Abstract
Objective: Psoriasis is an immune mediated disorder associated with T cell activation and cardiovascular disease (CVD). We explored the association of inflammation with left ventricular (LV) remodelling in psoriasis patients receiving treatment with the tumour necrosis factor-α (TNF-α) blocker infliximab. Methods: Psoriasis patients (n = 47, age 47 ± 14 years, 66% men) and 99 control subjects without psoriasis (age 47 ± 11 years, 72% men) were examined by echocardiography in a cross-sectional study. LV remodelling was assessed by LV mass index for height in the allometric power of 2.7. Serum concentrations of C-reactive protein (CRP), serum amyloid A (SAA), neopterin, kynurenine:tryptophan ratio (KTR) and the pyridoxic acid ratio (PAr) index were measured. Results: Serum concentration of neopterin (p = .007) was higher in psoriasis patients, while the other inflammatory biomarkers had similar levels. LV mass index was lower in patients than controls (35.6 ± 9.6 g/m2.7 vs. 40.3 ± 9.8 g/m2.7, p = .008). In the total study population, serum SAA (β = 0.18, p = .02), KTR (β = 0.20, p = .02) and the PAr index (β = 0.26, p = .002) were all associated with higher LV mass index independent of age, sex, body mass index, hypertension, smoking, renal function and psoriasis. Also in psoriasis patients, higher SAA level (β = 0.34, p = .02), KTR (β = 0.32, p = .02) and the PAr index (β = 0.29, p = .05) were associated with higher LV mass index independent of body mass index, hypertension and diabetes. Conclusion: Higher levels of the inflammatory biomarkers SAA, KTR and the PAr index were associated with greater LV mass index in psoriasis patients, indicating a role of chronic inflammation in LV remodelling evident even during treatment with TNF-α blockers.
Keywords: psoriasis, infliximab, echocardiography, left ventricular mass, biomarker, inflammation
Introduction
The activation of T cells is central in the pathogenesis if the immune mediated skin disorder psoriasis.1,2 Complex pathways of pro-inflammatory cytokines released from T cells stimulate keratinocyte proliferation, increase angiogenic mediators and promote immune cell infiltration in the skin.1,3 The predominant cytokine pathways are thought to be driven by interleukins-17,-22,-23, but also by tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).1,3 The degree of T cell activation, reflecting disease severity, could be assessed by circulating biomarkers. IFN-γ released from activated T cells is also a potent activator of the enzyme indoleamine 2,3-dioxygenase 1 (IDO). 4 Indoleamine 2,3-dioxygenase 1 regulates the first and rate-limiting step of the degradation of the essential amino acid tryptophan into metabolites collectively called kynurenines. INF-γ produced by activated T cells also stimulates the macrophages to produce neopterin, and together, neopterin and kynurenines serves as biomarkers of T cell mediated inflammation, which is of particular interest in psoriasis.4,5
The risk of atherosclerotic cardiovascular disease (CVD) is increased in psoriasis and related to disease severity. 6 Disease modifying treatment with TNF-α inhibitors is frequently used in the treatment of moderate to severe psoriasis, 7 and has been associated with reduction in atherosclerotic CVD in these patients.8,9 Furthermore, growing evidence suggests that inflammation is associated with left ventricular (LV) remodelling10–12 and LV dysfunction.13,14 Presence of structural LV remodelling is a strong predictor of future CVD, independent of co-presence of other CVD risk factors.15–18 In particular, increased LV mass impacts all-cause mortality as well as major cardiovascular events including coronary artery disease, cardiac arrhythmias and heart failure.15–18 In psoriasis, we recently demonstrated that higher disease severity at initiation of systemic treatment with infliximab was associated with persistent LV remodelling after 4.9 years of treatment. 19 In this substudy, we hypothesized that levels of circulating biomarkers of inflammation, and in particular, markers of T cell mediated inflammation, are associated with LV remodelling in psoriasis patients even after treatment with TNF-α inhibitors. Thus, the study purpose was to explore the association of inflammatory biomarkers with LV remodelling in psoriasis patients receiving chronic treatment with TNF-α inhibitors, and in controls subjects without psoriasis.
Methods
Patient population
In 2017, all patients with psoriasis receiving systemic treatment with the TNF-α inhibitor infliximab at the Department of Dermatology, Haukeland University Hospital, Bergen, Norway were asked to participate in the present cross-sectional study. 19 Exclusion criteria were previous myocardial infarction, cardiac surgery or heart failure, severe psychiatric disorders or low expected compliance. In total, 55 patients were screened, and 53 patients were included in the study. For the present analysis, five patients were excluded because biobank samples were not available, and one patient was excluded because of a clinical infection at the time when the biobank samples were drawn, leaving 47 patients for the present analyses.
Controls without psoriasis were included from the FAT associated CardiOvasculaR dysfunction (FATCOR) cohort. Details on the FATCOR study have been published previously.20,21 In short, the study was conducted at the Department of Heart Disease, Haukeland University Hospital between 2009 and 2017 (ClinicalTrials.gov Identifier: NCT02805478) and included 618 study subjects between 30 and 65 years of age with a body mass index >27.0 kg/m2 and free from clinical CVD. Control subjects were matched with psoriasis patients in a 2:1 manner for age, sex and body mass index. In total, 106 controls were included, of whom seven were excluded from the present analysis because of missing echocardiographic data, leaving 99 control subjects. Written, informed consent was signed by all study participants in agreement with the Declaration of Helsinki, and the study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics division West (2016/2194).
Echocardiography
All echocardiographic examinations of patients and controls were performed at the Department of Heart Disease, Haukeland University Hospital following a standardized protocol on a GE Vivid E9 scanner. The examinations were analysed offline with Image Arena software version 4.1 (Tom Tec Imaging Systems GmbH, Unterschleissheim, Germany) at the Echocardiography Core Laboratory at the University of Bergen, Bergen, Norway. Analysis of LV chamber dimensions was done in accordance with the joint European Association of Echocardiography and American Society of Echocardiography guidelines. 22 Left ventricular mass was indexed for height2.7 and LV hypertrophy was defined as LV mass index exceeding 47 g/m2.7 in women and 50 g/m2.7 in men. 15 Relative wall thickness was determined as 2 × the posterior wall thickness/LV internal diameter at end-diastole. 22 LV remodelling was defined by LV mass index and relative wall thickness. Intra- and interobserver variability were assessed by repeated analysis of LV mass in 20 randomly selected patients.
Biobank analyses
Non-fasting blood samples were collected at the Biobank at the Department of Dermatology, Haukeland University hospital and stored at −80°C before analysis at the Bevital laboratory, Bergen, Norway (www.bevital.no). The following inflammatory biomarkers were measured: the acute phase reactants serum amyloid A (SAA) and C-reactive protein (CRP), the markers of T cell mediated inflammation neopterin and kynurenine-tryptophan ratio (KTR), as well as the novel marker of cellular inflammation, the pyridoxic acid ratio (PAr) index. Serum levels of neopterin, tryptophan and kynurenines, 4-pyridoxic acid, pyridoxal 5’-phosphate and pyridoxal were measured by liquid chromatography tandem mass spectrometry. 23 Kynurenine:tryptophan ratio was calculated as the ratio between kynurenine (in nmol) to the concentration of tryptophan (in µmol). The PAr index was calculated as the ratio of the vitamin B6 metabolites 4-pyridoxic acid divided by the sum of pyridoxal 5’-phosphate and pyridoxal. 24 CRP and SAA were analysed by Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight mass spectrometry (MALDI-TOF MS). 25
Cardiovascular risk factors and psoriasis severity
Self-reported information about CVD risk factors, actual medication and medical history was collected on a standardized questionnaire and quality ensured against the patients’ medical hospital records at the time of the echocardiographic examination. Office blood pressure was measured according to European guidelines using a Welch Allyn ProBPTM 2000 digital apparatus (Skaneateles Falls, NY, USA). 15 The 24-h ambulatory blood pressure measurement was done using a Diasys Integra II device (Novacor, Cedex, France) with appropriately sized cuff in the individual participant. 19 Hypertension was defined as history of hypertension, use of antihypertensive medications or elevated ambulatory blood pressure (≥130/80 mmHg). Diabetes was defined as known diabetes or use of antidiabetic treatment. Psoriasis severity was assessed by the treating dermatologist based on the Psoriasis Area Severity Index (PASI) and Dermatology Life Quality Index (DLQI).26,27
Statistical analyses
IBM SPSS statistical program version 26.0 (IBM, Armonk, New York, USA) was used for statistical analyses. Continuous data were reported as mean ± standard deviation (SD) or median (interquartile range) for normally and non-normally distributed data, respectively. Categorical variables were given as numbers (percentages). The comparisons between groups were analysed by the two-sample Student’s t-test or Chi-square test as appropriate. Non-normally distributed variables (CRP, SAA, neopterin, KTR, PAr) were log transformed before analysis. Uni- and multivariable associations were assessed by linear regression using an enter method and collinearity tools, and the results reported as multiple R2 for models and standardized beta coefficients for individual variables. Due to collinearity between inflammatory markers, the association between each marker and LV remodelling was tested in separate multivariable models, adjusted for significant variables of LV remodelling identified in univariable analysis and/or known clinical confounders. Reproducibility of the echocardiographic measurements was assessed by the intraclass correlation coefficient (ICC) with 95% confidence intervals (CI) of LV mass. A two-tailed p-value of < .05 was considered statistically significant in all analyses.
Results
Clinical characteristics
Psoriasis disease duration was on average 25 years ranging from 4 to 52 years. The duration of infliximab treatment was on average 5 ± 4 years, and 85% of psoriasis patients received additional treatment with methotrexate. At the time of the echocardiographic examination, patients had an average PASI score of 0.8 ± 0.8 and a DQLI score of 0.7 ± 1.4 indicating very well treated psoriasis. Except for more patients than controls being active smokers, the CVD risk factor profile was comparable between groups (Table 1). The neopterin concentration was higher in psoriasis patients (p = .007), while the other inflammatory biomarkers showed similar serum levels between patients and controls (Table 1).
Table 1.
Clinical and echocardiographic characteristics of psoriasis patients and controls.
| Psoriasis (n = 47) | Controls (n = 99) | p | |
|---|---|---|---|
| Age, years | 47.3 ± 14.3 | 47.0 ± 10.6 | .90 |
| Male sex, n (%) | 31 (66) | 71 (72) | .48 |
| Body mass index, kg/m2 | 29.3 ± 5.8 | 29.9 ± 3.9 | .54 |
| Office SBP, mmHg | 137 ± 17 | 132 ± 17 | .14 |
| Office DBP, mmHg | 86 ± 8 | 82 ± 9 | .01 |
| Creatinine, µg/L | 75 ± 14 | 77 ± 12 | .43 |
| Psoriasis duration, years | 24.6 ± 13.2 | na | na |
| Psoriasis arthritis, n (%) | 15 (32) | na | na |
| Hypertension, n (%) | 27 (66) | 57 (61) | .57 |
| Current smoking, n (%) | 17 (38) | 15 (17) | .006 |
| Obesity, n (%) | 16 (34) | 33 (33) | .93 |
| Diabetes, n (%) | 3 (7) | 5 (7) | 1.0 |
| Treated hypertension, n (%) | 13 (48) | 20 (35) | .25 |
| Statin treatment, n (%) | 5 (11) | 11 (11) | .90 |
| Inflammatory biomarkers | |||
| CRP, median (IQR), µg/ml | 1.0 (0.2, 2.7) | 1.1 (0.6, 3.2) | .22 |
| Serum amyloid A, median (IQR), µg/ml | 2.1 (1.6, 3,6) | 2.6 (2.0, 3.8) | .41 |
| Neopterin, median (IQR), nmol/L | 23.7 (18.5, 32.9) | 21.3 (16.7, 25.4) | .007 |
| KTR, median (IQR), nmol/µmol | 24.8 (20.8, 31.7) | 25.0 (22.9, 28.9) | .96 |
| PAr, median (IQR) | 0.34 (0.27, 0.53) | 0.38 (0.29, 0.48) | 1.00 |
| Echocardiographic measurements | |||
| Interventricular septum, end-diastole, cm | 1.0 ± 0.2 | 1.1 ± 0.2 | .005 |
| LV posterior wall, end-diastole, cm | 0.9 ± 0.1 | 0.8 ± 0.2 | .08 |
| LV internal diameter, end-diastole, cm | 4.8 ± 0.6 | 5.1 ± 0.5 | .001 |
| Relative wall thickness | 0.38 ± 0.09 | 0.34 ± 0.07 | .001 |
| LV mass, g | 163 ± 48 | 187 ± 56 | .01 |
| LV mass index, g/m2.7 | 35.6 ± 9.6 | 40.3 ± 9.8 | .008 |
| LV hypertrophy, n (%) | 4 (9) | 14 (14) | .33 |
| Ejection fraction, % | 65 ± 5 | 62 ± 5 | <.001 |
CRP: C-reactive protein; DPB: diastolic blood pressure; KTR: kynurenine:tryptophan ratio; LV: left ventricular; PAr: pyridoxic acid ratio; SBP: systolic blood pressure; IQR: interquartile range.
Left ventricular remodelling and inflammatory biomarkers
Total study population
The patients had lower LV end-diastolic interventricular septum thickness and LV internal diameter resulting in higher LV relative wall thickness and lower LV mass index compared to controls (Table 1). LV mass reproducibility was excellent with intraobserver variability (ICC 0.92, 95% CI 0.79–0.97) and interobserver variability (ICC 0.92, 95% CI 0.79–0.97).
Clinical variables associated with higher LV mass index in univariable analyses were greater body mass index, male sex and hypertension. Having psoriasis was associated with lower LV mass index both in univariable (β−0.22, p = .008) and in multivariable analyses (β−0.21, p = .007) after adjustment for age, sex, body mass index and hypertension.
In univariable analyses, higher serum KTR and PAr index were associated with higher LV mass index, while there was a borderline significant association with SAA (Table 2). In subsequent multivariable analyses adjusting for presence of psoriasis and significant clinical variables, KTR, PAr index and SAA remained associated with higher LV mass index (Table 2). There was a significant association between neopterin and LV relative wall thickness (β 0.19, p = .02) in univariable analysis, but after adjusting for age and sex, this association became non-significant (β 0.14, p = .08).
Table 2.
Association of inflammatory biomarkers with LV mass index in the total study population.
| Univariable | Model 1 a | Model 2 b | Model 3 c | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| SAA, µg/ml | 0.16 | .05 | 0.17 | .04 | 0.13 | .09 | 0.18 | .02 |
| CRP, µg/ml | 0.07 | .41 | 0.06 | .44 | 0.04 | .61 | 0.06 | .48 |
| Neopterin, nmol/L | 0.03 | .69 | 0.01 | .86 | 0.08 | .29 | 0.11 | .17 |
| KTR, nmol/µmol | 0.18 | .03 | 0.20 | .02 | 0.19 | .01 | 0.20 | .02 |
| PAr index | 0.26 | .002 | 0.27 | .001 | 0.26 | .001 | 0.26 | .002 |
The association between each inflammatory marker and LV mass index was tested in separate multivariable models adjusted for aage and sex; bage, sex, psoriasis, body mass index, hypertension; cage, sex, psoriasis, body mass index, hypertension, smoking, kidney function.
CRP: C-reactive protein; KTR: kynurenine:tryptophan ratio; LV: left ventricular; PAr: pyridoxic acid ratio; SAA: serum amyloid A.
Patients with psoriasis
Significant covariables of higher LV mass index in univariable analyses were higher body mass index, hypertension and diabetes (all p < .05). Psoriasis duration or PASI score was not associated with higher LV mass index or serum levels of inflammatory markers (p > .05 for both). Both higher SAA (β 0.36, p = .01) and KTR (β 0.32, p = .03) were significantly associated with higher LV mass index in univariable analyses, while higher neopterin had a borderline association (β 0.29, p = .05) (Table 3). In multivariable analyses, higher SAA (β 0.34, p = .02) and KTR (β 0.32, p = .02) both remained independently associated with higher LV mass index after adjustment for body mass index, hypertension and diabetes (Table 3). The PAr index was not associated with higher LV mass index in univariable analyses, but a significant association was found in multivariable analyses after adjustment for body mass index, hypertension and diabetes (β 0.29, p = .048) (Table 3). There was no association between any of the inflammatory markers and higher LV relative wall thickness in psoriasis patients.
Table 3.
Association of inflammatory biomarkers with LV mass index among psoriasis patients.
| Univariable | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| SAA, µg/ml | 0.36 | .01 | 0.36 | .02 | 0.34 | .02 |
| CRP, µg/ml | 0.05 | .73 | 0.04 | .79 | 0.16 | .27 |
| Neopterin, nmol/L | 0.29 | .05 | 0.31 | .04 | 0.24 | .10 |
| KTR, nmol/µmol | 0.32 | .03 | 0.32 | .03 | 0.32 | .02 |
| PAr index | 0.23 | .12 | 0.26 | .10 | 0.29 | .05 |
The association between each inflammatory marker and LV mass index was tested in separate multivariable models adjusted for aage, sex, bbody mass index, hypertension, diabetes.
CRP: C-reactive protein; KTR: kynurenine:tryptophan ratio; LV: left ventricular; PAr: pyridoxic acid ratio; SAA: serum amyloid A.
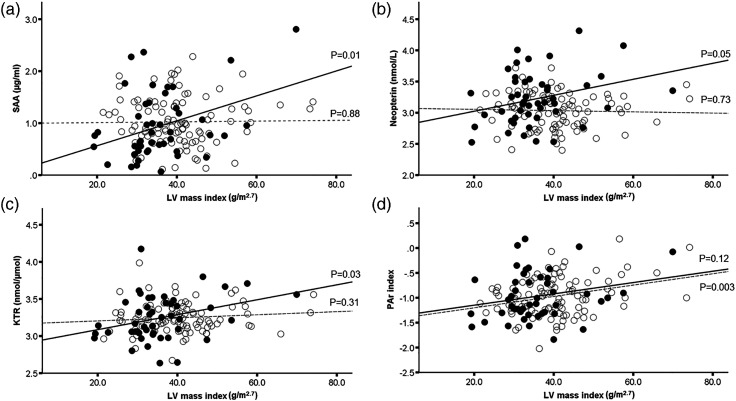
The univariable association between inflammatory biomarkers and LV mass index was also tested in separate models in patients and controls (Figure 1). For SAA, neopterin and KTR, there were significant associations with higher LV mass index for psoriasis patients, but not in controls (Figure 1). The PAr index was associated with higher LV mass index in controls, but this association was not statistically significant for patients in univariable analysis.
Figure 1.
Association between serum amyloid A (Panel A), neopterin (Panel B), KTR (Panel C) and the PAr index (Panel D) with LV mass index in psoriasis patients (solid lines and filled rings) and control subjects (broken line and open rings). CRP: C-reactive protein; KTR: kynurenine:tryptophan ratio; LV: left ventricular; PAr: pyridoxic acid ratio; SAA: serum amyloid A.
Discussion
This study is the first to assess circulating inflammatory markers in relation to LV remodelling in patients with psoriasis receiving systemic treatment with the TNF-α inhibitor infliximab. Although the serum levels of inflammatory markers were comparable between patients and controls, an independent association of higher levels of serum SAA, KTR and PAr index with unfavourable increase in LV mass index could be demonstrated in psoriasis patients, pointing to the role of inflammation in LV remodelling in psoriasis that is evident even during chronic treatment with TNF-α blockers.
Traditionally, increased LV mass index has been perceived as secondary to cardiac haemodynamic load, where pressure or volume overload results in cardiomyocyte and interstitial hypertrophy and fibrosis. 28 However, growing evidence links inflammation to LV remodelling.12,29 In cardiac TNF-α overexpressing mice, neutralization of T cells with anti-CD3 antibodies blocked LV hypertrophy development. 12 In a clinical trial of 150 psoriasis patients, treatment with the interleukin-17 inhibitor secukinumab resulted in better LV function compared to cyclosporine and methotrexate treatment. 30 Using cardiac magnetic resonance imaging, it has been documented that myocardial fibrosis increases with inflammatory activity in rheumatoid arthritis patients. 31 In a community-based study of 1564 subjects, electrocardiographic confirmed LV hypertrophy at baseline was associated with higher CRP levels over 6 years follow-up. 29 However, in another population-based cohort of 1370 subjects, CRP was only associated with LV mass in women, but not in men. 32 Further, the present study demonstrated that the markers of T cell mediated inflammation like KTR and neopterin were particularly associated with higher LV mass index in psoriasis patients. The ratio of tryptophan to kynurenine, KTR, is both a marker of IFN-γ mediated T cell inflammation and of IDO activity. 5 Recent experimental research has demonstrated that there is upregulation of IDO in hypertrophic hearts of both human and mice, as well as in hypertrophic rat cardiomyocytes. 33 Of note, IDO overexpression was alone not sufficient to induce cardiac hypertrophy, but needed presence of elevated angiotensin II. 33 In another study of 136 subjects with primary hyperparathyroidism, higher LV mass index was associated with activation of the tryptophan-kynurenine pathway as well as higher levels of CRP. 34 In psoriasis, it has been demonstrated that the expression of IDO varies with disease activity and is paradoxically lower in severe disease. 35 As IDO is an immunoregulatory enzyme capable of blunting T cell response, it has been hypothesized that this is a potential pathophysiological mechanism of the dysregulated immunity in psoriasis. 35 Another marker of cellular inflammation, the PAr index, was also associated with higher LV mass index in the present study. The PAr index reflects vitamin B6 catabolism during inflammatory states. It has been associated with increased risk of stroke in a community-based cohort of 6891 subjects,36,37 as well as with higher all-cause mortality in 7796 subjects with established CVD. 38 Taken together, the present results suggest that T cell driven inflammation is associated with LV remodelling in psoriasis.
The acute phase protein SAA was particularly associated with higher LV mass index in psoriasis patients. SAA may aggregate into fibrils and accumulate in tissues if concentrations are elevated over time causing LV hypertrophy and amyloid A (AA) amyloidosis. 39 In an elegant study of transgenic mice with dermatitis and sustained elevated levels of interleukin-1, extensive amyloid deposition in internal organs as well as LV hypertrophy were demonstrated. 40 The researchers were able to show that these abnormalities were ameliorated with use of antibodies to interleukin-1, establishing a direct link from the inflamed skin to LV hypertrophy in mice. 40 From a nationwide Japanese study of 199 patients with histopathological confirmed AA amyloidosis, rheumatic diseases were the underlying condition in two thirds of patients. 41 In psoriasis, casuistic reports of AA cardiac amyloidosis have been published; 42 however, larger studies are lacking. In the current study, psoriasis patients had a low prevalence of LV hypertrophy and lower LV mass index compared to controls, but psoriasis patients were under aggressive anti-inflammatory treatment with infliximab and methotrexate. It is therefore particularly striking that an association of higher serum levels of SAA with higher LV mass index could still be demonstrated in the psoriasis patients. Of note, a similar association between SAA and LV mass index was not found in controls without psoriasis.
Study limitations and strengths
This is a cross-sectional study, and causality between inflammatory markers and LV mass index cannot be claimed. Since the study only included patients receiving infliximab treatment, the effect of infliximab on inflammatory markers could not be tested. It is also possible that infliximab treatment has attenuated the association between some markers of inflammation with LV remodelling, explaining why several of the biomarkers tested did not show a significant association with LV mass index or LV relative wall thickness. Unfortunately, biomarkers were not measured before initiation of infliximab treatment. Prospective studies addressing the longitudinal effect of systemic biological treatment on inflammatory heart disease in psoriasis are warranted. Further, because of the limited sample size in the current study, we cannot rule out any type 2 error. The strengths of the current study include usage of a core laboratory for the echocardiographic analyses, as recommended in European echocardiography guidelines. 43 Furthermore, biomarkers were analysed simultaneously in patients and controls at Bevital AS to minimize analytical variations, and the control group had a similar CVD risk factor burden as the patients.
Conclusion
Higher levels of the inflammatory biomarkers SAA, KTR and the PAr index were associated with greater LV mass index in psoriasis patients, indicating a role of chronic inflammation in LV remodelling evident even during treatment with TNF-α blockers.
Acknowledgements
The authors are grateful to Liv Himle RN, Marina Kokorina MD and staff engineer Hilde Jacobsen for invaluable help with patient management and data handling in the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: For this study, we received grants from Hjertefondet, University of Bergen and The Western Regional Health Authority of Norway. The echocardiograph used in the study was donated by the Grieg Foundation, Bergen Norway. And this work was supported by the Universitetet i Bergen.
Ethics approval: Informed, written consent was signed prior to participation in the study in accordance to the Declaration of Helsinki, and the research protocol was approved by the Norwegian Regional Committees for Medical and Health Research Ethics division West (2016/2194).
Informed consent: Written informed consent was obtained from all subjects before inclusion in the study.
Data availability statement: Data are not publically available, as the Regional committee for medical and health research ethics does not grant public deposition of the data.
ORCID iDs
Helga Midtbø https://orcid.org/0000-0002-3558-9914
Ester Kringeland https://orcid.org/0000-0002-9015-741X
References
- 1.Armstrong AW, Read C. (2020) Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 323(19): 1945–1960. [DOI] [PubMed] [Google Scholar]
- 2.Zwain A, Aldiwani M, Taqi H. (2021) The association between psoriasis and cardiovascular diseases. European Cardiology 16: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestle FO, Kaplan DH, Barker J. (2009) Psoriasis. The New England Journal of Medicine 361(5): 496–509. [DOI] [PubMed] [Google Scholar]
- 4.Sulo G, Vollset SE, Nygard O, et al. (2013) Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. International Journal of Cardiology 168(2): 1435–1440. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen ER, Tuseth N, Eussen SJ, et al. (2015) Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arteriosclerosis, Thrombosis, and Vascular Biology 35(2): 455–462. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong EJ, Harskamp CT, Armstrong AW. (2013) Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. Journal of the American Heart Association 2(2): e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blandizzi C, Gionchetti P, Armuzzi A, et al. (2014) The role of tumour necrosis factor in the pathogenesis of immune-mediated diseases. International Journal of Immunopathology and Pharmacology 27(1 suppl l): 1–10. [DOI] [PubMed] [Google Scholar]
- 8.Ahlehoff O, Skov L, Gislason G, et al. (2013) Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: A Danish real-world cohort study. Journal of Internal Medicine 273(2): 197–204. [DOI] [PubMed] [Google Scholar]
- 9.Nast A, Gisondi P, Ormerod AD, et al. (2015) European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. Journal of the European Academy of Dermatology and Venereology 29(12): 2277–2294. [DOI] [PubMed] [Google Scholar]
- 10.Midtbø H, Gerdts E, Kvien TK, et al. (2015) Disease activity and left ventricular structure in patients with rheumatoid arthritis. Rheumatology 54(3): 511–519. [DOI] [PubMed] [Google Scholar]
- 11.Midtbø H, Gerdts E, Berg IJ, et al. (2018) Ankylosing spondylitis is associated with increased prevalence of left ventricular hypertrophy. The Journal of Rheumatology 45(9): 1249–1255. [DOI] [PubMed] [Google Scholar]
- 12.Frieler RA, Mortensen RM. (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131(11): 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonomidis I, Papadavid E, Makavos G, et al. (2017) Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor-a antagonism or cyclosporine in psoriasis. Circulation Cardiovascular Imaging 10(9): e006283. [DOI] [PubMed] [Google Scholar]
- 14.Ahlehoff O, Hansen PR, Gislason GH, et al. (2016) Myocardial function and effects of biologic therapy in patients with severe psoriasis: A prospective echocardiographic study. Journal of the European Academy of Dermatology and Venereology 30(5): 819–823. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal; 39(33): 3021–3104. [DOI] [PubMed] [Google Scholar]
- 16.Gerdts E, Cramariuc D, de Simone G, et al. (2008) Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study). European Heart Journal – Cardiovascular Imaging 9(6): 809–815. [DOI] [PubMed] [Google Scholar]
- 17.Gerdts E, Izzo R, Mancusi C, et al. (2018) Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network). International Journal of Cardiology; 258: 257–261. [DOI] [PubMed] [Google Scholar]
- 18.Levy D, Garrison RJ, Savage DD, et al. (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. The New England Journal of Medicine 322(22): 1561–1566. [DOI] [PubMed] [Google Scholar]
- 19.Linde A, Gerdts E, Tveit KS, et al. (2021) Subclinical cardiac organ damage in patients with moderate to severe psoriasis. Journal of Clinical Medicine 10(11): 2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halland H, Lonnebakken MT, Pristaj N, et al. (2018) Sex differences in subclinical cardiac disease in overweight and obesity (the FATCOR study). Nutrition, Metabolism, and Cardiovascular Diseases 28(10): 1054–1060. [DOI] [PubMed] [Google Scholar]
- 21.Halland H, Matre K, Einarsen E, et al. (2019) Effect of fitness on cardiac structure and function in overweight and obesity (the FATCOR study). Nutrition, Metabolism, and Cardiovascular Diseases 29(7): 710–717. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Mor-Avi V, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging 16(3): 233–270. [DOI] [PubMed] [Google Scholar]
- 23.Midttun Ø, Hustad S, Ueland PM. (2009) Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry 23(9): 1371–1379. [DOI] [PubMed] [Google Scholar]
- 24.Ulvik A, Midttun Ø, Pedersen ER, et al. (2014) Evidence for increased catabolism of vitamin B-6 during systemic inflammation. American Journal of Clinical Nutrition 100(1): 250–255. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Meyer K, Borucki K, et al. (2018) Multiplex immuno-MALDI-TOF MS for targeted quantification of protein biomarkers and their proteoforms related to inflammation and renal dysfunction. Analytical Chemistry 90(5): 3366–3373. [DOI] [PubMed] [Google Scholar]
- 26.Fredriksson T, Pettersson U. (1978) Severe psoriasis--oral therapy with a new retinoid. Dermatologica 157(4): 238–244. [DOI] [PubMed] [Google Scholar]
- 27.Finlay AY, Khan GK. (1994) Dermatology life quality index (DLQI)--a simple practical measure for routine clinical use. Clinical and Experimental Dermatology 19(3): 210–216. [DOI] [PubMed] [Google Scholar]
- 28.Devereux RB, Roman MJ. (1999) Left ventricular hypertrophy in hypertension: Stimuli, patterns, and consequences. Hypertension Research: Official Journal of the Japanese Society of Hypertension 22(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 29.Bo S, Mandrile C, Milanesio N, et al. (2012) Is left ventricular hypertrophy a low-level inflammatory state? A population-based cohort study. Nutrition, Metabolism, and Cardiovascular Diseases 22(8): 668–676. [DOI] [PubMed] [Google Scholar]
- 30.Makavos G, Ikonomidis I, Andreadou I, et al. (2020) Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Canadian Journal of Cardiology 36(1): 100–111. [DOI] [PubMed] [Google Scholar]
- 31.Ntusi NA, Piechnik SK, Francis JM, et al. (2015) Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovascular Imaging 8(5): 526–536. [DOI] [PubMed] [Google Scholar]
- 32.Medenwald D, Dietz S, Tiller D, et al. (2014) Inflammation and echocardiographic parameters of ventricular hypertrophy in a cohort with preserved cardiac function. Open Heart 1(1): e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Li S, Gao Z, et al. (2021) Indoleamine 2, 3-dioxygenase 1 (IDO1) promotes cardiac hypertrophy via a PI3K-AKT-mTOR-dependent mechanism. Cardiovascular Toxicology 21(8): 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Verheyen N, Meinitzer A, Grübler MR, et al. (2017) Low-grade inflammation and tryptophan-kynurenine pathway activation are associated with adverse cardiac remodeling in primary hyperparathyroidism: The EPATH trial. Clinical Chemistry and Laboratory Medicine; 55(7): 1034–1042. [DOI] [PubMed] [Google Scholar]
- 35.Llamas-Velasco M, Bonay P, José Concha-Garzón M, et al. (2017) Immune cells from patients with psoriasis are defective in inducing indoleamine 2, 3-dioxygenase expression in response to inflammatory stimuli. The British Journal of Dermatology 176(3): 695–704. [DOI] [PubMed] [Google Scholar]
- 36.Ueland PM, McCann A, Midttun Ø, et al. (2017) Inflammation, vitamin B6 and related pathways. Molecular Aspects of Medicine 53: 10–27. [DOI] [PubMed] [Google Scholar]
- 37.Zuo H, Tell GS, Ueland PM, et al. (2018) The PAr index, an indicator reflecting altered vitamin B-6 homeostasis, is associated with long-term risk of stroke in the general population: The Hordaland Health Study (HUSK). American Journal of Clinical Nutrition 107(1): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulvik A, Pedersen ER, Svingen GF, et al. (2016) Vitamin B-6 catabolism and long-term mortality risk in patients with coronary artery disease. American Journal of Clinical Nutrition 103(6): 1417–1425. [DOI] [PubMed] [Google Scholar]
- 39.Westermark GT, Fandrich M, Westermark P. (2015) AA amyloidosis: Pathogenesis and targeted therapy. Annual Review of Pathology: Mechanisms of Disease 10(321–344. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka K, Nakanishi T, Saito H, et al. (2014) Persistent release of IL-1s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: The potential of anti-IL-1 therapy for systemic inflammatory diseases. PloS One 9(8): e104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okuda Y, Yamada T, Ueda M, et al. (2018) First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Internal Medicine 57(23): 3351–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanabe H, Maki Y, Urabe S, et al. (2015) Myopathy in a patient with systemic AA amyloidosis possibly induced by psoriasis vulgaris: An autopsy case. Muscle and Nerve 52(6): 1113–1117. [DOI] [PubMed] [Google Scholar]
- 43.Galderisi M, Henein MY, D'Hooge J, et al. (2011) Recommendations of the European association of echocardiography: How to use echo-doppler in clinical trials: Different modalities for different purposes. European Journal of Echocardiography 12(5): 339–353. [DOI] [PubMed] [Google Scholar]