Abstract
Astrocytes are critical components of neural circuits positioned in close proximity to the synapse, allowing them to rapidly sense and respond to neuronal activity. One repeatedly observed biomarker of astroglial activation is an increase in intracellular Ca2+ levels. These astroglial Ca2+ signals are often observed spreading throughout various cellular compartments from perisynaptic astroglial processes, to major astrocytic branches and on to the soma or cell body. Here we review recent evidence demonstrating that astrocytic Ca2+ events are remarkably heterogeneous in both form and function, propagate through the astroglial syncytia, and are directly linked to the ability of astroglia to influence local neuronal activity. As many of the cellular functions of astroglia can be linked to intracellular Ca2+ signaling, and the diversity and heterogeneity of these events becomes more apparent, there is an increasing need for novel experimental strategies designed to better understand the how these signals evolve in parallel with neuronal activity. Here we review the recent advances that enable the characterization of both subcellular and population-wide astrocytic Ca2+ dynamics. Additionally, we also outline the experimental design required for simultaneous in vivo Ca2+ imaging in the context of neuronal or astroglial manipulation, highlighting new experimental strategies made possible by recent advances in viral vector, imaging, and quantification technologies. Through combined usage of these reagents and methodologies, we provide a conceptual framework to study how astrocytes functionally integrate into neural circuits and to what extent they influence and direct the synaptic activity underlying behavioral responses.
Keywords: Astrocytes, Morphology, Ca2+ imaging, Genetic indicators, Viral vectors
1. Introduction
Unlike neurons, astroglia do not require rapid transmission of electrical signals in the form of action potentials to communicate with nearby cells [1], and as a result they were initially considered to be passive players in brain function with a limited influence on synaptic communication. Yet, the structural complexity and number of astroglia increase along the evolutionary timeline, reaching their apex in the human brain [2], [3]. Moreover, the morphological properties of astrocytes enable just a single astrocyte to contact and monitor hundreds of dendrites and by extension hundreds of thousands of synapses [4], [5], [6], [7]. We now know that astrocytes respond directly to neuronal neurotransmitter release [8], [9], and react to bouts of synaptic activity with homeostatic regulation of ion buffering [10], clearance of neurotransmitters [11], and with the release of various neuroactive chemicals [12], [13], [5], [14]. These properties allow astroglia to differentiate and uniquely respond to activity at diverse synaptic inputs [15]. Astrocytes are responsible for the vast majority of GABA and glutamate clearance from the synaptic cleft following neuronal release, a cellular function that is mediated via patterned expression of various transporters including GAT3 (GABA) and GLT-1 (glutamate), with modifications in the expression or localization of transporters serving as a means to directly influence neurotransmission and synaptic plasticity [16], [17], [18]. In addition to neurotransmitter uptake systems, astrocytes express a myriad of ionotropic and metabotropic neurotransmitter receptors which allow them to sense and respond to synaptic activity [19], [20]. Moreover, the majority of astrocytes contact blood vessels, serving as an intermediary between neurons and the neurovascular network [21], both to provide metabolic support and to deliver the required precursors for the generation of GABA and glutamate, a cellular function that is required for normal synaptic communication and physiological processes [22]. Because of the large body of work detailing their role in modulating synaptic plasticity, astrocytes are now appreciated as active components of neural networks with diverse and functional roles in neuronal processing.
Among other intracellular processes [23], astroglia respond to bouts synaptic activity with intracellular calcium (Ca2+) elevations, occurring across multiple cellular compartments, including the astrocytic cell body or soma, the astroglial primary branches, and the fine membranous peripheral perisynaptic astroglial processes (PAPs) [6], [24]. Generally, elevated intracellular astrocytic Ca2+ levels are considered the cellular substrate of astrocytic action, a phenomenon often linked to a biological response in astroglia, often thought as somewhat of a proxy for the electrical excitation and action potentials observed in neurons [24], [25]. Indeed, many of the cellular functions of astroglia, including aspects of the homeostatic support of neurons and their ability to influence synaptic communication, rely on intracellular Ca2+ signaling [26]. Consequently, a considerable body of work now demonstrates that tightly regulated astroglia Ca2+ dynamics and the related homeostatic function of astroglia are required for many normal physiological processes [27], [28], with disruption of these cellular systems linked to the pathophysiology of neuropsychiatric diseases, including substance use disorders [22], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]. As we progress further in understanding the role of astroglia in shaping neural communication, there is an increasing need to evaluate astrocytic spatiotemporal Ca2+ dynamics to better understand the mechanistic underpinnings of how astrocytes influence local neurotransmission at both the single cell and circuit-level in vivo. To date, a significant amount of pioneering work in this field has been done ex vivo, which has provided a solid understanding of the bidirectional chemical communication between neurons and astrocytes, including the astroglial intracellular signaling pathways leading to astrocytic Ca2+ dynamics, and the neuroactive chemicals released by astroglia as a result of this intracellular signaling modality [25], [26], [46], [47], [48]. However, current strategies, such as in vivo two-photon calcium imaging in behaving animals [48], [49], [50], combined with advanced analysis pipelines [51], [52], now enable the study of astrocytes as potential computational entities, allowing for a more complete understanding of if and how astroglia shape neuronal activity patterns during complex behaviors, including learning and motivated responses. Importantly, while astrocytic Ca2+ events are remarkably heterogeneous (discussed in length below) and can occur at spatially restricted subcellular domains within individual cells [53], these events can also expand across multiple cells in astroglial networks [54], [55], which likely reflects their ability to influence neuronal network activity. Further, evidence suggests that the functional outcomes of these spatiotemporally distinct Ca2+ events are themselves functionally unique [56]. Accordingly, care must be taken to interpret Ca2+ signals in context of astrocyte morphology, given the complex structure–function relationship exhibited by astroglia. As an example, the spatiotemporal specifics of Ca2+ dynamics enable astrocytes to gate and influence neurotransmission in a variety of modalities, including through the dynamic structural remodeling of their physical synaptic presence and by extension the regulation of the literal space that neurotransmission occurs in [57]. Apart from this, Ca2+ dynamics have also been directly linked to the release of glial-derived neuromodulators and adaptations in ion buffering or transmitter uptake [26], [47], [58], underlining the need to better understand Ca2+ signaling in astroglia and how it is linked to alterations in synaptic plasticity.
1.1. Examination of astroglial structural plasticity
As described above, astrocytic structural complexity increases across the evolutionary timeline reaching its apex in the human brain, with human astrocytes displaying a more ramified and complex overall structure that is accompanied by more efficient and rapid induction of intracellular Ca2+ signaling as compared to their rodent counterparts [59]. Armed with this information, the Nedergaard laboratory performed a study utilizing chimeric mice where human glial cell progenitors were engrafted into the murine forebrain, including the hippocampus and cortex. Remarkably, the human astroglial progenitors became mature astrocytes that fully integrated into the mouse brain and extant glial syncytium, forming functional gap junction connections with murine host cells, yet retaining the larger structural profile and enhanced overall complexity that typifies hominoid astroglia. Interestingly, the hominoid astrocytes displayed more rapid induction of Ca2+ events and functionally enhanced hippocampal long-term potentiation, resulting in enhanced behavioral performance in a variety of cognitive and conditioning tasks [60]. This study not only speaks to the structure–function relationship evident in astroglial cells, but also establishes that enhanced glial cell complexity and Ca2+ signaling efficacy can be directly linked to the synaptic plasticity underlying cognitive performance and learning, demonstrating that astroglia functionally influence neural communication as it pertains to learning and memory. Despite being less complex than their human counterparts, rodent astroglia still serve as an excellent model system to examine the functional influence of astroglia on neural networks. Among other complex functions, rodent astrocytes display experience-dependent structural remodeling in a Ca2+-dependent manner, enabling astrocytes to regulate the extent of their interaction with neighboring active synapses and to facilitate neurotransmission [57]. Taken together, data from studies of both hominid and rodent astroglia support the general hypothesis through their structural complexity, interaction with synapses, and their ability to chemically respond to neural activity, astrocytes actively regulate and tune neurobiological processes. These data highlight the need for the continued examination of astrocyte physiology, adaptations in transporter expression, morphology, synaptic interaction, and Ca2+ dynamics to better understand their role in directing and refining synaptic plasticity.
Initially, studies examining rodent and human astroglial numbers and overall morphology focused on alterations in glial fibrillary acidic protein (GFAP), a cytoskeletal protein found in the primary astrocytic branches, often used as a canonical astrocytic marker. However, this approach has significant limitations given reports that GFAP expression is not present in all astroglia [61], [62], [63] and that GFAP, when present, only constitutes about 15 % of total cell volume. Accordingly, the signal attained from GFAP staining provides a partial and largely incomplete representation of the complex membranous structure of astrocytes and vastly underestimates the extent of their presence at the tripartite synapse [64]. The use of dye filling strategies [64], [65] and viral vectors utilizing GFAP promoter-driven cytosolic or membrane-targeted fluorescent probes, such as LCK-GFP, in combination with high-resolution microscopy now allows for a more complete visualization of astroglia including their perisynaptic ramifications and fine membranous peripheral processes [66]. Further, employing the highest resolution imaging strategies, including stimulation emission depletion (STED) microscopy or electron microscopy, allows for detailed visualization of astrocytes in the context of the synaptic microenvironment and the extent of PAP coverage of the pre- and post-synaptic cell [6], [67], [68], [69]. Using these techniques, it is now possible for Ca2+ events in the astroglial leaflets to be mapped onto super-resolution micrographs to detail nodes of activity in the tripartite synapse [6]. By examining astroglia with these strategies, it has become evident that astrocytes tile the parenchyma, occupying largely non-overlapping domains or territories [64], and have an active synaptic presence [6], [69]. Akin to neuronal processes, astroglial processes are also subject to experience-dependent plasticity, that occurs in combination with modulation of nearby neurotransmission [27], [57], [70]. In parallel with the experience-dependent synaptic plasticity and concordant morphological plasticity observed at dendritic spines, synapse-associated astroglial processes exhibit enhanced motility following induction of LTP, demonstrating that astroglia also exhibit brain-state dependent structural plasticity [57]. Interestingly, LTP-induced astroglial structural plasticity has direct consequences for excitatory neurotransmission, as LTP induction protocols can cause GLT-1-rich PAPs to retract from potentiated synapses, allowing for glutamate spillover and enhanced glutamatergic signaling, possibly extending to intrasynaptic crosstalk [70]. These data demonstrate that astroglial structural plasticity can directly impact excitatory neurotransmission. In keeping with these findings, our laboratory and others have demonstrated drug- and withdrawal-dependent reductions in the morphometric features of astrocytes and reduced synaptic interaction within the nucleus accumbens, phenomenon that has been directly linked to relapse vulnerability following drug exposure and withdrawal. Additionally, re-exposure to drug-conditioned cues also induces astrocytic structural plasticity and restores astrocytic synaptic presence in the nucleus accumbens, with these astrocytic changes critical in limiting cue-drug seeking [30], [31], [43], [33], [71]. Changes in astroglial structure and synaptic interaction are often accompanied by additional functional adaptations including downregulation of GFAP and the glutamate transporter, GLT-1, resulting in impaired glutamate homeostasis at the tripartite synapse [30], [72]. Collectively, these results demonstrate that astroglial plasticity and adaptations in their synaptic presence are directly linked to the neuroplastic adaptations that underlie relapse vulnerability and cue-induced drug seeking.
Many studies, including the ones discussed above, employ viral vectors to selectively engage astrocytic expression of a membrane-targeted fluorescent molecule [lymphocyte-specific protein tyrosine kinase (LCK)-GFP] to assess adaptations in astroglial morphometric features (surface area, volume) and changes in the extent of astrocyte-synapse interactions. Inclusion of LCK allows for the fluorophore to be trafficked to the cellular membrane [73], and enables a more complete visualization of the fine astrocytic processes that would otherwise be lost using cytosolic markers [66]. Fig. 1 illustrates the level of detail gained from employing an adeno associated viral (AAV) construct packaged under the truncated GfaABC1D promoter to express the membranous LCK-GFP (AAV2/5-GFAP-LCK-GFP) in concert with a cytosolic tdTomato (AAV2/5-GFAP-tdTomato). The combination of AAV serotype 2/5 and GfaABC1D promoter is a commonly used strategy for viral transduction of mammalian astrocytes [74], [75], although the AAV8 serotype and/or ALDH1 promoter have also been used to effectively and selectively transduce astroglia [76], [77]. As the methodologies for labeling and manipulation of astroglia have become established and refined, expression of optogenetic constructs [27], [78], [79], [80], chemogenetic constructs [28], [81], [82], [83], [84], [85], and various genetic fluorescent indicators for Ca2+ [18], [48], [49], [86], [87], as well as various fluorescent neurotransmitter indicators [75], [88] have become more routinely employed. As these tools are being utilized for the study of astroglial function as well as astroglial Ca2+ dynamics both ex and in vivo, the heterogeneity and complexity of astroglial Ca2+ dynamics, and the underlying functional relevance of this signaling modality, is beginning to be elucidated.
Fig. 1.
Comparison of membrane targeted vs cytosolic astroglial expression of fluorescent proteins. A) Low magnification confocal images of membrane-targeted GFP expression via AAV2/5-GFAP-LCK-GFP (green) vs cytoplasmic expression of tdTomato via AAV2/5-GFAP-tdTomato (red) in a field of cortical astroglia. B) High magnification images of a single astrocyte with co-expression of membrane targeted GFP (green) and cytosolic tdTomato. Note the differences in overall appearance of these cellular compartments. Scale bars depict 100 μm in A) and 10 μm in B). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
1.2. Astroglial calcium dynamics and their subcellular localization
As described above, astrocytes typically respond to bouts of synaptic activity with Ca2+ transients in various cellular compartments including astroglial leaflets or microdomains within the cellular architecture [6], [87]. These calcium events can then expand to encompass the somatic region of the cell, or the entirety of the astrocyte itself. Beyond Ca2+ propagation within individual cells, astrocytic Ca2+ dynamics can spread across individual territories to adjacent cells within the astroglial syncytium, an effect that occurs as a result of gap junction connectivity, cytoplasmic continuity, and diffusion of second messengers like inositol 1,4,5-triphosphate (IP3), ATP, and diacylglycerol [55]. This phenomenon is often described as Ca2+ waves [54], [89], and has been linked to synchronization of neuronal activity [46], [58]. In addition to the regional diversity within the cell structure, astrocytic Ca2+ signals also display temporal heterogeneity, with the duration of Ca2+ events lasting from hundreds of milliseconds to tens of seconds [90], [91], with rapid microdomain Ca2+ linked to vasodilatation [92], cerebral blood flow [86], as well as neuromodulation via astroglial-derived chemicals [24], [93].
Early explorations into the mechanistic underpinnings of astroglial Ca2+ dynamics and the functional relevance of Ca2+ mobilization from internal stores were somewhat inconclusive. While organism-wide activation of astroglial G-protein coupled receptors (GPCRs) via chemogenetic manipulation was shown to impact autonomous nervous system function and various behaviors tied to locomotion, deletion of an IP3 receptor subtype most prominently expressed in astroglia (IP3R2) and subsequent blockade of GPCR action via disruption of IP3 signaling did not prevent this behavioral phenotype [94]. Moreover, selective genetic deletion of astroglial IP3R2 did not appear to dramatically influence neuronal activity, anxiety- or depression-like behaviors, or learning and memory [94], [95]. In parallel, early means for measuring astrocytic Ca2+ dynamics were carried out via bulk loading of Ca2+ indicator dyes, such as OGB-AM or Fluro-AM, methods that have considerable limitations [96], including low signal-to-background noise ratio and restricted visualization of astrocytic calcium dynamics outside of the somatic region or the larger primary branches [90]. As such, the data obtained from these early studies indicated that intracellular Ca2+ elevations may not directly cause astrocyte-derived neurochemical release and subsequent adaptations in neuronal activity, and thus likely did not consequently impact or direct the neural activity underlying learning or complex behaviors [97], [98]. However, the advent of ultra-sensitive genetically encoded calcium indicators (GECIs) [99], [100] and discovery of additional IP3 receptor subtypes (IP3R1 and 3) in astrocytes has substantially refined our understanding of the diversity and temporal dynamics of astrocytic Ca2+ responses and the cellular systems that regulate intracellular Ca2+ elevations in astroglia [101]. Specifically, astrocytic Ca2+ elevations in the somatic region and primary branches are evoked in large part through IP3R2 stimulation [101], yet microdomain Ca2+ dynamics are often attributed to other IP3 receptor subtypes, including IP3R1 or IP3R3, or additional non IP3R-mediated means for Ca2+ flux including transient receptor potential ankyrin 1 [102], l-type voltage gated Ca2+ channels [103], the sodium/calcium exchanger [104], transient receptor potential canonical channels [105], and Ca2+ mobilization from mitochondria [87]. For a comprehensive review of this topic, including discussion of various IP3R knock out animal models and analyses of the resulting impact on astrocytic Ca2+ dynamics, see [106]. Collectively, these findings demonstrate that astrocytic Ca2+ dynamics are more uniquely heterogenous in their magnitude and subcellular distribution than we had originally perceived, with heterogeneity also evident in the intracellular signaling required to evoke astroglial Ca2+ dynamics and the functional consequences derived from of each modality of intracellular Ca2+ events (discussed at length below) [86], [87], [91], [93].
As expected, microdomain-level Ca2+ events are thought to be largely dictated by activity patterns at nearby synapses, where astroglia both perform their traditional homeostatic roles [107], and act to specifically tune excitatory or inhibitory neurotransmission through release of gliotransmitters or adaptations in their physical presence at the synapse [11], [53]. Specifically, microdomain Ca2+ dynamics enable cytoarchitecture plasticity, actin-mediated cytoskeletal dynamics, and growth towards or retraction from the synaptic cleft, a process directly linked to adaptations in neurotransmitter reuptake, gliotransmitter release, and synaptic regulation [108]. Importantly, Ca2+-dependent release and clearance of neuroactive chemicals by astrocytes can bidirectionally modulate neuronal activity depending on the type of gliotransmitter released, the type of receptors present at tripartite synapse, the characteristics of the pre- and postsynaptic neurons, and the preference for and presence of astroglial physical interaction at the pre- or postsynapse [69], [109], [110], [111], [112]. Evidence now demonstrates that Ca2+ elevations in astrocytic microdomains as a result of neurotransmission occur on rapid timescales, comparable to neuronal calcium dynamics [49], and often propagate to the parent branch and somatic region, culminating in cell-wide Ca2+ elevations and subsequent glial-derived release of neuroactive chemicals, which can also influence Ca2+ dynamics in neighboring astroglia [108] as a means to influence neuronal network activity [58]. As described above, astrocytic gap junction hemichannels direct intracellular communication via cytoplasmic continuity [113] and as such are permeable to charged molecules (Ca2+, NAD+), second messengers (IP3, ATP), and even glutamate [55]. Thus, Ca2+ dynamics that occur in the PAPs of an individual astrocyte could quickly be translated to a Ca2+ activity within neighboring, functionally connected cells and occur on a timescale that is consistent with an ability to influence local synaptic transmission [49]. Taken together, these data support the hypothesis that astrocytes shape the formation of neuronal ensembles and influence long-range circuit communication. The rapid nature of PAP Ca2+ dynamics also establishes the possibility that astrocytes themselves can store information and participate in encoding discrete environmental stimuli. These hypotheses are directly supported by work done by Poskanzer and Yuste, which established that astrocytic activation regulates circuit UP states, a phenomenon that can be described as a period of time (hundreds of milliseconds) in which neurons are depolarized and fire a multitude of action potentials [46]. These studies demonstrate that stimulation of an individual astrocyte is sufficient to increase Ca2+ activity throughout the astroglial network, and that astroglial-derived glutamatergic and purinergic signals direct the formation of circuit UP states ex vivo [46]. Moreover, Poskanzer and Yuste also found that astrocytes control cortical circuit state switching in vivo, with astroglial glutamatergic signaling responsible for the shift from high-frequency neuronal firing towards synchronized, low-frequency circuit activity [58]. Taken together, it is apparent from these studies that astrocytes sense neuronal activity and respond with rapid microdomain Ca2+ events that can translate into long-range Ca2+ waves through the astroglial syncytia, ultimately culminating in the coordinated release of astroglial-derived neuroactive chemicals to gate neuronal network activity.
Despite these well-documented outcomes of astrocytic Ca2+ signaling and subsequent modulation of neuronal activity, the extent of Ca2+-dependent astrocyte-derived neuroactive chemical release under physiological conditions remains controversial [98]. For example, experiments examining either increases or decreases in astrocytic Ca2+ levels through stimulation of Gq-GPCRs or deletion of components in the IP3 signaling pathway provide evidence that these manipulations do not alter neurotransmission and synaptic plasticity in vivo, and that some methodologies used to manipulate astrocytic activity in vivo and stimulate gliotransmission can be considered not physiologically relevant [98]. Moreover, the presence of SNARE proteins in astrocytes and Ca2+-dependent vesicular release of neuroactive chemicals remains disputed. While these observations support the opinion astrocytes lack the machinery for Ca2+-dependent vesicular release of gliotransmitters, it is important to consider gliotransmitter release has been reported to occur through several vesicular and non-vesicular modalities. Moreover, gliotransmission likely does not exhibit 1:1 parity with analogous molecular mechanisms dictating release of neuroactive chemicals from neurons [114]. In a well-written perspective, Savtchouk and Volterra (2018) counter the argument against in vivo gliotransmission and outline some oversimplifications may have contributed to this perspective [114], including simply focusing on a single neurotransmitter system or calcium source to trigger gliotransmission. Accordingly, these perspectives do not provide a wholistic view to what is happening in an intact, in vivo system. Further, the authors argue that it is difficult to equate Ca2+-dependent vesicular transmitter release in astrocytes and neurons. Evidence does indeed exist supporting astroglial expression of low levels of glutamate-containing vesicles and alternative isoforms of SNARE proteins that support vesicular release [114], implying that astrocytes are capable of Ca2+-dependent mechanisms of gliotransmitter release. Furthermore, as discussed above, astrocytic regulation of synaptic activity also extends beyond vesicular or non-vesicular gliotransmission, as astrocytes are responsible for (1) the delivery of the precursors needed to generate key neurotransmitters, (2) the reuptake of neuroactive chemicals from the synaptic cleft and cessation of synaptic activity, (3) the shaping the physical space that neurotransmission occurs in, (4) the ion buffering required to support action potential generation, and (5) the metabolic support of local neurons [1], [22], with the several of these processes occurring via Ca2+-dependent mechanisms. As outlined throughout this review, our perspective is that Ca2+ dynamics are essential for astroglia to respond to and influence the synaptic environment and regulate the local neuronal activity and circuit-level communication underlying complex behavioral responses. This viewpoint is supported by the studies highlighted herein, as well as in our discussion outlining the need to study the spatiotemporal outcomes of astrocytic Ca2+ signaling using sophisticated methodologies.
1.3. Quantification of astroglial action
An increased appreciation of the diversity of Ca2+ signals in astrocytes has led to a parallel refinement of strategies for the quantification and analyses of how astrocytes respond to various stimuli. Importantly, the methodologies for quantification of astrocytic Ca2+ events are also applicable to the analysis of fluorescent signals from recently developed neurotransmitter-specific genetic indicators that, when expressed in astrocytes, allow for the detection of astoglial receipt of glutamate [115], norepinephrine [116], GABA, and dopamine [117], [118]. The progression, characteristics, and advantages of the various analysis pipelines used for Ca2+- and/or neurotransmitter-linked fluorescent imaging in astrocytes has recently been reviewed, for a more complete discussion on these topics see [119], [90]. Briefly, astrocyte-specific computational imaging analysis toolkits have evolved in parallel with our increasing ability to visualize and detect localized Ca2+ dynamics in astrocytes, and the continued refinement of computer-aided region of interest (ROI) detection. One early open-source toolkit developed by the Khakh laboratory is GECIquant, an ImageJ-based pipeline for the analysis of 2D + time data that allows for the estimation of an individual astrocyte territory, and subsequent separation and quantification of somatic, wave-like, and microdomain-specific Ca2+ dynamics [120]. When combined with expression of GCaMP6f in astrocytes, the Khakh laboratory was able to use this quantification method to effectively demonstrate that while somatic Ca2+ responses were not present in astroglia of IP3R2 KO mice, these IP3R2 KO astrocytes still displayed microdomain-level Ca2+ oscillations both in slice and during a startle response in vivo, aiding in the development of a more complete understanding of the functional ramifications of somatic vs microdomain Ca2+ signaling in astroglia [120]. Another fundamental step forward was the development of “accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology” or AQuA by the Poskanzer laboratory [52]. This ROI-based methodology employs machine learning to bring a fine level of detail to the quantification, tracking, propagation and directionality of astrocytic microdomain-level Ca2+ events. In the initial description of AQuA, Wang et al. also mention the advantage of using an intersectional viral vector approach that employs a “dynamic” Ca2+ or neurotransmitter genetic indicator used in combination with a “static” cytosolic label (tdTomato is suggested as most Ca2+ and neurotransmitter indicators utilize GFP). This experimental strategy allows for a more direct assessment of individual astrocyte territories and the repeated tracking of Ca2+ activity in the same astroglia over time. For an example of a co-expressed chemical indicator and static cytosolic label in astrocytes see (Fig. 2). A more recent software package developed to assess intercellular activity within astrocytic networks was developed by Dzyubenko et al (2021), deemed Astral, and is geared at examining astrocyte-astrocyte communication at the population level [51]. The Astral software package employs pipelines for data processing and signal extraction in 3-D + time data sets and groups adjacent pixels together with their change in intensity to evaluate the existence of a calcium waves, evaluated in light of background noise, based on their standard deviation. This quantification strategy is a powerful tool geared towards examining activity patterns in the astroglial syncytia in live-imaging preparations [51], and if coupled with in vivo 2-photon calcium imaging, could aid in elucidating how astrocytic networks adapt during various behaviors. Through the continual advances in these toolkits and the use of intersectional viral vector strategies to define individual astrocytic territories, microdomain Ca2+ and transmitter events are now being quantified within individual cells over time, with these toolkits also allowing the quantification of how these signals are translated across the astroglial network. These advances have fundamentally improved the ability to evaluate how astroglial signaling events are refined over time and will aid in establishing their functional relevance.
Fig. 2.
Comparison of iGluSnFR vs cytosolic astroglial expression of tdTomato. A) Low magnification confocal images of the fluorescent glutamate neurotransmitter indicator iGluSnFR via AAV2/5-GFAP-iGluSnFR (green) vs cytoplasmic expression of tdTomato via AAV2/5-GFAP-tdTomato (red) in a field of cortical astroglia. B) High magnification images of a several astrocytes with co-expression of iGluSnFR (green) and cytosolic tdTomato. Note the differences in the overall appearance of these cellular compartments, which resembles Fig. 1 above. Scale bars depict 200 μm in A) and 30 μm in B). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
1.4. How do astrocytes respond to and influence neuronal circuit activity?
Astrocytic response to and influence on synaptic activity provides regulation of the neuronal circuit dynamics related to memory, cognition, and behavior. As an example, chemogenetic disruption of calcium dynamics in hippocampal astrocytes can lead to reduced output-selective neuronal activity patterns, reduced downstream neuronal recruitment, and impaired memory recall [28]. Additionally, astrocytes differentiate and decode information from afferent neuronal projections in a Ca2+-dependent manner, and subsequently release gliotransmitters to influence local synaptic activity [121]. Yet, precisely how astrocytes interrogate incoming information, influence local neurotransmission, and gate outgoing projections in a synapse-specific manner remains to be completely understood. Further, it also remains to be determined if and how these cells influence formation of neuronal ensembles that encode information related to salient environmental stimuli and/or behavioral output. Fortunately, this can now be addressed as astrocytic and neuronal dynamics can be simultaneously visualized and manipulated using cutting-edge techniques in awake, behaving animals.
1.4.1. In vivo calcium imaging
The continued refinement of viral-mediated in vivo imaging and manipulation strategies now allows for the concomitant measurement and control of cell-type specific activity, an advance that is required for an improved understanding of how astrocytes shape local and circuit-level neuronal dynamics during behavior. Towards this end, it is necessary to employ strategies that allow visualization and longitudinal tracking of astrocytic activity at both the single cell and population level during complex behavioral tasks. Many laboratories routinely employ strategies such as head-mounted miniscope imaging [122], [123], [124] and head-fixed 2-photon imaging [125], [126], [127], [128], [129], [130] with neuronal GECIs to characterize Ca2+ activity patterns in different cellular compartments (soma or cell body, dendrite, axon) in awake behaving animals. While miniscopes are light-weight and allow for visualization of deep brain neuronal dynamics in freely moving animals [131], head-fixed 2-photon imaging provides higher resolution imaging that can be used to capture subcellular Ca2+ events [128], [132]. As there is a need to design experiments that incorporate astrocytes into systems neuroscience [133], 2-photon microscopy can be used to visualize and quantify diverse subcellular astrocytic Ca2+ dynamics and relate astrocytic Ca2+ events to neuronal activity patterns in vivo [49], [58]. While it is important to consider that the dimensionality of astrocytic Ca2+ events, including the spatial and temporal profile, may be more difficult to assess in vivo given resolution limitations and Ca2+ buffering in GECI-expressing astrocytes [134], this is a necessary trade off as repeated measurement of concomitant astrocytic and neuronal activity patterns will be required to understand how activity patterns in each cell type relate to learning, cognitive function, and complicated behaviors like reward seeking.
As in vivo two-photon microscopy with concurrent mouse behavioral assays becomes a more common means to measure and longitudinally track neuronal Ca2+ dynamics, methods for analyzing these data have also become more sophisticated [125], [126], [127], [128], [129], [135]. These methods include principal components analysis (PCA) and clustering algorithms, which are used to identify unique neuronal ensembles that differentially encode information related to environmental stimuli and behavioral output [125], [135]. For example, our laboratory has identified unique neuronal ensembles that emerge during training and stabilize after learning in a Pavlovian sucrose conditioning task, with each cluster encoding specialized information related to the sucrose reward, reward-predictive stimuli, and the behavioral response to the stimuli [135]. Importantly, these neuronal ensembles are not present early in learning, but emerge across training to encode learned information. This method could be extended to define astroglial ensembles that may act to shape neuronal activity and complex behaviors, such as learning and reward seeking. Specifically, by combining astrocytic GECIs with 2-photon imaging, it is possible to longitudinally track Ca2+ dynamics in individual astrocytes and time-lock astrocytic Ca2+ dynamics to behaviorally relevant events. Given the evidence supporting the role of astrocytic Ca2+ signaling and concordant gliotransmission in altered neuronal activity and state-switching in vivo [58], it is reasonable to hypothesize astrocytes are engaged during learning, influence neuronal activity (through excitation and/or inhibition), and coordinate ensemble formation. When combined with means to manipulate neuronal and astroglial activity, future experimentation will allow direct testing of the hypothesis that astroglia function as computational entities that encode discrete stimuli to shape neuronal activity.
The advent of non-GFP based genetic Ca2+ indicators now allow for concomitant expression of GECIs in astrocytes and neurons, which provides an experimental platform to simultaneously evaluate neuronal and astroglial Ca2+ dynamics in vivo. An example of this strategy would be to employ an astrocytic GCaMP alongside a neuronal RCaMP (red wavelength GECIs; [136]), which would allow for examination and tracking of activity dynamics in both cellular populations, simultaneously, in repeated behavioral sessions [49], [137]. It was through this strategy that Stobart et al. identified that Ca2+ transients in the astroglial leaflets occurred on a rapid timescale (∼120 ms) following neuronal activity [49], indicating astrocytic activity is indeed quick enough to respond to and modulate neurotransmission and linked behavioral responses. Moreover, similar strategies have been used to identify Ca2+ dynamics in astroglia that encode spatial information related to navigation through a virtual environment [137]. These astrocytic Ca2+ events were topographically organized in subcellular compartments, including the processes and soma, and complimented activity patterns of neighboring neurons during navigation [137]. The combination of multiphoton imaging during various behavioral paradigms in concert with a multiplexed GECI approach will enable researchers to longitudinally track activity patterns of both neurons and astrocytes with single-cell resolution, as they adapt throughout behavior, and relate to each other. In this way, astrocytes could be integrated into a variant of PCA with multidimensional modeling that includes both adaptations in activity of neurons and astrocytes. A multidimensional PCA coupled with clustering algorithms could aid in elucidating how activity in the astroglial syncytia relates to synchronization of neuronal activity and recruitment of nearby neurons into functional neuronal ensembles that orchestrate behavioral outputs.
1.4.2. Viral strategies for manipulation of cellular responses and concomitant in vivo imaging
By combining in vivo Ca2+ imaging in astrocytes with viral constructs that allow for the manipulation of neurons, experimentation aimed at understanding how astrocytes respond to incoming neurotransmitter release or local neuronal activity have become possible in awake, behaving animals. These data will allow for a better mechanistic understanding of precisely how Ca2+ signals are evoked in astroglia and if they are generated predominantly via afferent activity (Fig. 3A), are generated in response to local neuron activity (Fig. 3B), or through some mixture of both (Fig. 3C). Additionally, the inverse experimental paradigm is also possible, utilizing in vivo 2-photon Ca2+ imaging in neurons combined with viral constructs that allow for the manipulation of astrocytic activity. Such tools include those that direct activation of astroglia including optogenetics [27], [58], [78], chemogenetics [28], [81], [82], [83], [138], as well as those used to decrease astroglial activity including CalEx [18], [139], iβark [138], as well as manipulation of intracellular IP3 signaling pathways (for an in-depth review of current tools to manipulate astrocytic Ca2+ see [75]). Furthermore, genetic strategies for selective knockdown of genes controlling astrocytic-neuronal interaction or astroglial structural plasticity, such as with the CRISPR/Cas9 system [140], [141], can be employed with 2-photon microscopy to examine the mechanisms by which astroglia respond to and direct synaptic transmission in vivo. These types of experiments will allow for a better understanding of how astrocytic activity shapes neuronal responses in awake, behaving animals.
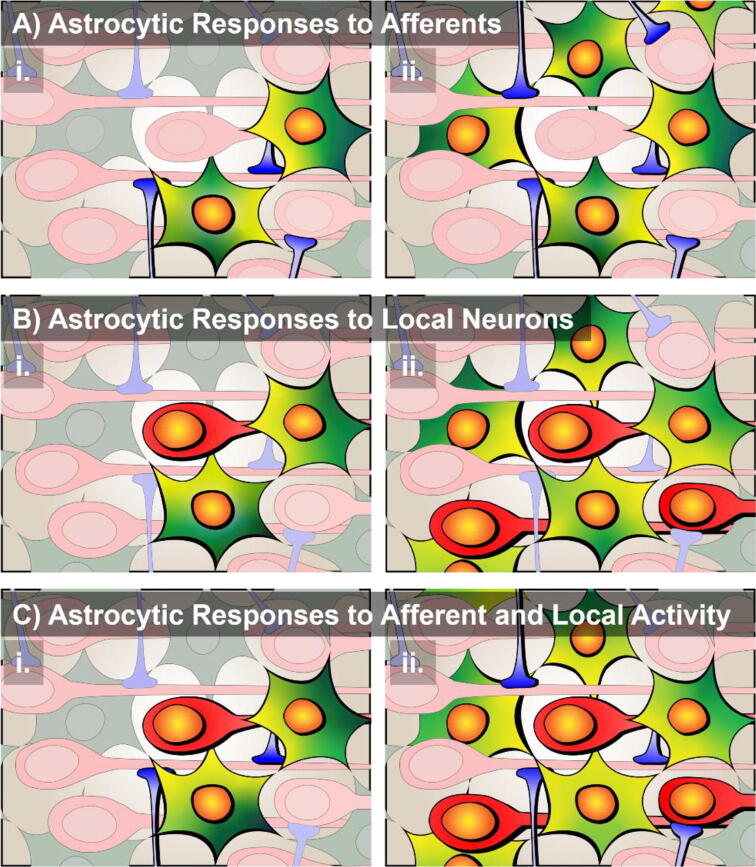
Fig. 3.
Astrocytes coordinate neuronal network activity. A) Astrocytes respond to afferent projections (blue) with microdomain-level calcium events (yellow) early in a behavioral task. Following training, as more afferents are active astrocytes can differentiate between inputs and decode information in a Ca2+-dependent manner. B) Peripheral astroglial processes engage because of nearby activated neurons (red) elicit microdomain Ca2+ events (yellow) that spread to neighboring astrocytes early in learning. Late in learning, astrocyte activity is refined and synchronized Ca2+ signaling coordinates local ensemble dynamics. C) Following training, astrocytes can integrate afferent information to influence local neuronal activity and formation of the neuronal ensemble dynamics that encode behaviorally relevant stimuli. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Viral strategies often used in neuronal circuits, such as anterograde tracing, now enable investigation of input-specific astrocytic populations. The unique serotype-specific properties of adenoviral vectors enable transduction of neurons by virtue of their projection targets or their inputs. As an example, AAV1-mediated anterograde transsynaptic activity can be used to interrogate input-specific populations of neurons [71], [142], [143], [144], [145]. Recently, the Kuhn laboratory established AAV1-mediated anterograde vectors also allow for the axo-astrocytic delivery of Cre at the tripartite synapse [146], making it possible to genetically tag astrocytes at anatomically-defined synapses and evaluate if these astrocytes uniquely respond to and modulate circuit activity. When combined with Cre-dependent astrocytic expression of GECIs, this strategy can be used to probe the spatiotemporal activity patterns of astrocytes in a synapse-specific manner [146].
These studies have already uncovered ultrafast astrocytic microdomain Ca2+ dynamics with synapse-specific resolution as it pertains to locomotion and vibrissa stimulation [146], reinforcing the idea that astrocytes uniquely respond to circuit-specific neuronal activity. Given that astrocytes display regional heterogeneity, with specialized gene expression profiles, Ca2+ responses, and morphometric profiles observed across and even within various brain regions [7], [147], [148], experiments can now directly characterize and manipulate astrocytic Ca2+ dynamics at various input-defined synapses using an AAV1-based experimental design. Axo-astrocytic viral transfer is an innovative strategy that can be used to create activity maps of astrocytes within distinct neuronal circuits during specific behavioral tasks and will prove crucial for evaluating how astrocytes gate circuit-level communication. The data depicted in Fig. 4 provides a glimpse into the remarkable segregation of subsets of astroglia, within a single brain region, dependent on the synapses that they are present at, hinting at potential functional subdomains within the astroglial syncytia. This approach can be coupled with CRE-dependent constructs to manipulate astrocytic activity or CRE-dependent genome editing tools to investigate the function of input-defined subgroupings of astrocytes and the mechanisms by which bidirectional chemical communication occurs between astrocytes and neurons.
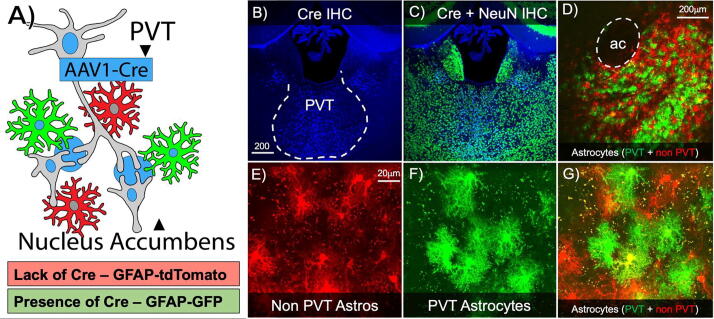
Fig. 4.
Axonal-Astrocyte anterograde transfer of CRE particles can be used to label astrocytes at distinct synapses. A) Schematic of surgical strategy: AAV1-CMV-CRE was microinjected into the paraventricular thalamus (PVT) and AAV5-GFAP-dlox-tdTomato-EGFP(rev)-dlox was microinjected into the nucleus accumbens (NAc). In the presence of CRE astrocytes will express GFP, in the absence they will express tdTomato. A representative 10x Z-stack of the PVT CRE expression (B; pseudocolored blue) in neurons (C; CRE depicted as blue, NeuN shown in green). D) A representative 10x Z-stack of the NAc illustrating anatomical segregation of astrocytes at PVT and non-PVT synapses in the core and the shell. In the absence of PVT input and anterograde CRE astrocytes express tdTomato (E), and when astrocytes are present at PVT synapses and CRE is delivered, they express GFP (F). G) A representative merged 63x Z-stack of NAc astrocytes demonstrating grouping of astrocytes at anatomically-defined synapses. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Through the combination of these new tools, the field is now poised to more precisely evaluate the hypothesis that subsets of astrocytes direct plasticity at key synapses, and that astrocytes themselves are functional components of neural circuits that integrate incoming information, participate in neuronal ensemble formation, and coordinate neuronal network activity to drive behavioral responses. For example, stimulation of neuronal terminals using red-shifted optogenetic constructs [149], [150] coupled with in vivo astrocytic Ca2+ imaging can be used to test how astrocytes respond to afferent information with subcellular and population-level resolution. This experimental design could also accomodate specific promoter- or CRE-driven constructs to target distinct neuronal populations and/or astrocytes at input-defined synapses. Moreover, single-cell optogenetic approaches exist for use in neurons, with stimulation of just two neurons sufficient to initiate ensemble dynamics and resulting licking behavior in mice [151]. As it has been demonstrated that stimulation of a single astrocyte is sufficient to generate an astrocytic calcium wave ex vivo and dictate neuronal circuit activity [46], future lines of research can employ single-cell optogenetic strategies to target individual astrocytes and quantify resulting activity patterns in the astroglial syncytia and local neuronal populations. These approaches can be coupled with genome editing tools, such as the CRISPR/Cas9 system, in astrocytes to selectively target receptors or gliotransmitter production/release pathways and determine the mechanism by which astrocytes integrate information from afferent neuronal projections and influence local neuronal activity, including ensemble formation, in behaving animals. Using the innovative approaches described above, researchers will be able to functionally tie afferent driven astrocytic Ca2+ signaling to recruitment of adjacent astrocytic nodes and local neuronal ensemble formation in awake, behaving animals.
2. Summary and outlook
With the continued refinement of neuroscience tools and analysis pipelines, we will soon be able to probe how astrocytes influences circuit communication in vivo. Despite the challenges that still exist while measuring astrocytic activity in awake behaving animals [134], investigation of astroglial Ca2+ signaling in an intact system during complex behavioral tasks is critical to assess how mammalian astrocytes encode environmental stimuli, relay the information to neighboring astroglia and neurons, and modulate neuronal communication from the synapse to the circuit. In summary, examining astroglial Ca2+ events in the context of their morphology have revealed complex signaling dynamics that coordinate neuronal activity patterns and orchestrate a range of behavioral outputs. As the field continues to develop, researchers will be able to place astrocytes as computational entities within neural networks and better understand their functional role in various behavioral responses.
Funding
This work was funded by R01-DA054154 (MDS), R01-DA051650 and R01-DA054271 (JMO), T32-DA007288 (JEP), the MUSC College of Medicine Enhancement of Team Science (COMETS) program (MDS and JMO), and NIDA Center on Cocaine and Opioid Addiction, P50-DA046373 (MDS).
CRediT authorship contribution statement
Jacqueline E. Paniccia: Conceptualization, Writing - original draft, Writing - review & editing. James M. Otis: Conceptualization, Writing - original draft, Writing - review & editing. Michael D. Scofield: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Allen N.J., Barres B.A. Neuroscience: Glia - more than just brain glue. Nature. 2009;457(7230):675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 2.Baxter P. Astrocytes: more than just glue. Dev Med Child Neurol. 2012;54(4):291. doi: 10.1111/j.1469-8749.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 3.Taber K.H., Hurley R.A. Astroglia: not just glue. J Neuropsychiatry Clin Neurosci. 2008;20(2):iv–129. doi: 10.1176/jnp.2008.20.2.iv. [DOI] [PubMed] [Google Scholar]
- 4.Agulhon C., et al. What Is the Role of Astrocyte Calcium in Neurophysiology? Neuron. 2008;59(6):932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada K., Kamiya T., Tsuboi T. Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front Neurosci. 2015;9:499. doi: 10.3389/fnins.2015.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arizono M., et al. Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat Commun. 2020;11(1):1906. doi: 10.1038/s41467-020-15648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai H., et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017;95(3):531–549.e9. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter J.T., McCarthy K.D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16(16):5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter J.T., McCarthy K.D. Adenosine receptors modulate [Ca2+]i in hippocampal astrocytes in situ. J Neurochem. 1995;65(4):1515–1523. doi: 10.1046/j.1471-4159.1995.65041515.x. [DOI] [PubMed] [Google Scholar]
- 10.Bellot-Saez A., et al. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci Biobehav Rev. 2017;77:87–97. doi: 10.1016/j.neubiorev.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Chung W.S., Allen N.J., Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015;7(9) doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert G., Schilling K., Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 13.Porter J.T., McCarthy K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51(4):439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 14.Agulhon C., et al. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front Pharmacol. 2012;3:139. doi: 10.3389/fphar.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea G., Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25(9):2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy-Royal C., et al. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci. 2015;18(2):219–226. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- 17.Boddum K., et al. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat Commun. 2016;7(1):13572. doi: 10.1038/ncomms13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X., et al. Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron. 2018;99(6):1170–1187.e9. doi: 10.1016/j.neuron.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018;98(1):239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira J.F., Araque A. Astrocyte regulation of neural circuit activity and network states. Glia. 2022;70(8):1455–1466. doi: 10.1002/glia.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hösli L., et al. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Reports. 2022;39(1) doi: 10.1016/j.celrep.2022.110599. [DOI] [PubMed] [Google Scholar]
- 22.Kruyer A., Kalivas P.W., Scofield M.D. Astrocyte regulation of synaptic signaling in psychiatric disorders. Neuropsychopharmacology. 2022 doi: 10.1038/s41386-022-01338-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armbruster M., et al. Neuronal activity drives pathway-specific depolarization of peripheral astrocyte processes. Nat Neurosci. 2022;25(5):607–616. doi: 10.1038/s41593-022-01049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volterra A., Liaudet N., Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci. 2014;15(5):327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 25.Khakh B.S., McCarthy K.D. Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol. 2015;7(4) doi: 10.1101/cshperspect.a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araque A., et al. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamsky A., et al. Astrocytic Activation Generates <em>De Novo</em> Neuronal Potentiation and Memory Enhancement. Cell. 2018 doi: 10.1016/j.cell.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Kol A., et al. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat Neurosci. 2020 doi: 10.1038/s41593-020-0679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scofield M.D., Kalivas P.W. Astrocytic Dysfunction and Addiction: Consequences of Impaired Glutamate Homeostasis. Neurosci: Rev J. Bring Neurobiol Neurol Psychiatry. 2014;20(6):610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scofield M.D., et al. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry. 2016;80(3):207–215. doi: 10.1016/j.biopsych.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Testen A., et al. Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front Cell Neurosci. 2018;12:246. doi: 10.3389/fncel.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healey K.L., et al. Enduring alterations in hippocampal astrocytesynaptic proximity following adolescent alcohol exposure: reversal by gabapentin. Neural Regen Res. 2020;15(8):1496–1501. doi: 10.4103/1673-5374.274339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruyer A., et al. Heroin Cue-Evoked Astrocytic Structural Plasticity at Nucleus Accumbens Synapses Inhibits Heroin Seeking. Biol Psychiatry. 2019;86(11):811–819. doi: 10.1016/j.biopsych.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H.G., Wheeler M.A., Quintana F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. 2022;21(5):339–358. doi: 10.1038/s41573-022-00390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., et al. Astrocytes in Neuropsychiatric Disorders: A Review of Postmortem Evidence. Adv Neurobiol. 2021;26:153–172. doi: 10.1007/978-3-030-77375-5_8. [DOI] [PubMed] [Google Scholar]
- 36.Maly I.V., Morales M.J., Pletnikov M.V. Astrocyte Bioenergetics and Major Psychiatric Disorders. Adv Neurobiol. 2021;26:173–227. doi: 10.1007/978-3-030-77375-5_9. [DOI] [PubMed] [Google Scholar]
- 37.Lyu S., et al. Downregulation of astroglial glutamate transporter GLT-1 in the lateral habenula is associated with depressive-like behaviors in a rat model of Parkinson's disease. Neuropharmacology. 2021;196 doi: 10.1016/j.neuropharm.2021.108691. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X., et al. Astrocyte, a Promising Target for Mood Disorder Interventions. Front Mol Neurosci. 2019;12:136. doi: 10.3389/fnmol.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariani J.N., Zou L., Goldman S.A. Human Glial Chimeric Mice to Define the Role of Glial Pathology in Human Disease. Methods Mol Biol. 2019;1936:311–331. doi: 10.1007/978-1-4939-9072-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S., et al. Activation of Astrocytes in the Dorsomedial Striatum Facilitates Transition From Habitual to Goal-Directed Reward-Seeking Behavior. Biol Psychiatry. 2020;88(10):797–808. doi: 10.1016/j.biopsych.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skupio U., et al. Astrocytes determine conditioned response to morphine via glucocorticoid receptor-dependent regulation of lactate release. Neuropsychopharmacology. 2020;45(2):404–415. doi: 10.1038/s41386-019-0450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruyer A., Scofield M.D. Astrocytes in Addictive Disorders. Adv Neurobiol. 2021;26:231–254. doi: 10.1007/978-3-030-77375-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siemsen B.M., et al. Effects of Methamphetamine Self-Administration and Extinction on Astrocyte Structure and Function in the Nucleus Accumbens Core. Neuroscience. 2019;406:528–541. doi: 10.1016/j.neuroscience.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schipke C.G., Heuser I., Peters O. Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J Psychiatr Res. 2011;45(2):242–248. doi: 10.1016/j.jpsychires.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Sanacora G., Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73(12):1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poskanzer K.E., Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci. 2011;108(45):18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parpura V., Haydon P.G. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. PNAS. 2000;97(15):8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shigetomi E., et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141(5):633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stobart J.L., et al. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron. 2018;98(4):726–735.e4. doi: 10.1016/j.neuron.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 50.Shigetomi E., Patel S., Khakh B.S. Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol. 2016;26(4):300–312. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dzyubenko E., et al. Analysing Intercellular Communication in Astrocytic Networks Using “Astral”. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.689268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., et al. Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat Neurosci. 2019;22(11):1936–1944. doi: 10.1038/s41593-019-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lia A., et al. Calcium Signals in Astrocyte Microdomains, a Decade of Great Advances. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.673433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuga N., et al. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31(7):2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sáez J.C., et al. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand. 2003;179(1):9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazargani N., Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19(2):182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Alvarez A., et al. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci: Off J Soc Neurosci. 2014;34(38):12738–12744. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poskanzer, K.E. and R. Yuste, Astrocytes regulate cortical state switching in vivo. Proc Nat Acad Sci, 2016. 113(19): p. E2675-E2684. [DOI] [PMC free article] [PubMed]
- 59.Oberheim N.A., et al. Uniquely hominid features of adult human astrocytes. J Neurosci: Off J Soc Neurosci. 2009;29(10):3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han X., et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walz W., Lang M.K. Immunocytochemical evidence for a distinct GFAP-negative subpopulation of astrocytes in the adult rat hippocampus. Neurosci Lett. 1998;257(3):127–130. doi: 10.1016/s0304-3940(98)00813-1. [DOI] [PubMed] [Google Scholar]
- 62.Tatsumi K., et al. Olig2-Lineage Astrocytes: A Distinct Subtype of Astrocytes That Differs from GFAP Astrocytes. Front Neuroanat. 2018;12 doi: 10.3389/fnana.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Escartin C., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24(3):312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bushong E.A., et al. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng K., et al. Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca2+ in Neurons and Astroglia. Neuron. 2015;88(2):277–288. doi: 10.1016/j.neuron.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benediktsson A.M., et al. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141(1):41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 67.Panatier A., Arizono M., Nägerl U.V. Dissecting tripartite synapses with STED microscopy. Philos Trans R Soc B: Biol Sci. 2014;369(1654):20130597. doi: 10.1098/rstb.2013.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arizono M., Nägerl U.V. Deciphering the functional nano-anatomy of the tripartite synapse using stimulated emission depletion microscopy. Glia. 2022;70(4):607–618. doi: 10.1002/glia.24103. [DOI] [PubMed] [Google Scholar]
- 69.Lehre K.P., Rusakov D.A. Asymmetry of Glia near Central Synapses Favors Presynaptically Directed Glutamate Escape. Biophys J. 2002;83(1):125–134. doi: 10.1016/S0006-3495(02)75154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henneberger C., et al. LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron. 2020;108(5):919–936.e11. doi: 10.1016/j.neuron.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siemsen B.M., et al. A Subset of Nucleus Accumbens Neurons Receiving Dense and Functional Prelimbic Cortical Input Are Required for Cocaine Seeking. Front Cell Neurosci. 2022;16 doi: 10.3389/fncel.2022.844243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reissner K.J., et al. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20(2):316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zlatkine P., Mehul B., Magee A.I. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110(Pt 5):673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]
- 74.Heffernan K.S., et al. Characterization of the GfaABC1D promoter to selectively target astrocytes in the rhesus macaque brain. J Neurosci Methods. 2022;372 doi: 10.1016/j.jneumeth.2022.109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu X., Nagai J., Khakh B.S. Improved tools to study astrocytes. Nat Rev Neurosci. 2020 doi: 10.1038/s41583-020-0264-8. [DOI] [PubMed] [Google Scholar]
- 76.Koh W., et al. AAV-Mediated Astrocyte-Specific Gene Expression under Human ALDH1L1 Promoter in Mouse Thalamus. Exp Neurobiol. 2017;26(6):350–361. doi: 10.5607/en.2017.26.6.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borodinova A.A., et al. Genetic Constructs for the Control of Astrocytes'. Activity Cells. 2021;10(7) doi: 10.3390/cells10071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mederos S., et al. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia. 2019;67(5):915–934. doi: 10.1002/glia.23580. [DOI] [PubMed] [Google Scholar]
- 79.Perea G., et al. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun. 2014;5:3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyon K.A., Allen N.J. From Synapses to Circuits, Astrocytes Regulate Behavior. Front Neural Circuits. 2022;15 doi: 10.3389/fncir.2021.786293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paniccia J.E., et al. Dorsal hippocampal neural immune signaling regulates heroin-conditioned immunomodulation but not heroin-conditioned place preference. Brain Behav Immun. 2018 doi: 10.1016/j.bbi.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones M.E., et al. Chemogenetic Manipulation of Dorsal Hippocampal Astrocytes Protects Against the Development of Stress-enhanced Fear Learning. Neuroscience. 2018;388:45–56. doi: 10.1016/j.neuroscience.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Scofield M.D., et al. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78(7):441–451. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nam M.H., et al. Signaling mechanisms of μ-opioid receptor (MOR) in the hippocampus: disinhibition versus astrocytic glutamate regulation. Cell Mol Life Sci. 2021;78(2):415–426. doi: 10.1007/s00018-020-03595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J.-H., et al. Chemogenetic stimulation of the G(i) pathway in astrocytes suppresses neuroinflammation. Pharmacol Res Perspect. 2021;9(6):e00822–e. doi: 10.1002/prp2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lind B.L., et al. Fast Ca(2+) responses in astrocyte end-feet and neurovascular coupling in mice. Glia. 2018;66(2):348–358. doi: 10.1002/glia.23246. [DOI] [PubMed] [Google Scholar]
- 87.Agarwal A., et al. Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron. 2017;93(3):587–605.e7. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haustein M.D., et al. Conditions and Constraints for Astrocyte Calcium Signaling in the Hippocampal Mossy Fiber Pathway. Neuron. 2014;82(2):413–429. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srinivasan R., et al. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron. 2016;92(6):1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Covelo A., Badoual A., Denizot A. Reinforcing Interdisciplinary Collaborations to Unravel the Astrocyte “Calcium Code”. J Mol Neurosci. 2022 doi: 10.1007/s12031-022-02006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Castro M.A., et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14(10):1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 92.Lind B.L., et al. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA. 2013;110(48):E4678–E4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panatier A., et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146(5):785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 94.Agulhon C., et al. Modulation of the autonomic nervous system and behaviour by acute glial cell G(q) protein-coupled receptor activation in vivo. J Physiol. 2013;591(Pt 22):5599–5609. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petravicz J., Boyt K.M., McCarthy K.D. Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci. 2014;8:384. doi: 10.3389/fnbeh.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reeves A.M.B., Shigetomi E., Khakh B.S. Bulk Loading of Calcium Indicator Dyes to Study Astrocyte Physiology: Key Limitations and Improvements Using Morphological Maps. J Neurosci. 2011;31(25):9353–9358. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agulhon C., Fiacco T.A., McCarthy K.D. Hippocampal Short- and Long-Term Plasticity Are Not Modulated by Astrocyte Ca<sup>2+</sup> Signaling. Science. 2010;327(5970):1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- 98.Fiacco T.A., McCarthy K.D. Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neurosci. 2018;38(1):3–13. doi: 10.1523/JNEUROSCI.0016-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen T.-W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye L., et al. Comparison of GCaMP3 and GCaMP6f for studying astrocyte Ca2+ dynamics in the awake mouse brain. PLoS One. 2017;12(7):e0181113. doi: 10.1371/journal.pone.0181113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sherwood M.W., et al. Astrocytic IP(3) Rs: Contribution to Ca(2+) signalling and hippocampal LTP. Glia. 2017;65(3):502–513. doi: 10.1002/glia.23107. [DOI] [PubMed] [Google Scholar]
- 102.Shigetomi E., et al. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15(1):70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Letellier M., et al. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc Natl Acad Sci USA. 2016;113(19):E2685–E2694. doi: 10.1073/pnas.1523717113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rose C.R., Ziemens D., Verkhratsky A. On the special role of NCX in astrocytes: Translating Na(+)-transients into intracellular Ca(2+) signals. Cell Calcium. 2020;86 doi: 10.1016/j.ceca.2019.102154. [DOI] [PubMed] [Google Scholar]
- 105.Shiratori-Hayashi M., et al. Astrocytic STAT3 activation and chronic itch require IP3R1/TRPC-dependent Ca(2+) signals in mice. J Allergy Clin Immunol. 2021;147(4):1341–1353. doi: 10.1016/j.jaci.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 106.Sherwood M.W., et al. Astrocytic IP3Rs: Beyond IP3R2. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.695817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oheim M., Schmidt E., Hirrlinger J. Local energy on demand: Are 'spontaneous' astrocytic Ca(2+)-microdomains the regulatory unit for astrocyte-neuron metabolic cooperation? Brain Res Bull. 2018;136:54–64. doi: 10.1016/j.brainresbull.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 108.Semyanov A. Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium. 2019;78:15–25. doi: 10.1016/j.ceca.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Ahmadpour N., Kantroo M., Stobart J.L. Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules. 2021;11(10) doi: 10.3390/biom11101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doengi M., et al. GABA uptake-dependent Ca<sup>2+</sup> signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci. 2009;106(41):17570–17575. doi: 10.1073/pnas.0809513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kruyer A., et al. Astrocytes in the ventral pallidum extinguish heroin seeking through GAT-3 upregulation and morphological plasticity at D1-MSN terminals. Mol Psychiatry. 2022;27(2):855–864. doi: 10.1038/s41380-021-01333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ibáñez I., et al. Activity dependent internalization of the glutamate transporter GLT-1 requires calcium entry through the NCX sodium/calcium exchanger. Neurochem Int. 2019;123:125–132. doi: 10.1016/j.neuint.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 113.Bennett M.V., et al. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26(11):610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Savtchouk I., Volterra A. Gliotransmission: Beyond Black-and-White. J Neurosci. 2018;38(1):14–25. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armbruster M., Dulla C.G., Diamond J.S. Effects of fluorescent glutamate indicators on neurotransmitter diffusion and uptake. eLife. 2020;9 doi: 10.7554/eLife.54441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng J., et al. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron. 2019;102(4):745–761 e8. doi: 10.1016/j.neuron.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patriarchi T., et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018;360(6396) doi: 10.1126/science.aat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marvin J.S., et al. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat Methods. 2019;16(8):763–770. doi: 10.1038/s41592-019-0471-2. [DOI] [PubMed] [Google Scholar]
- 119.Gorzo K.A., Gordon G.R. Photonics tools begin to clarify astrocyte calcium transients. Neurophotonics. 2022;9(2) doi: 10.1117/1.NPh.9.2.021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srinivasan R., et al. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18(5):708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santello M., Toni N., Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22(2):154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 122.Cai D.J., et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534(7605):115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghosh K.K., et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8(10):871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zong W., et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods. 2017;14(7):713–719. doi: 10.1038/nmeth.4305. [DOI] [PubMed] [Google Scholar]
- 125.Namboodiri V.M.K., et al. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat Neurosci. 2019;22(7):1110–1121. doi: 10.1038/s41593-019-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McHenry J.A., et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20(3):449–458. doi: 10.1038/nn.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Otis J.M., et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543(7643):103–107. doi: 10.1038/nature21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Otis J.M., et al. Paraventricular Thalamus Projection Neurons Integrate Cortical and Hypothalamic Signals for Cue-Reward Processing. Neuron. 2019;103(3):423–431.e4. doi: 10.1016/j.neuron.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rossi M.A., et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science. 2019;364(6447):1271–1274. doi: 10.1126/science.aax1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang W., et al. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife. 2018;7:e32671. doi: 10.7554/eLife.32671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Silva A.J. Miniaturized two-photon microscope: seeing clearer and deeper into the brain. Light Sci Appl. 2017;6(8):e17104–e. doi: 10.1038/lsa.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Broussard G.J., et al. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat Neurosci. 2018;21(9):1272–1280. doi: 10.1038/s41593-018-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kastanenka K.V., et al. A roadmap to integrate astrocytes into Systems Neuroscience. Glia. 2020;68(1):5–26. doi: 10.1002/glia.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Semyanov A., Henneberger C., Agarwal A. Making sense of astrocytic calcium signals — from acquisition to interpretation. Nat Rev Neurosci. 2020;21(10):551–564. doi: 10.1038/s41583-020-0361-8. [DOI] [PubMed] [Google Scholar]
- 135.Grant R.I., et al. Specialized coding patterns among dorsomedial prefrontal neuronal ensembles predict conditioned reward seeking. eLife. 2021;10:e65764. doi: 10.7554/eLife.65764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dana H., et al. Sensitive red protein calcium indicators for imaging neural activity. Elife. 2016;5 doi: 10.7554/eLife.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Curreli S., et al. Complementary encoding of spatial information in hippocampal astrocytes. PLoS Biol. 2022;20(3):e3001530. doi: 10.1371/journal.pbio.3001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nagai J., et al. Specific and behaviorally consequential astrocyte G(q) GPCR signaling attenuation in vivo with iβARK. Neuron. 2021;109(14):2256–2274.e9. doi: 10.1016/j.neuron.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu X., Moye S.L., Khakh B.S. Local and CNS-Wide Astrocyte Intracellular Calcium Signaling Attenuation In Vivo with CalEx(flox) Mice. J Neurosci. 2021;41(21):4556–4574. doi: 10.1523/JNEUROSCI.0085-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takano T., et al. Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature. 2020;588(7837):296–302. doi: 10.1038/s41586-020-2926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meneghini V., et al. Delivery Platforms for CRISPR/Cas9 Genome Editing of Glial Cells in the Central Nervous System. Front Genome Editing. 2021;3 doi: 10.3389/fgeed.2021.644319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kitanishi T., et al. Intersectional, anterograde transsynaptic targeting of neurons receiving monosynaptic inputs from two upstream regions. Commun Biol. 2022;5(1):149. doi: 10.1038/s42003-022-03096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zingg B., et al. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron. 2017;93(1):33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zingg B., et al. Application of AAV1 for Anterograde Transsynaptic Circuit Mapping and Input-Dependent Neuronal Cataloging. Curr Protoc. 2022;2(1):e339. doi: 10.1002/cpz1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zingg B., et al. Synaptic Specificity and Application of Anterograde Transsynaptic AAV for Probing Neural Circuitry. J Neurosci. 2020;40(16):3250–3267. doi: 10.1523/JNEUROSCI.2158-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Georgiou L., et al. Ca(+) activity maps of astrocytes tagged by axoastrocytic AAV transfer. Sci Adv. 2022;8(6) doi: 10.1126/sciadv.abe5371. p. eabe5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khakh B.S., Sofroniew M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18(7):942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khakh B.S., Deneen B. The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci. 2019;42(1):187–207. doi: 10.1146/annurev-neuro-070918-050443. [DOI] [PubMed] [Google Scholar]
- 149.Zhang F., et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11(6):631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mager T., et al. High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics. Nat Commun. 2018;9(1):1750. doi: 10.1038/s41467-018-04146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carrillo-Reid L., et al. Controlling Visually Guided Behavior by Holographic Recalling of Cortical Ensembles. Cell. 2019;178(2):447–457.e5. doi: 10.1016/j.cell.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]