and data from tick surveillance, tickborne pathogen testing, and/or tick control programs are communicated. The most commonly indicated forms of program information sharing were providing results to partner agencies within the state (n = 41, 29.3%), drafting public information materials (n = 34, 24.3%), and disseminating information to local health departments (n = 32, 22.9%). Reporting data to the CDC through databases or cooperative agreement progress reports (e.g., Emerging Infections Program or Epidemiology and Laboratory Capacity cooperative agreements) was only indicated by 20 (14.3%) respondents.
Barriers to Program Development and Operations
Respondents were asked to identify the most significant barriers to developing and/or enhancing tick surveillance and control programs in their jurisdiction, including barriers to effectively disseminating data and other material (Table 4). The most commonly identified barriers to program development or enhancement for both tick surveillance and tick control were funding constraints (n = 91, 65.0%; n = 65, 46.4%, respectively) and competing priorities for limited program resources (n = 76, 54.3%; n = 54, 38.6%, respectively). In addition, the lack of evidence-based, large-scale tick management practices (n = 49, 35.0%) was also frequently indicated as a barrier to tick control program development or enhancement. Comparing responses between state and sub-state jurisdiction levels, a significantly higher proportion of state-level respondents indicated funding constraints (χ2 = 8.984, P = 0.003) as a barrier to tick surveillance program development or enhancement. There were no other statistically significant differences between state and sub-state respondents on barriers to tick surveillance and control program development.
Table 4.
Reported barriers to tick surveillance and control program development by jurisdiction
| Barrier category | Barriers to tick surveillance | Barriers to tick control | ||
|---|---|---|---|---|
|
|
|
|||
| Respondent jurisdictiona | Respondent jurisdictiona | |||
|
|
|
|||
| Sub-state (n = 73) | State (n = 67) | Sub-state (n = 73) | State (n = 67) | |
|
| ||||
| Funding Constraintsb | 39 (53.4%) | 52 (77.6%) | 32 (43.8%) | 33 (49.3%) |
| Lack of trained personnel | 28 (38.4%) | 22 (32.8%) | 19 (26.0%) | 20 (29.9%) |
| Competing priorities for limited program resources | 38 (52.1%) | 38 (56.7%) | 31 (42.5%) | 23 (34.3%) |
| Limitations in facilities/equipment | 22 (30.1%) | 19 (28.4%) | 19 (26.0%) | 17 (25.4%) |
| Lack of access to testing labs/resources | 23 (31.5%) | 20 (29.9%) | 11 (15.1%) | 10 (14.9%) |
| Coordination among agencies/units | 13 (17.8%) | 19 (28.4%) | 12 (16.4%) | 12 (17.9%) |
| Lack of guidelines for best practices | 22 (30.1%) | 15 (22.4%) | 24 (32.9%) | 15 (22.4%) |
| Lack of evidence-based, large-scale tick mgmt. practices | 18 (24.7%) | 8 (11.9%) | 24 (32.9%) | 25 (37.3%) |
Sub-state respondents include those working at either a local or county agency. State respondents include those working at either a state or federal agency.
Statistical testing for jurisdictional subgroups conducted through χ2 analysis. Statistically significant difference between jurisdictional subgroups detected only for funding constraints as a barrier to tick surveillance (χ2 = 8.984, P = 0.003).
Funding sources for tick surveillance programs closely aligned with the jurisdiction level at which programs operate (Table 5). Compared with sub-state level jurisdictions, state-level programs were significantly more likely to receive funding through state appropriations (33.3 vs. 6.1%, χ2 = 8.514, P = 0.004) and federal agency grants or cooperative agreements (52.9 vs. 6.1%, χ2 = 19.480, P < 0.001). Conversely, sub-state programs were more likely to receive funding from county or municipal taxes (66.7 vs. 3.9%, χ2 = 38.651, P < 0.001). Twenty-five (29.8%) respondents indicated that their program had other funding outside of the options listed in Table 5. These responses primarily indicated that programs either had no funding (n = 12), gained revenue through fee-for-service pathogen testing (n = 4), or through small grants from local universities or private entities (n = 7). There were no differences in the distribution of ‘other’ responses by jurisdiction level.
Table 5.
Funding Sources for tick surveillance programs by jurisdiction
| Funding source | Respondent Jurisdictiona | χ2 (P-value) | |
|---|---|---|---|
|
|
|||
| Sub-state (n = 33) | State (n = 51) | ||
|
| |||
| State funding through appropriations | 2 (6.1%) | 17 (33.3%) | 8.514 (0.004) |
| State funding through grants | 3 (9.1%) | 8 (15.7%) | 0.766 (0.382) |
| Federal funding through agency grants/cooperative agreements | 2 (6.1%) | 27 (52.9%) | 19.480 (0.000) |
| Federal funding through appropriations | 0 (0.0%) | 2 (3.9%) | 1.326 (0.250) |
| County/municipal tax-based funding | 22 (66.7%) | 2 (3.9%) | 38.651 (0.000) |
Sub-state respondents include those working at either a local or county agency. State respondents include those working at either a state or federal agency.
Respondents were asked to propose methods to resolve program barriers via open-ended questions, with 77 respondents (55.0%) providing written responses regarding tick surveillance and 66 (47.1%) providing responses regarding tick control. Responses for tick surveillance barriers generally centered on a need for consistent, stable funding (n = 41, 53.3%), training for personnel (n = 21, 27.3%), and availability of standardized guidance and protocols (n = 14, 18.2%). Respondents also indicated that administrative support for program development, access to pathogen testing laboratories or services, and a unified community of practice across jurisdictional agencies would help to address barriers to tick surveillance program development. One respondent found the ‘proliferation of nonaccredited labs doing tick testing’ to be a serious concern.
Responses for tick control barriers generally centered on a need for consistent, stable funding (n = 28, 42.4%), availability of cost-effective tick control strategies (n = 14, 21.2%), training for personnel (n = 11, 16.7%), and availability of standardized guidance and protocols (n = 10, 15.2%). Several respondents also mentioned that tick control activities fell outside of their jurisdictional mandate (n = 11, 16.7%) and that their program primarily focused on public education for tick exposure prevention. Respondents also indicated that public concerns about tick control implementation and gaps in research on effective control options would need to be addressed prior to adopting tick control activities.
The largest barriers to sharing tick-related information with the public were lack of funds to develop public-facing materials (mean rating 1.15, SE 0.090), and lack of time (mean rating 1.32, SE 0.077). There were no statistically significant differences in mean ratings for public communication barriers by state and sub-state jurisdictions. The largest barriers to sharing tick surveillance, testing, and/or control data with partners or stakeholders were time and effort costs of preparing data for sharing (mean rating 1.21, SE 0.087), followed by lack of standardized protocols across agencies (mean rating 0.93, SE 0.097) and lack of trained personnel (mean rating 0.85, SE 0.091). The mean barrier rating for lack of minimum data set requirements (mean rating state 0.55 (SE 0.11) vs. sub-state 1.0 (SE 0.15), P = 0.017) and lack of standardized protocols across agencies (mean rating state 0.74 (SE 0.12) vs. sub-state 1.14 (SE 0.151), P = 0.044) were significantly higher among sub-state respondents compared with state-level respondents. The mean barrier rating for intellectual property rights/data ownership concerns was significantly higher among state-level respondents (mean rating state 0.70 (SE 0.12) vs. sub-state 0.34 (SE 0.102), P = 0.026). Additional information on barriers to program communication and data sharing practices is available in Supp Tables 4 and 5 (online only).
Discussion
Public health and vector control agencies across the United States aim to predict and manage vector-borne disease threats, including those spread through tick bites. Ultimately, these efforts can reduce the incidence of tickborne illnesses and enable response to outbreaks (Rosenberg et al. 2018, Petersen et al. 2019). Our survey revealed that many jurisdictions were engaged to some degree in tick surveillance, and several had a desire to expand the capacity of their tick surveillance and control programs. Still, their ability to do so was impeded by constraints on consistent funding, limited infrastructure, guidance on best practices, lack of training opportunities for personnel, and limited institutional capacity to perform these functions. Our results support the need for a systematic national tick and pathogen surveillance and control program. Such a program can serve as the foundation for strategies to increase the involvement and support of additional employment sectors while standardizing guidelines for data collection and sharing. Our results suggest the need to build support for sub-state level jurisdictions, who serve as integral parts of their communities while collaborating with state and academic partners in tick and pathogen surveillance.
While our survey revealed that most respondents were engaged in some form of tick surveillance, larger proportions of respondents indicated that their program conducts irregular, ad hoc tick, or passive tick surveillance, but not routine active tick surveillance. The majority of routinely implemented active tick surveillance programs were located in regions with endemic Lyme disease and associated tick vectors (Northeast, Upper Midwest, and Northwest). This may be due to greater funding specifically targeting Lyme disease, which is the most commonly reported human vector-borne disease in the United States, despite substantial underreporting (Hinckley et al. 2014).
The most commonly identified tick surveillance program objectives were the detection (presence) of ticks by species, monitoring tick distribution by species, and detection of pathogens in ticks. Despite these being listed as the most common objectives, 16–35% of respondents indicated that their programs were not currently able to work on these fundamental program objectives. Although medically important ticks and their associated pathogens are found in every state in the continental United States, tick surveillance objectives with direct impact on public health decision-making, such as monitoring the distribution of ticks of public health importance, evaluation of pathogen prevalence in ticks, and calculation of public health risk of tickborne infection, were currently implemented by less than half of respondents’ programs. Furthermore, only a quarter of survey respondents reported that their program was able to support the testing of ticks or other samples for tickborne pathogens.
The interest in ticks and tickborne diseases across employment sectors, such as public health, mosquito control, cooperative extension, natural resources, and agriculture (Supp Table 1 [online only]), suggests that there are opportunities for building multi-disciplinary and multi-occupation collaborative networks to address national needs on tick and tickborne pathogen surveillance. The majority of respondents referenced partnerships with academic organizations to implement their tick surveillance and pathogen testing program operations. This response suggests that supporting collaborations between academic organizations involved in vector-borne disease research and environmental sectors may have significant public health synergism.
With some exceptions, tick species targeted by programs across climate regions and jurisdictions aligned with the known geographic distributions of species of medical importance, indicating a good understanding of relevant public health threats to communities across the nation. However, an area of discrepancy across both climate regions and jurisdictions was the importance placed on detecting the emergence of new species, which was more highly rated in NOAA regions concentrated on the east coast and among sub-state respondents. Such a regional interest could be driven by the recent detection of Haemaphysalis longicornis in the Northeast and mid-Atlantic regions just prior to distribution of this survey (Beard et al. 2018, Rainey et al. 2018). Collections of more than 95 species of exotic ticks imported into the United States in the last half of the 20th century (Keirans and Durden 2001), concomitant growth in animal trade and exotic animal introductions (Marano et al. 2007), and continuous risk of introductions through transboundary pathways (Estrada-Peña et al. 2007, Pérez de León et al. 2012) also suggest detection of new species is an ever-present concern. These findings highlight the need for national guidance on tick surveillance to be flexible to serve local public health priorities, while providing standardization in methods and collected outcomes across jurisdictions.
A relatively small proportion of respondents (35%) was able to comment on tick control operations in their jurisdictions, with only 17 programs financially supporting the implementation of control efforts. Methods for tick control included vegetation modification (20.4%) and host-targeted treatments such as the 4-poster systems for deer and rodent-targeted bait boxes (22.5%). Thus very few programs across the United States are directly involved in tick control, and activities undertaken by these programs favor approaches suited to public lands and open spaces (Stafford 2007, Eisen and Dolan 2016). Recent reports indicate that private commercial pest control firms play an important role in control of ticks in peridomestic settings, often based on application of synthetic acaricides (Jordan and Schulze 2020).
Respondents repeatedly indicated both through discrete questions and open-ended responses that the lack of consistent and sustainable funding sources was the primary factor limiting their ability to conduct regular tick surveillance activities or to expand existing capacity. The lack of program continuity due to funding constraints can result in gaps in tick surveillance activities across years, as well as the loss of tick records due to unidentified ticks in tick collections or uncatalogued and unpublished data (Gilliam et al. 2020). Although in some cases year-to-year monitoring is not required to meet surveillance objectives (e.g., documenting the presence of established tick populations), in other cases, lack of continuity inhibits the ability to monitor changes in risk of exposure to ticks and tickborne pathogens over time (e.g., phenology studies, or monitoring changes in tick abundance or pathogen prevalence in areas of recent emergence). In addition, lack of funding for personnel affects the sharing of tick-related information when staff time is limited for the development of public-facing materials. However, our respondents did indicate an effort to obtain support from private entities and other collaborators; these efforts speak to the commitment, interest, and dedication of respondents.
Lack of infrastructure and institutional capacity was another common barrier identified by our survey. Programs historically focused on mosquito surveillance and control, or other issues of public health importance such as bed bugs and communicable disease response, have limited ability to expand their scope of work. The capacity of the public health infrastructure must be expanded in order for tick surveillance and control activities to operate at a similar level to other priority areas. Respondents also recognized the need for innovations in tick monitoring and control. Findings from the 2018 Tick Borne Disease Working group highlight that innovative research projects on tick surveillance and control are continually underfunded in the United States (Tick-Borne Disease Working Group 2018). Recognizing that tick life cycles span multiple years and intervention trials often require at least 3–5 yr to complete, sustained funding for tick and tickborne disease control studies are critically important (Eisen and Dolan 2016).
Availability of tickborne pathogen testing was identified as a significant barrier to tick surveillance programs. Since the time this survey was implemented, CDC has offered laboratory support to state health departments for testing Ixodes spp. for known human pathogens. Nonetheless, testing is constrained across the nation, particularly for non-Ixodes tick species, creating a gap in our knowledge of tickborne pathogen presence and impeding our ability to accurately assess risk across communities. Increased and sustained funding, coupled with implementation of, or adherence to, pathogen testing quality standards, is required to expand the capacity to conduct responsive tickborne pathogen testing as more programs are initiated.
Beyond funding, there is a need for nuanced recommendations and training programs in surveillance and control practices. This survey was disseminated in the months prior to the publication of the CDC’s guidance documents for I. scapularis or I. pacificus and their associated pathogens (Centers for Disease Control 2019a). A valuable next step in this direction will be the development of nuanced guidance and recommendations for the surveillance of additional tick species of medical importance that address differences in their behaviors and the habitats where these vectors live. Besides, developing new training and reference materials, and widening the distribution of existing materials—available through a variety of modalities—can enhance the ability of state and sub-state respondents across different agencies to implement tick surveillance following standardized practices, and support the sharing of information to multiple stakeholder audiences.
This survey is the first of its kind to be undertaken at a national level and has several limitations. While it would be helpful to know the response rate for our survey, we cannot determine this due to the distribution method used. Some states had missing data or low response rates, which resulted in sample sizes too small for meaningful statistical analysis across state or geographic subgroups. Thus, we do not fully understand the tick surveillance and control activities ongoing in no- or low-response states. Future surveys should attempt to measure response rates and increase sample sizes.
Ticks and tickborne diseases are a growing problem associated with globalization and climate change (Jongejan and Uilenberg 2004, Dantas-Torres 2015). While this survey addressed ticks and tickborne diseases of public health importance, the threat of tickborne diseases is a One Health challenge. Geographic ranges of vectors are expanding with rising temperature, which affects not only vector behaviors such as biting and reproduction, but also pathogen vitality and transmission rates(U.S. Global Change Research Program 2015, Eisen et al. 2016b). Ticks are particularly impacted by humidity and changes in rainfall (Campbell-Lendrum et al. 2015), and in many areas, the seasonal duration of vector exposure is lengthening. Furthermore, new pathogens of public health importance are emerging or are newly recognized (Eisen et al. 2017, Petersen et al. 2019). In order to stem the tide of increasing tickborne diseases, we need to improve surveillance capacity and tick control efforts through recognition of these activities as a national priority. Here, we have determined the range in capacity to support tick surveillance and control in the contiguous United States as of September 2018, and have identified barriers to building and improving capacity.
Our study results can serve as a baseline for understanding current nationwide practices and challenges for managing tickborne diseases. We document regional tick and tickborne disease concerns, challenges for workers, and outline recommendations for improved delivery of tick control programs. Tick surveillance and control programs would have a more significant impact on human health with increased coordination among programs and stakeholders, increased funding from multiple jurisdiction levels, and through careful planning and development of mission-focused protocols. In addition, this study shows that research to develop and evaluate new control tools is desperately needed.
Supplementary Material
Acknowledgments
We would like to acknowledge the support of the Regional Centers for Excellence in Vector-Borne Diseases for survey development and dissemination of the initial survey instrument developed by the Northeast Regional Center for Excellence in Vector-Borne Diseases. We would also like to thank James Burtis, Jenna Bjork, Vicki Kramer, Melissa Yoshimizu, Kerry Padgett, Matt Frye, and Lars Eisen for beta testing the survey and providing guidance during the data analysis process. This work was supported through Cooperative Agreement Number 1U01CK000509-01 between the Centers for Disease Control and Prevention (CDC) and Cornell University/Northeast Regional Center for Excellence in Vector-Borne Diseases. C.G. acknowledges funding support from the Southeastern Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000510). J.E.F. acknowledges funding support from the Pacific Southwest Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516). R.L.S. and N.M.P. acknowledge funding support from the Midwest Center of Excellence for Vector-Borne Diseases funded by the Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000505). P.D.T. acknowledges funding support from the Western Gulf Center of Excellence for Vector-Borne Diseases funded by the Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000512). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
References Cited
- Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, and Bertone MA 2018. Multistate infestation with the exotic disease—vector tick Haemaphysalis longicornis—United States, August 2017–September 2018. Morb. Mortal. Wkly Rep 67: 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacki P, and Waldorf D. 1981. Snowball sampling: problems and techniques of chain referral sampling. Sociol. Methods Res 10: 141–163. [Google Scholar]
- Campbell-Lendrum D, Manga L, Bagayoko M, and Sommerfeld J. 2015. Climate change and vector-borne diseases: what are the implications for public health research and policy? Phil. Trans. R. Soc. B: Biol. Sci 370: 20130552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. 2019a. Tick surveillance. https://www.cdc.gov/ticks/surveillance/index.html. Accessed 26 November 2019.
- Centers for Disease Control. 2019b. Vector-Borne Disease Regional Centers of Excellence. https://www.cdc.gov/ncezid/dvbd/about/prepare-nation/coe.html. Accessed 26 November 2019.
- Crouse T, and Lowe PA 2018. Snowball sampling. In Frey BB (ed.), The SAGE encyclopedia of educational research, measurement, and evaluation. SAGE Publications, Thousand Oaks, CA. [Google Scholar]
- Dantas-Torres F. 2015. Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. Int. J. Parasitol. Parasites Wildl 4: 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, and Dolan MC 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol 53: 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, and Eisen L. 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34: 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, and Beard CB 2016a. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53: 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, and Beard CB 2016b. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol 53: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, and Paddock CD 2017. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. Ilar J. 58: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI. 2018. ArcGIS desktop: release 10.6.1. Environmental Systems Research Institute, Redlands, CA. [Google Scholar]
- Estrada-Peña A, Pegram RG, Barré N, and Venzal JM 2007. Using invaded range data to model the climate suitability for Amblyomma variegatum (Acari: Ixodidae) in the New World. Exp. Appl. Acarol 41: 203–214. [DOI] [PubMed] [Google Scholar]
- Gilliam B, Gronemeyer P, Chakraborty S, Winata F, Lyons LA, MillerHunt C, Tuten HC, Debosik S, Freeman D, O’Hara-Ruiz M, et al. 2020. Impact of unexplored data sources on the historical distribution of three vector tick species in Illinois. J. Med. Entomol 57: 872–883. doi: 10.1093/jme/tjz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golnar AJ, Martin E, Wormington JD, Kading RC, Teel PD, Hamer SA, and Hamer GL 2019. Reviewing the potential vectors and hosts of african swine fever virus transmission in the United States. Vector Borne Zoo. Dis 19: 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, and Mead PS 2014. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis 59: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F, and Uilenberg G. 2004. The global importance of ticks. Parasitology. 129 Suppl: S3–14. [DOI] [PubMed] [Google Scholar]
- Jordan RA, and Schulze TL 2020. Availability and nature of commercial tick control services in three Lyme Disease endemic states. J. Med. Entomol 57: 807–814. doi: 10.1093/jme/tjz215. [DOI] [PubMed] [Google Scholar]
- Junker L. 2020. ESA position statement on tick-borne diseases. Ann. Entomol. Soc. Am 113: 62–63. [Google Scholar]
- Karl T, and Koss WJ 1984. Regional and national monthly, seasonal, and annual temperature weighted by area, 1895–1983, National Climate Data Center. https://repository.library.noaa.gov/view/noaa/10238. Accessed 24 December 2019. [Google Scholar]
- Keirans JE, and Durden LA 2001. Invasion: exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. J. Med. Entomol 38: 850–861. [DOI] [PubMed] [Google Scholar]
- Kugeler KJ., Farley GM, Forrester JD, and Mead PS. 2015. Geographic distribution and expansion of human Lyme Disease, United States. Emerg. Infect. Dis 21: 1455–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano N, Arguin PM, and Pappaioanou M. 2007. Impact of globalization and animal trade on infectious disease ecology. Emerg. Infect. Dis 13: 1807–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration National Centers for Environmental Information. 2019. U.S. Climate Regions. https://www.ncdc.noaa.gov/monitoring-references/maps/us-climate-regions.php. Accessed 24 December 2019.
- Pérez de León AA, Teel PD, Auclair AN, Messenger MT, Guerrero FD, Schuster G, and Miller RJ 2012. Integrated strategy for sustainable cattle fever tick eradication in USA is required to mitigate the impact of global change. Front. Physiol 3: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Beard CB, and Visser SN 2019. Combatting the increasing threat of vector-borne disease in the United States with a national vector-borne disease prevention and control system. Am. J. Trop. Med. Hyg 100: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R, Barker S, Cobos M, Barker D, Teo E, Foley D, Nakao R, Lawrence K, Heath A, and Peterson A. 2019. Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci. Rep 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey T, Occi JL, Robbins RG, and Egizi A. 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol 55: 757–759. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.org. [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, and Kersh G. 2018. Vital signs: trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly Rep 67: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team. 2019. RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com. [Google Scholar]
- Sanders DM, Schuster AL, McCardle PW, Strey OF, Blankenship TL, and Teel PD 2013. Ixodid ticks associated with feral swine in Texas. J. Vector Ecol 38: 361–373. [DOI] [PubMed] [Google Scholar]
- Siegel JT, and Jones ND 2018. Survey methods, pp. 1639–1642. In Frey BB (ed.), The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation. SAGE Publications, Thousand Oaks, CA. [Google Scholar]
- SPSS Inc. 2017. SPSS statistics for windows, Version 25.0. IBM Corp, Armonk, NY. [Google Scholar]
- Stafford KC 2007. Tick Management Handbook; an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. Connecticut Aricultural Experiment Station, New Haven, CT. [Google Scholar]
- Tick-Borne Disease Working Group. 2018. Tick-Borne Disease Working Group 2018 Report to Congress. https://www.hhs.gov/sites/default/files/tbdwg-report-to-congress-2018.pdf. Accessed 7 February 2020.
- U.S. Global Change Research Program. 2015. Climate and Health Assessment. https://health2016.globalchange.gov/about. Accessed 7 February 2020.
- Villarreal Z, Stephenson N, and Foley J. 2018. Possible northward introgression of a tropical lineage of Rhipicephalus sanguineus ticks at a site of emerging rocky mountain spotted fever. J. Parasitol 104: 240–245. [DOI] [PubMed] [Google Scholar]
- Wisely SM, and Glass GE 2019. Advancing the science of tick and tick-borne disease surveillance in the United States. Insects 10: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington JD, Golnar A, Poh KC, Kading RC, Martin E, Hamer SA, and Hamer GL 2019. Risk of African swine fever virus sylvatic establishment and spillover to domestic swine in the United States. Vector Borne Zoo. Dis 19: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.