Abstract
Background:
Lifestyle interventions are a cornerstone in the treatment of chronic ischaemic heart disease (CIHD) and type 2 diabetes mellitus (T2DM). This study aimed at identifying differences in clinical characteristics between categories of the common lifestyle intervention targets BMI, exercise capacity (peak V̇O2) and health literacy (HL).
Methods:
Cross-sectional baseline characteristics of patients enrolled in the LeIKD trial (Clinicaltrials.gov NCT03835923) are presented in total, grouped by BMI, %-predicted peak V̇O2 and HL (HLS-EU-Q16), and compared to other clinical trials with similar populations.
Results:
Among 499 patients (68.3±7.7 years; 16.2% female; HbA1c, 6.9±0.9%), baseline characteristics were similar to other trials and revealed insufficient treatment of several risk factors (LDL-C 92±34 mg/dl; BMI, 30.1±4.8 kg/m2; 69.6% with peak V̇O2<90% predicted). Patients with lower peak V̇O2 showed significantly higher (p < 0.05) CIHD and T2DM disease severity (HbA1c, CIHD symptoms, coronary artery bypass graft). Obese patients had a significantly higher prevalence of hypertension and higher triglyceride levels, whereas in patients with low HL both quality of life components (physical, mental) were significantly reduced.
Conclusions:
In patients with CIHD and T2DM, peak V̇O2, BMI and HL are important indicators of disease severity, risk factor burden and quality of life, which reinforces the relevance of lifestyle interventions.
Keywords: Lifestyle, exercise capacity, chronic coronary syndrome, secondary prevention, cardiorespiratory fitness, disease management
Key messages:
• Exercise capacity is linked to disease severity in patients with CIHD and T2DM
• Medical and lifestyle-related disease management in Germany is still insufficient despite the high-risk classification for patients with CIHD and T2DM
• There is an urgent need for sustainable lifestyle intervention strategies in this multi-morbid and heterogeneous patient population with CIHD and T2DM
Introduction
In 2019, ischaemic heart disease was the leading cause of death accounting for 8.9 million cases or approximately 16% of total deaths worldwide. 1 In chronic ischaemic heart disease (CIHD), diabetes mellitus type 2 (T2DM) is a frequent companion associated with an elevated risk for cardiovascular (CV) events and increased mortality, classifying these patients as a very high-risk population.2,3 Both diseases share several pathophysiological mechanisms and risk factors: besides age, behavioural risk factors such as smoking, poor dietary patterns, and a sedentary lifestyle promote obesity, impaired glycaemic control, hypertension and hyperlipidaemia, which causes a chronic systemic inflammatory state and leads to progressive vascular alterations and the development of arteriosclerosis complicated by CV events and death.4,5
Despite evidence based international guidelines on the prevention and treatment of CIHD and T2DM, the implementation of lifestyle recommendations and the achievement of risk factor control are still not at a satisfactory level as recently demonstrated by the results of the EUROASPIRE V survey (European Action on Secondary and Primary Prevention by Intervention to Reduce Events, EAV). 5 Regular screening, risk assessment and adequate disease management are highly recommended2,3,6 since lifestyle-related changes in physical activity and nutrition are a cornerstone of the treatment of CIHD and T2DM. 7 In recent years, health literacy (HL), which has been shown to be low in patients with CV diseases, 8 has gained increasing attention and might be important to implement sustainable lifestyle changes. Low-threshold and cost-effective strategies are urgently needed to improve risk factor control and prognosis in these patients.
Therefore, the LeIKD trial (Lifestyle Intervention in Chronic Ischaemic Heart Disease and Type 2 Diabetes) aimed to improve CV risk factors through exercise training, nutritional recommendations and the increase of HL through a telemedical lifestyle intervention programme. 9 So far, only few trials performed cardiopulmonary exercise testing (CPET) in patients with both CIHD and T2DM and data on several clinical and lifestyle-related aspects in this patient population is still lacking. To improve the characterisation of these high-risk patients, baseline characteristics of the LeIKD study population are presented next to similar lifestyle or pharmacological trials and compared within different subgroups of obesity, exercise capacity and HL.
Methods
Study design
LeIKD (Clinicaltrials.gov identifier: NCT03835923) was a multicentre, randomised controlled trial aiming to improve CV risk factors by promoting a healthy lifestyle via a telemedical supported lifestyle intervention in patients with CIHD and T2DM.
The study design has been published before. 9 In brief, patients with CIHD (ICD-10: I20-I25) and T2DM (ICD-10: E11; HbA1c ≥ 6.5% or anti-diabetic medication at the time of screening) were randomised (1:1) to either lifestyle intervention (LS) or usual care (UC). The LS group received an individual home-based exercise training programme, tailored nutrition counselling and health related information to increase HL, while the UC group received general lifestyle recommendations according to CIHD and T2DM treatment guidelines. The intervention was performed using smartphone applications in combination with telemedical self-tracking devices (pedometer, heart rate and blood glucose monitor). Participants without a smartphone were provided with a free loaner device. All patients were insured at the statutory health insurance fund TK (Techniker Krankenkasse, Hamburg, Germany) and were already included in a disease management program (DMP) for CIHD or T2DM.
The primary endpoint is the difference in change in HbA1c after 6 months between groups. Initial sample size calculation was based on the results of the ENHANCE trial (mean difference in HbA1c of 0.4±1.8%, 80% statistical power, 5% significance level, estimated dropout rate of 15%). 10 Accordingly, a number of 750 patients would have been required to detect a significant difference in the primary endpoint between groups. Due to a planned comparison between rural and urban areas, the calculated sample size was initially doubled to 1500. In consequence of delays in the start of the project, insufficient recruitment and a fixed project duration, sample size calculation had to be adjusted. The comparison of rural and urban areas was omitted and based on a small to medium effect size of 0.305, 11 80% statistical power, 5% significance level for two-sided t-test and an estimated drop-out rate of 30%, we aimed to include a minimum of 486 patients. The study was conducted in 11 study sites across Germany and the study protocol was approved by the ethics committee of the Technical University of Munich (reg. number: 144/18-S) and at all participating study sites. LeIKD was performed according to the Declaration of Helsinki.
Recruitment/assessments
The recruitment was conducted as a three-step procedure: Potential study participants were first selected and contacted by the TK health insurance to inquire about a potential interest to participate in the trial. Interested patients were then contacted by the corresponding local study site to receive further information and arrange a screening appointment. After the screening visit, eligible patients were included into the trial.
Assessments contained a comprehensive anamnesis including medical history and current medication, anthropometric measurements, physical examination, blood draw and a resting electrocardiogram (ECG). Participants were not required to be in a fasting state at the time of blood collection and were asked to take their daily prescribed medication as usual. Participants performed CPET including exercise ECG on a bicycle ergometer according to a prescribed ramp protocol. If CPET was not possible, patients carried out a symptom-limited exercise testing with ECG. After 4 min at rest and a warm-up phase of 2 min at 20W, the work rate was continuously increased by 8W, 12W or 18W per minute until exhaustion. The increment was chosen based on the estimated exercise capacity with the aim to reach maximal exhaustion within 8–12 min. CPET data was assessed breath-by-breath and every data set was verified and analysed by the CPET core laboratory in Munich. Peak oxygen consumption (peak V̇O2) was obtained as the highest 30 s average during exercise, predicted values (% pred. peak V̇O2) were calculated according to reference values of the SHIP study. 12 Ventilatory threshold (VT1) was determined by the V-slope method. 13 All examinations were conducted according to standard operating procedures.
Sociodemographic data was collected by questionnaire. Education was classified according to International Standard Classification of Education 2011 and categorised in low (level 1-2), medium (level 3-4) and high (level 5-6). Study participants also completed validated questionnaires for the assessment of HL and quality of life (QoL). HL was assessed with the German version of HLS-EU-Q16. Based on four dimensions (16 items on a four-point Likert scale) covering the ability to obtain, understand, process and apply health-related information, HL is categorised as “sufficient”, “problematic” or “inadequate”. 8 For score creation, responses were dichotomized. Missing values were coded 0 and cases were excluded, if more than two items were missing. 14 Health-related QoL was measured with SF-36 including 36 items on 8 dimensions (General Health, Physical Performance, Physical Limitations, Physical Pain, Vitality, Mental Health, Emotional Impairment and Social Impairment) and summarised as “Physical Component Score” (PCS) and “Mental Component Score” (MCS). 15
Statistical analysis
Baseline characteristics are presented for the overall study population and within subgroups of body mass index (BMI) [“non-obese” (BMI ≤ 30 kg/m2) vs “obese” (BMI > 30 kg/m2)], exercise capacity [“severely restricted” (<75% of pred. peak V̇O2) vs “mildly to moderately restricted” (75–89.9% of pred. peak V̇O2) vs “normal” (≥90% of pred. peak V̇O2)] and HL [“non-sufficient HL” (problematic/inadequate HL) vs “sufficient HL”], as these parameters reflect the main components of the LeIKD intervention (nutritional advice, exercise training and health literacy training, respectively). Continuous variables are presented as mean and standard deviation, categorical variables are displayed as absolute and relative frequencies. For comparison of subgroups, t-tests or ANOVAs were calculated for continues variables, and Chi-square-tests or F-tests for categorical variables. p-values below 0.05 determine statistical significance. All analyses were performed using the statistics software R (Version 4.0.2).
Comparison to other study populations
In order to ensure generalisability, LeIKD baseline characteristics were compared to other trials selected to include pharmacological and lifestyle interventions that have similar study populations focusing on T2DM and CV risk and present a large set of comparable baseline characteristics. Based on these criteria, LeIKD baseline characteristics are compared to the pharmacological intervention trials CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes)16,17 and a subsample with established CV disease of the CARMELINA trial (CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin), 18 the lifestyle intervention trial EXCADI (Exercise Training in Patients with Coronary Heart Disease and Type 2 Diabetes) 19 and the landmark lifestyle intervention study Look AHEAD (Action for Health in Diabetes). 20
Results
Recruitment
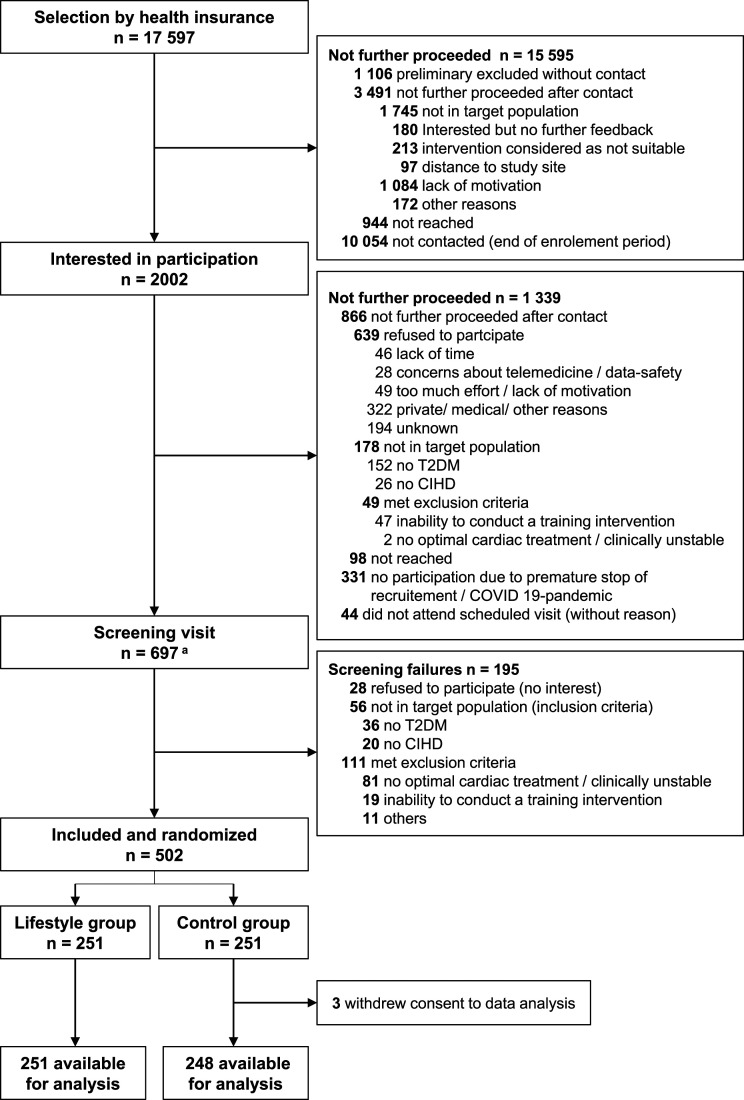
The recruitment period lasted from February 2019 to March 2020. TK health insurance selected 17 597 potential participants with ICD-10: I20-I25 for CIHD and ICD-10: E11 for T2DM (Figure 1). After initial contact by TK, 2002 patients were interested in participation and were referred to the corresponding local study sites. A total of 697 screening visits were performed, including 195 screening failures. The main reason for screening failure was meeting the exclusion criteria “no optimal cardiac treatment or not clinically stable within the last 4 weeks” which applied to 81 patients. With the onset of the COVID-19 pandemic in Germany that significantly blunted the recruitment rate due to cancellations of appointments and lock-down of study sites, the recruitment phase was prematurely stopped after randomisation of 502 patients. In the UC group 3 patients withdrew consent to study participation and data analysis. Thus, baseline data from 499 patients was available for analysis.
Figure 1.
Patient recruitment in the LeIKD study. Abbreviations: CIHD, chronic ischaemic heart disease; T2DM, type 2 diabetes mellitus; aincluding re-screenings (initial screening failures may have been included during re-screening, n = 34).
Baseline characteristics
Baseline characteristics including CV and anti-diabetic medication of the enrolled study population are summarized in Table 1. The mean age was 68.3 ± 7.7 years and 16.2% of patients were female. Mean duration of CIHD was 8.8 ± 7.3 years and 231 (46.3%) patients had a CIHD affecting at least two vessels. Previous myocardial infarction was reported by 34.3%, coronary revascularisation by 54.9% and coronary bypass graft implantation by 16.4% of patients. Furthermore, 81.1% reported no angina pectoris-related symptoms, while 14.7% and 4.2% reported symptoms according to the Canadian Cardiovascular Society (CCS) score Grade I and II-IV, respectively. Mean duration of T2DM was 12.3 ± 8.4 years, mean HbA1c was 6.9 ± 0.9% with 27.5% prescribed with insulin, 83.0% using oral anti-diabetic medication (72.5% metformin) and 12.6% without any anti-diabetic medication.
Table 1.
Baseline characteristics of LeIKD study population with T2DM and CIHD.
| Overall [n = 499] a | |
|---|---|
| Age at baseline (years) | 68.3 ± 7.7 |
| Female sex | 81 (16.2) |
| Body height (cm) | 175 ± 9 |
| Weight (kg) | 91.8 ± 16.7 |
| BMI (kg/m2) | 30.1 ± 4.8 |
| Obese with BMI > 30 kg/m2 | 217 (43.5) |
| Waist circumference (cm) | 108 ± 12 [480] |
| Hip circumference (cm) | 107 ± 11 [480] |
| Systolic blood pressure (mmHg) | 137 ± 17 |
| Diastolic blood pressure (mmHg) | 79 ± 10 |
| Heart rate (bpm) | 70 ± 11 [498] |
| Smoking | |
| Never | 185 (37.1) |
| Current | 55 (11.0) |
| Former | 259 (51.9) |
| Alcoholic drinks per week | 4.1 ± 7.3 [495] |
| CIHD and T2DM | |
| Duration of CIHD (years) | 8.8 ± 7.3 [397] |
| CIHD classification | [498] |
| No relevant stenosis (<50%) | 87 (17.5) |
| 1-vessel coronary disease | 115 (23.1) |
| 2-vessel coronary disease | 84 (16.9) |
| 3-vessel coronary disease | 138 (27.7) |
| main coronary disease | 9 (1.8) |
| Unknown | 65 (13.1) |
| CIHD symptoms | [498] |
| Asymptomatic | 404 (81.1) |
| CCS score grade I b | 73 (14.7) |
| CCS score grade II - IV | 21 (4.2) |
| Previous myocardial infarction | 171 (34.3) |
| Coronary revascularisation | 274 (54.9) |
| Coronary artery bypass graft | 82 (16.4) |
| Duration of T2DM (years) | 12.3 ± 8.4 [363] |
| HbA1c (%) | 6.9 ± 0.9 [496] |
| Additional medical history | |
| Hypertension | 461 (92.4) |
| Hyperlipidaemia | 429 (86.0) |
| Atrial fibrillation | 96 (19.2) |
| Peripheral artery disease | 35 (7.0) |
| Cerebrovascular disease | 40 (8.0) |
| COPD | 22 (4.4) |
| Depression | 31 (6.2) |
| Chronic kidney disease | 59 (11.8) |
| Heart failure | 120 (24.0) |
| HFpEF (LVEF > 50%) | 68 (13.6) |
| HFmrEF (LVEF 40–49%) | 30 (6.0) |
| HFrEF (LVEF < 40%) | 17 (3.4) |
| Exercise performance c | |
| Peak work load (Watt) | 129 ± 42 [497] |
| Peak V̇O2 (ml/kg/min) | 18.6 ± 4.6 [473] |
| Pred. peak V̇O2 (%) | 81.9 ± 16.8 [473] |
| Peak RER | 1.09 ± 0.10 [473] |
| Additional laboratory analysis | |
| Haemoglobin (g/dl) | 14.5 ± 1.3 [487] |
| Total cholesterol (mg/dl) | 157 ± 39 [495] |
| HDL-C (mg/dl) | 48 ± 13 [498] |
| LDL-C (mg/dl) | 92 ± 34 [496] |
| Triglycerides (mg/dl) | 182 ± 100 [497] |
| NT-proBNP (ng/l) | 255 ± 358 [487] |
| Current medication | |
| Anti-diabetic medication | |
| Oral anti-diabetics | 414 (83.0) |
| Metformin | 362 (72.5) |
| DPP4-inhibitors | 115 (23.0) |
| SGLT2-inhibitors | 109 (21.8) |
| Sulfonylurea | 26 (5.2) |
| GLP-1-analoga | 65 (13.0) |
| Insulin | 137 (27.5) |
| Cardiovascular medication | |
| ACE inhibitor/AR blocker | 423 (84.8) |
| Anti-platelet | 349 (69.9) |
| ASA | 329 (65.6) |
| Clopidogrel | 36 (7.2) |
| Ticagrelor | 11 (2.2) |
| Prasugrel | 5 (1.0) |
| Lipid-lowering drugs | 420 (84.2) |
| Statins | 412 (82.6) |
| Atorvastatin/Rosuvastatin | 241 (48.3) |
| Other statins | 171 (34.3) |
| Other lipid-lowering drugs | 81 (16.2) |
| Diuretics | 196 (39.3) |
| Aldosterone antagonists | 52 (10.4) |
| Beta-blocker | 364 (72.9) |
| Anticoagulation | |
| Phenprocoumon | 26 (5.2) |
| NOAC | 72 (14.4) |
| Nitrates/Ranolazin | 25 (5.0) |
| Sacubitril/Valsartan | 8 (1.6) |
| Health Literacy d & Quality of Life e | |
| HLS group | |
| Inadequate | 73 (15.9) |
| Problematic | 122 (26.6) |
| Sufficient | 264 (57.5) |
| QoL-PCS | 44.5 ± 9.8 |
| QoL-MCS | 52.2 ± 9.2 |
BMI: body mass index; CIHD: chronic ischaemic heart disease; T2DM: type 2 diabetes mellitus; CCS scale: Canadian Cardiovascular Society angina grading scale; HbA1c: glycated haemoglobin; COPD: chronical obstructive pulmonary disease; HFpEF: heart failure with preserved ejection fraction; LVEF: left ventricular ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; HFrEF: heart failure with reduced ejection fraction; V̇O2: oxygen consumption; RER: respiratory exchange ratio; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; DPP4: dipeptidyl peptidase-4; SGLT2: sodium/glucose cotransporter 2; GLP-1: glucagon-like peptide-1; ACE: angiotensin-converting-enzyme; AR: androgen receptor; ASA: acetylsalicylic acid; NOAC: novel oral anticoagulants; HLS: health literacy score; QoL: quality of life; PCS: physical component score; MCS: mental component score.
aData are presented as mean ± standard deviation or absolute and (relative) frequency. In case of missing data, corrected sample size is described as [No.].
bCanadian Cardiovascular Society angina grading scale describes the severity of angina pectoris. 0= no symptoms, I = angina pectoris with strenuous exertion, II-IV= angina with moderate/mild exertion or at rest.
cCardiopulmonary exercise testing was performed in 473 patients, exercise testing in 25 patients, 1 patient none.
dHLS-EU-Q16 data available from 459 patients.
eSF-36 data available from 393 patients.
Mean BMI was 30.1 ± 4.8 kg/m2 and 259 participants (51.9%) were identified as former and 55 (11.0%) as current smokers. The prevalence of hypertension was 92.4% with a mean systolic blood pressure of 137 ± 17 mmHg and a mean diastolic blood pressure of 79 ± 10 mmHg. Hyperlipidaemia was reported by 429 patients (86.0%) with 420 participants (84.2%) using lipid lowering drugs (82.6% prescribed with statins). Mean total cholesterol was 157 ± 39 mg/dl, LDL cholesterol (LDL-C) was 92 ± 34 mg/dl and triglycerides were 182 ± 100 mg/dl. CPET was performed in 473 patients, symptom-limited exercise testing in 25 patients. Mean peak workload was 129 ± 42 W with a peak V̇O2 of 18.6 ± 4.6 mL/kg/min according to 81.9 ± 16.8% of mean predicted peak V̇O2. HL data was available from 459 patients and only 264 patients (57.5%) showed a sufficient HL.
Subgroup analyses
Baseline characteristics according to subgroups by BMI, exercise capacity and HL are presented in Table 2. Among the study population, 282 patients were non-obese with a mean BMI of 26.7 ± 2.1 kg/m2 and 217 patients were classified as obese (mean BMI 34.5 ± 3.6 kg/m2). Obese patients were younger (66.4 ± 7.5 vs 69.8 ± 7.6 years, p < 0.001) and had a lower educational status than non-obese patients (p = 0.014). Furthermore, the diagnoses of T2DM (54.4 ± 10.4 vs 56.5 ± 9.7 years, p = 0.050) and CIHD (57.2 ± 9.4 vs 60.9 ± 10.0 years, p < 0.001) were made at a younger age. No differences in HbA1c and blood lipids could be observed except for higher mean triglycerides in the obese subgroup (204 ± 103 vs 166 ± 94 mg/dl, p < 0.001). Obese participants were also more likely to suffer from hypertension (96.8 vs 89.0%, p = 0.002). The non-obese group showed a significantly lower absolute peak V̇O2 (1610 ± 413 vs 1770 ± 464 mL/min, p < 0.001). However, relative to body weight, peak V̇O2 was significantly higher than in the obese subgroup (19.8 ± 4.5 vs 17.1 ± 4.2 mL/kg/min, p < 0.001), and %-predicted peak V̇O2 was not significantly different between the subgroups (81.4 ± 16.2 vs 82.6 ± 17.2%, p = 0.466). Non-obese patients had a significantly higher QoL-PCS (46.3 ± 8.8 vs 42.3 ± 10.5, p < 0.001) but no significant differences in QoL-MCS.
Table 2.
Baseline characteristics grouped by body mass index, exercise capacity and health literacy.
| Obesity a BMI (kg/m2) | Exercise capacity
b
Predicted peak V̇O2 (%) |
Health literacy c (HLS-EU-Q16) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-obese [n = 282] | Obese [n = 217] | p-value | Severely restricted [n = 165] | Mildly to moderately restricted [n = 164] | Normal [n = 144] | p-value | Not sufficient [n = 195] | Sufficient [n = 264] | p-value | |
| Age (years) | 69.8 ± 7.6 | 66.4 ± 7.5 | <0.001 | 67.6 ± 8.4 | 69.9 ± 6.6 | 67.8 ± 6.7 | 0.806 | 67.6 ± 8.3 | 68.3 ± 7.3 | 0.317 |
| Female sex | 41 (14.5) | 40 (18.4) | 0.295 | 24 (14.5) | 27 (16.5) | 23 (16.0) | 0.884 | 29 (14.9) | 42 (15.9) | 0.862 |
| Education | ||||||||||
| Low | 3 (1.1) | 9 (4.5) | 0.014 | 5 (3.2) | 3 (1.9) | 3 (2.2) | 0.268 | 8 (4.1) | 4 (1.5) | 0.226 |
| Medium | 116 (43.6) | 102 (50.5) | 78 (50.6) | 68 (43.9) | 52 (38.8) | 88 (45.4) | 121 (46.0) | |||
| High | 147 (55.3) | 91 (45.0) | 71 (46.1) | 84 (54.2) | 79 (59.0) | 98 (50.5) | 138 (52.5) | |||
| Duration T2DM (years) | 12.8 ± 8.2 | 11.5 ± 8.7 | 0.154 | 12.1 ± 8.3 | 13.0 ± 8.9 | 11.8 ± 8.9 | 0.800 | 12.1 ± 8.3 | 12.4 ± 8.6 | 0.780 |
| Age T2DM diagnosis (years) | 56.5 ± 9.7 | 54.4 ± 10.4 | 0.050 | 54.8 ± 10.7 | 56.4 ± 9.45 | 55.7 ± 9.97 | 0.428 | 54.9 ± 9.3 | 55.4 ± 10.2 | 0.708 |
| HbA1c (%) | 6.8 ± 0.8 | 6.9 ± 1.0 | 0.281 | 7.0 ± 1.0 | 6.8 ± 0.9 | 6.7 ± 0.9 | 0.002 | 6.9 ± 0.9 | 6.8 ± 0.9 | 0.962 |
| Duration CIHD | 8.5 ± 6.9 | 9.1 ± 7.9 | 0.480 | 8.7 ± 7.6 | 9.1 ± 7.4 | 8.0 ± 7.4 | 0.551 | 9.6 ± 7.8 | 8.2 ± 7.0 | 0.077 |
| Age CIHD diagnosis (years) | 60.9 ± 10.0 | 57.2 ± 9.4 | <0.001 | 58.1 ± 10.8 | 60.6 ± 8.78 | 59.8 ± 9.83 | 0.152 | 57.7 ± 9.9 | 59.8 ± 9.8 | 0.039 |
| CIHD classification | ||||||||||
| ≤1-vessel coronary disease/unknown | 142 (50.5) | 125 (57.6) | 0.139 | 77 (46.7) | 92 (56.1) | 84 (58.3) | 0.087 | 108 (55.4) | 136 (51.7) | 0.494 |
| ≥2-vessel coronary disease | 139 (49.5) | 92 (42.4) | 88 (53.3) | 72 (43.9) | 60 (41.7) | 87 (44.6) | 127 (48.3) | |||
| CIHD symptoms d | ||||||||||
| Asymptomatic | 228 (81.1) | 176 (81.1) | 0.559 | 123 (75.0) | 132 (80.5) | 129 (89.6) | 0.015 | 157 (80.9) | 215 (81.4) | 0.515 |
| CCS I | 39 (13.9) | 34 (15.7) | 31 (18.9) | 27 (16.5) | 13 (9.0) | 31 (16.0) | 36 (13.6) | |||
| CCS II-IV | 14 (5) | 7 (3.2) | 10 (6.1) | 5 (3.0) | 2 (1.4) | 6 (3.1) | 13 (4.9) | |||
| Previous myocardial infarction | 102 (36.2) | 69 (31.8) | 0.355 | 67 (40.6) | 49 (29.9) | 44 (30.6) | 0.074 | 73 (37.4) | 89 (33.7) | 0.468 |
| Coronary revascularisation | 159 (56.4) | 115 (53.0) | 0.507 | 94 (57.0) | 95 (57.9) | 71 (49.3) | 0.258 | 107 (54.9) | 146 (55.3) | 1.000 |
| Coronary artery bypass graft | 47 (16.7) | 35 (16.1) | 0.969 | 36 (21.8) | 27 (16.5) | 13 (9.0) | 0.009 | 31 (15.9) | 49 (18.6) | 0.536 |
| Heart failure | 62 (22.0) | 58(26.7) | 0.261 | 50 (30.3) | 34 (20.7) | 29 (20.1) | 0.057 | 41 (21.0) | 68 (25.8) | 0.286 |
| Risk factors | ||||||||||
| BMI (kg/m2) | 26.7 ± 2.1 | 34.5 ± 3.6 | <0.001 | 30.1 ± 4.6 | 29.5 ± 4.7 | 30.4 ± 4.7 | 0.596 | 30.0 ± 4.7 | 30.1 ± 4.8 | 0.897 |
| Hypertension | 251 (89.0) | 210 (96.8) | 0.002 | 154 (93.3) | 155 (94.5) | 131 (91.0) | 0.468 | 177 (90.8) | 245 (92.8) | 0.537 |
| Blood pressure (mmHg) | ||||||||||
| systolic | 137 ± 18 | 139 ± 17 | 0.244 | 137 ± 17 | 137 ± 17 | 140 ± 17 | 0.079 | 136 ± 18 | 138 ± 17 | 0.365 |
| diastolic | 78 ± 10 | 80 ± 10 | 0.003 | 78 ± 10 | 79 ± 9 | 80 ± 9 | 0.073 | 79 ± 10 | 78 ± 9 | 0.328 |
| Hyperlipidaemia | 242 (85.8) | 187 (86.2) | 1.000 | 141 (85.5) | 147 (89.6) | 120 (83.3) | 0.258 | 169 (86.7) | 226 (85.6) | 0.851 |
| Total Cholesterol (mg/dl) | 156 ± 40 | 159 ± 37 | 0.421 | 151 ± 39 | 158 ± 40 | 162 ± 40 | 0.013 | 154 ± 37 | 160 ± 42 | 0.103 |
| LDL-C (mg/dl) | 91 ± 36 | 94 ± 32 | 0.377 | 88 ± 35 | 91± 36 | 96 ± 36 | 0.041 | 90 ± 30 | 94 ± 38 | 0.186 |
| Triglycerides (mg/dl) | 166 ± 94 | 204 ± 103 | <0.001 | 187 ± 100 | 183 ± 113 | 175 ± 113 | 0.314 | 175 ± 93 | 189 ± 106 | 0.154 |
| Smoking | ||||||||||
| Never | 115 (40.8) | 70 (32.3) | 0.148 | 52 (31.5) | 63 (38.4) | 57 (39.6) | 0.002 | 75 (38.5) | 94 (35.6) | 0.755 |
| Current | 29 (10.3) | 26 (12.0) | 31 (18.8) | 9 (5.5) | 12 (8.3) | 22 (11.3) | 28 (10.6) | |||
| Former | 138 (48.9) | 121 (55.8) | 82 (49.7) | 92 (56.1) | 75 (52.1) | 98 (50.3) | 142 (53.8) | |||
| Alcoholic drinks per week | 4.2 ± 7.2 | 4.0 ± 7.5 | 0.843 | 4.6 ± 8.2 | 3.9 ± 5.2 | 4.3 ± 5.2 | 0.753 | 3.3 ± 5.0 | 4.8 ± 8.5 | 0.026 |
| Exercise capacity e | ||||||||||
| Peak V̇O2 (ml/kg/min) | 19.8 ± 4.5 | 17.1 ± 4.2 | <0.001 | 14.8 ± 2.7 | 18.7 ± 2.8 | 23.0 ± 2.8 | <0.001 | 19.1 ± 4.9 | 18.3 ± 4.3 | 0.077 |
| Peak V̇O2 (ml/min) | 1610 ± 413 | 1770 ± 464 | <0.001 | 1360 ± 259 | 1650 ± 294 | 2080 ± 434 | <0.001 | 1720 ± 441 | 1669 ± 442 | 0.155 |
| Pred. peak V̇O2 (%) | 81.4 ± 16.2 | 82.6 ± 17.6 | 0.466 | 64.5 ± 8.0 | 82.3 ± 4.2 | 101 ± 10.2 | <0.001 | 83.3 ± 17.3 | 80.7 ± 16.7 | 0.114 |
| Work load (Watt) | 128 ± 42 | 130 ± 42 | 0.598 | 101 ± 26 | 128 ± 30 | 166 ± 30 | <0.001 | 132 ± 43 | 127 ± 41 | 0.291 |
| RER | 1.10 ± 0.09 | 1.07 ± 0.10 | <0.001 | 1.06 ± 0.10 | 1.11 ± 0.10 | 1.10 ± 0.10 | <0.001 | 1.09 ± 0.10 | 1.09 ± 0.10 | 0.639 |
| VT1 not determinable | 11 (4.1) | 12 (5.9) | 0.468 | 17 (10.3) | 5 (3.0) | 1 (0.7) | <0.001 | 9 (4.8) | 12 (4.8) | 1.000 |
| V̇O2 at VT1 (ml/min/kg) | 11.7 ± 2.3 | 10.3 ± 1.9 | <0.001 | 9.7 ± 1.8 | 10.8 ± 1.5 | 12.7 ± 1.5 | <0.001 | 11.2 ± 2.5 | 11.1 ± 2.09 | 0.765 |
| Medication | ||||||||||
| Antidiabetic medication | 249 (88.3) | 187 (86.2) | 0.497 | 148 (89.7) | 145 (88.4) | 127 (88.2) | 0.915 | 170 (87.2) | 231 (87.5) | 1.000 |
| Number oral anti-diabetics | ||||||||||
| 0 | 44 (15.6) | 40 (18.4) | 0.725 | 25 (15.2) | 26 (15.9) | 23 (16.0) | 0.095 | 33 (16.9) | 44 (16.7) | 0.172 |
| 1 | 114 (40.4) | 91 (41.9) | 62 (37.6) | 60 (36.6) | 74 (51.4) | 89 (45.6) | 100 (37.9) | |||
| 2 | 91 (32.3) | 66 (30.4) | 57 (34.5) | 57 (34.8) | 38 (26.4) | 59 (30.3) | 87 (33.0) | |||
| ≥3 | 32 (11.3) | 20 (9.2) | 21 (12.7) | 20 (12.2) | 9 (6.2) | 14 (7.2) | 33 (12.5) | |||
| Oral anti-diabetics | 237 (84.0) | 177 (81.6) | 0.484 | 140 (84.8) | 137 (83.5) | 121 (84.0) | 0.974 | 162 (83.1) | 220 (83.3) | 1.000 |
| Metformin | 207 (73.4) | 155 (71.4) | 0.650 | 125 (75.8) | 119 (72.6) | 105 (72.9) | 0.805 | 141 (72.3) | 195 (73.9) | 0.790 |
| DPP4-inhibitors | 78 (27.7) | 37 (17.1) | 0.007 | 43 (26.1) | 46 (28.2) | 24 (16.7) | 0.044 | 43 (22.1) | 62 (23.5) | 0.803 |
| SGLT2-inhibitors | 64 (22.7) | 45 (20.7) | 0.663 | 36 (21.8) | 39 (23.9) | 29 (20.1) | 0.724 | 36 (18.5) | 63 (23.9) | 0.202 |
| Sulfonylurea | 15 (5.3) | 11 (5.1) | 1.000 | 12 (7.3) | 8 (4.9) | 5 (3.5) | 0.318 | 8 (4.1) | 14 (5.3) | 0.708 |
| GLP-1-analoga | 29 (10.3) | 36 (16.6) | 0.054 | 24 (14.5) | 22 (13.5) | 15 (10.4) | 0.539 | 21 (10.8) | 40 (15.2) | 0.219 |
| Insulin | 67 (23.8) | 70 (32.3) | 0.047 | 56 (33.9) | 40 (24.4) | 38 (26.4) | 0.137 | 56 (28.7) | 73 (27.7) | 0.884 |
| Cardiovascular medication | ||||||||||
| ACE inhibitor/AR blocker | 230 (81.6) | 193 (88.9) | 0.032 | 136 (82.4) | 141 (86.0) | 123 (85.4) | 0.635 | 165 (84.6) | 225 (85.2) | 0.961 |
| Anti-platelet | 205 (72.7) | 144 (66.4) | 0.152 | 119 (72.1) | 108 (65.9) | 104 (72.2) | 0.362 | 142 (72.8) | 185 (70.1) | 0.591 |
| ASA | 194 (68.8) | 135 (62.2) | 0.149 | 108 (65.5) | 104 (63.4) | 99 (68.8) | 0.613 | 132 (67.7) | 177 (67.0) | 0.964 |
| Clopidogrel | 23 (8.2) | 13 (6.0) | 0.452 | 20 (12.1) | 11 (6.7) | 5 (3.5) | 0.014 | 13 (6.7) | 21 (8.0) | 0.734 |
| Ticagrelor | 5 (1.8) | 6 (2.8) | 0.544 | 5 (3.0) | 1 (0.6) | 5 (3.5) | 0.172 | 8 (4.1) | 2 (0.8) | 0.021 |
| Prasugrel | 2 (0.7) | 3 (1.4) | 0.657 | 3 (1.8) | 1 (0.6) | 1 (0.7) | 0.629 | 5 (2.6) | 0 (0) | 0.013 |
| Lipid-lowering drugs | 234 (83.0) | 186 (85.7) | 0.480 | 139 (84.2) | 142 (86.6) | 120 (83.3) | 0.710 | 169 (86.7) | 219 (83.0) | 0.339 |
| Statins | 228 (80.9) | 184 (84.8) | 0.302 | 136 (82.4) | 138 (84.1) | 119 (82.6) | 0.903 | 167 (85.6) | 214 (81.1) | 0.244 |
| Atorvastatin/Rosuvastatin | 138 (48.9) | 103 (47.5) | 0.814 | 82 (49.7) | 72 (43.9) | 76 (52.8) | 0.282 | 92 (47.2) | 128 (48.5) | 0.855 |
| Other statins | 90 (31.9) | 81 (37.3) | 0.243 | 54 (32.7) | 66 (40.2) | 43 (29.9) | 0.136 | 75 (38.5) | 86 (32.6) | 0.227 |
| Other lipid-lowering drugs | 44 (15.6) | 37 (17.1) | 0.755 | 26 (15.8) | 35 (21.3) | 19 (13.2) | 0.145 | 32 (16.4) | 42 (15.9) | 0.987 |
| Diuretics | 86 (30.5) | 110 (50.7) | <0.001 | 70 (42.4) | 61 (37.2) | 53 (36.8) | 0.515 | 71 (36.4) | 106 (40.2) | 0.473 |
| Aldosterone antagonists | 25 (8.9) | 27 (12.4) | 0.251 | 25 (15.2) | 14 (8.5) | 9 (6.2) | 0.025 | 20 (10.3) | 30 (11.4) | 0.822 |
| Beta-blocker | 203 (72.0) | 161 (74.2) | 0.654 | 128 (77.6) | 121 (73.8) | 96 (66.7) | 0.094 | 138 (70.8) | 197 (74.6) | 0.417 |
| Anticoagulation | 57 (20.2) | 41 (18.9) | 0.800 | 42 (25.5) | 34 (20.7) | 19 (13.2) | 0.026 | 32 (16.4) | 56 (21.2) | 0.241 |
| Phenprocoumon | 17 (6.0) | 9 (4.1) | 0.463 | 12 (7.3) | 10 (6.1) | 4 (2.8) | 0.205 | 6 (3.1) | 18 (6.8) | 0.117 |
| NOAC | 40 (14.2) | 32 (14.7) | 0.961 | 30 (18.2) | 24 (14.6) | 15 (10.4) | 0.156 | 26 (13.3) | 38 (14.4) | 0.851 |
| Nitrates/Ranolazin | 13 (4.6) | 12 (5.5) | 0.795 | 7 (4.2) | 10 (6.1) | 6 (4.2) | 0.661 | 10 (5.1) | 14 (5.3) | 1.000 |
| Sacubitril/Valsartan | 6 (2.1) | 2 (0.9) | 0.429 | 6 (3.6) | 0 (0) | 2 (1.4) | 0.022 | 2 (1.0) | 5 (1.9) | 0.704 |
| Quality of life f | ||||||||||
| PCS | 46.3 ± 8.8 | 42.3 ± 10.5 | <0.001 | 42.2 ± 10.4 | 45.2 ± 9.0 | 47.4 ± 9.1 | <0.001 | 43.1 ± 10.4 | 45.6 ± 9.1 | 0.013 |
| MCS | 52.4 ± 8.1 | 52.1 ± 10.4 | 0.692 | 52.1 ± 9.7 | 53.1 ± 8.1 | 51.8 ± 8.1 | 0.844 | 50.5 ± 10.0 | 53.6 ± 8.2 | <0.001 |
BMI: body mass index; V̇O2: oxygen consumption; HLS-EU-Q16: Health literacy score; T2DM: type 2 diabetes mellitus; HbA1c: glycated haemoglobin; CIHD: chronic ischaemic heart disease; CCS scale: Canadian Cardiovascular Society angina grading scale; LDL-C: low density lipoprotein cholesterol; RER: respiratory exchange ratio; VT: ventilatory threshold; DPP4: dipeptidyl peptidase-4; SGLT2: sodium/glucose cotransporter 2; GLP-1: glucagon-like peptide-1; ACE: angiotensin converting-enzyme; AR: androgen receptor; ASA: acetylsalicylic acid; NOAC: novel oral anticoagulants; PCS: physical component score; MCS: mental component score.
Data are presented as mean ± standard deviation or absolute and (relative) frequency.
aGrouped according to BMI; BMI ≤ 30 kg/m2 “non-obese”, BMI >30 kg/m2 “obese”.
bGrouped according to exercise capacity; predicted peak V̇O2 < 75% “severely restricted”, 75–89.9% “mildly to moderately restricted” and ≥ 90% “normal”.
cGrouped according to HLS; HLS > 12 “sufficient HL”, “not sufficient HL” otherwise.
dCanadian Cardiovascular Society angina grading scale describes the severity of angina pectoris. 0= no symptoms, I = angina pectoris with strenuous exertion, II-IV= angina with moderate/mild exertion or at rest.
eCardiopulmonary exercise testing was performed in 473 patients, exercise testing in 25 patients, 1 patient none.
fMeasured via SF-36 questionnaire. Available data from 393 patients.
Among 473 patients with available CPET data, 165 (34.9%) had a severely restricted, 164 (34.7%) a mildly to moderately restricted and 144 (30.4%) a normal exercise capacity. Mean duration of CIHD and T2DM were not significantly different between groups, however, mean HbA1c was highest in the severely restricted group (7.0 ± 1.0 vs 6.8 ± 0.9 vs 6.7 ± 0.9%, p = 0.002). Similarly, these patients were most likely to have CIHD symptoms (p = 0.015) and the highest prevalence of previous coronary bypass graft implants (21.8 vs 16.5 vs 9.0%, p = 0.009). Total cholesterol (151 ± 39 vs 158 ± 40 vs 162 ± 40 mg/dl, p = 0.013) and LDL-C (88 ± 35 vs 91 ± 36 vs 96 ± 36 mg/dl, p = 0.041) were significantly different with the highest values in the fittest subgroup. QoL-PCS was higher with increasing exercise capacity (42.2 ± 10.4, 45.2 ± 9.0 and 47.4 ± 9.1, p < 0.001) while no significant difference in QoL-MCS could be observed.
Data on HL was available from 459 participants and 195 (42.5%) patients were classified with a non-sufficient HL. Both groups showed no significant differences in age, sex, education, CIHD or T2DM history and duration. Also, regarding risk factors and exercise capacity, no further statistically significant differences could be observed. However, patients with sufficient HL were diagnosed with CIHD at an older age (59.8 ± 9.8 vs 57.7 ± 9.9 years, p = 0.039) and reported a higher QoL with higher mean values in PCS (45.6 ± 9.1 vs 43.1 ± 10.4, p = 0.013) and MCS (53.6 ± 8.2 vs 50.5 ± 10.0, p < 0.001).
LeIKD in context of other studies on T2DM and CV risk
In comparison to selected trials on T2DM and CIHD or CV risk (Table 3), patients included in the present study were notably older. Sex distribution in LeIKD was similar to the exercise intervention trial EXCADI, but Look AHEAD, CAROLINA and CARMELINA included a higher proportion of women. Besides CARMELINA, LeIKD enrolled patients with the longest average duration of T2DM.
Table 3.
LeIKD in context of other studies on T2DM and CV risk.
| Lifestyle intervention trials | Clinical drug-intervention trials | ||||
|---|---|---|---|---|---|
| Look AHEAD a | EXCADI | LeIKD | CAROLINA | CARMELINA d | |
| Primary study aims | Long-term effects of an intensive lifestyle-intervention on weight reduction and CV outcome | Effects of a 12-month exercise training on HbA1c and peak V̇O2 | Effects of an individual telemedicine-based lifestyle intervention on CV risk factors and health literacy | Effects of treatment with linagliptin vs glimepiride on CV safety in patients with early T2DM and increased CV risk or established atherosclerotic CVD | Effects of linagliptin on CV and kidney outcomes in a study population enriched for cardio-renal risk |
| Key eligibility criteria | T2DM, overweight (BMI ≥ 25 kg/m2; if on insulin, BMI ≥ 27 kg/m2), blood pressure < 160/100 mmHg, HbA1c ≤ 11%, triglycerides < 600 mg/dl, passed exercise test | T2DM and CIHD | CIHD (ICD-10: I20-I25), T2DM (ICD-10: E11), HbA1c ≥ 6.5% or antidiabetic medication, permission to do physical exercise | T2DM, HbA1c 6.5 - 8.5%, pre-existing CVD OR specified diabetes end-organ damage OR age ≥ 70 years OR two or more specified CV risk factors | T2DM, HbA1c of ≥ 6.5% and ≤ 10.0%, BMI ≤ 45 kg/m2, high risk of CV events |
| Number | 5145 | 137 | 499 | 6041 | 3990 |
| Years screening | 2001–2004 | 2010–2012 | 2019–2020 | 2010–2012 | 2013–2016 |
| Baseline characteristics of randomised cohort | |||||
| Age (years) | 59 ± 6.8 | 63.1 ± 7.9 | 68.3 ± 7.7 | 64.0 ± 9.5 | 64.8 ± 8.8 |
| Female sex | 3058 (59.4) | 22 (16.1) | 81 (16.2) | 2419 (40) | 1238 (31.0) |
| BMI (kg/m2) | 35.9 ± 5.8 | 29.2 ± 5.0 | 30.1 ± 4.8 | 30.1 ± 5.1 | 31.0 ± 5.1 |
| Blood pressure (mmHg) | |||||
| Systolic | 129 ± 17 | 139 ± 17 | 137 ± 17 | 136 ± 16 | 139.2 ± 16.8 |
| Diastolic | 70 ± 10 | — | 79 ± 10 | 79 ± 10 | 78.2 ± 10.2 |
| Smokers | 228 (4.4) | 23 (17) | 55 (11.0) | 1188 (19.7) b | 478 (12.0) |
| Hypertension | 4132 (80.3) | 100 (73) | 461 (92.4) | 5418 (90) b | — |
| Heart failure | — | 11 (8) | 120 (24.0) | 271 (4.5) b | — |
| Myocardial infarction | — | 62 (45) | 171 (34.3) | — | — |
| Duration of T2DM (years) | 6.8 ± 6.5 | 9 [5 – 15] | 12.3 ± 8.4 | 6.2 [2.9 – 11.0] | 13.8 ± 9.3 |
| HbA1c (%) | 7.28 ± 1.2 | 7.4 [6.8 – 8.3] | 6.9 ± 0.9 | 7.2 ± 0.6 | 8.0 ± 1.0 |
| History of CVD/CIHD | 726 (14) (CVD) | 137 (100) (CIHD) | 502 (100) (CIHD) | 6014 (99.7) (CVD) 1905 (31.7) b (CIHD) |
3990 (100) (CVD) |
| Cholesterol (mg/dl) | 191 ± 38 | 152 [132 – 179] | 157 ± 39 | 177 ± 44 | 169 ± 48 |
| LDL-C (mg/dl) | 112 ± 32 | 78 [62 – 101] | 92 ± 34 | 95 ± 36 | 89 ± 39 |
| Triglycerides (mg/dl) | 181 ± 118 | 124 [97 – 168] | 182 ± 100 | 144 [105 – 198] | 185 ± 134 |
| Medication | |||||
| Statins | 2221 (43.2) | 128 (93) | 412 (82.6) | 3900 (64.8) b | 2979 (74.7) |
| Beta-blocker | 957 (18.6) | 106 (77) | 364 (72.9) | 2352 (39.1) b | 2563 (64.2) |
| ACE/ARBs | 1620 (31.5)/578 (11.2) | 97 (70) | 423 (84.8) | 2472 (41.1) c /1884 (31.3) b | 3214 (80.6) |
| Diuretics | 1467 (28.5) | — | 196 (39.3) | 2236 (37) b | 1848 (46.3) |
| Metformin | 2688 (52.2) | 101 (74) | 362 (72.5) | 4982 (82.5) | 2657 (66.6) |
| Sulfonylurea | 2295 (44.6) | 48 (35) | 26 (5.2) | 1728 (28.6) | 1486 (37.2) |
| Insulin | 750 (14.6) | 26 (19) | 137 (27.5) | 0 (0) c | 2077 (52.1) |
| Exercise capacity | |||||
| Peak V̇O2 (ml/kg/min) | — | 24.7 ± 5.9 | 18.6 ± 4.6 | — | — |
| VT (ml/kg/min) | — | 18.9 ± 4.1 | 11.1 ± 2.3 | — | — |
BMI: body mass index; T2DM: type 2 diabetes mellitus; HbA1c: glycated haemoglobin; CV: cardiovascular; CVD: cardiovascular disease; CIHD: chronic ischaemic heart disease; LDL-C: low density lipoprotein cholesterol; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; VO2: oxygen consumption; VT: ventilatory threshold. Data are reported as mean ± standard deviation, absolute and relative (%) frequency or median [1st quartile – 3rd quartile].
aIf mean ± standard deviation was only available for separate treatment groups, overall values were calculated as follows: Mean Overall = (M1 x N1 + M2 x N2)/(N1 + N2) √SD2 = (1/(N1 + N2 – 1) x ((N1 – 1) x SD12 + (N2 – 1) x SD22 + (N1 x N2)/(N1 + N2) x (M1 – M2)2) M = mean; N = number; SD = standard deviation.
bData originate from main results publication, n = 6033.
cinsulin was an exclusion criteria.
dReported data is from a subsample with established CV disease, defined according to study inclusion criteria as albuminuria (UACR ≥30 mg/g or ≥30 μg albumin/min or ≥30 mg albumin/24 h) and prevalent macrovascular disease (one or more of the following: confirmed history of myocardial infarction; advanced coronary artery disease; high-risk single-vessel coronary artery disease; history of ischemic or haemorrhagic stroke; presence of carotid artery disease; presence of peripheral artery disease.
Baseline HbA1c was lower than in the compared trials, even in trials with similar inclusion criteria regarding HbA1c values like CAROLINA and CARMELINA. Common risk factors for CV diseases (e.g. blood pressure, history of smoking, blood lipids, BMI) were similar between the trials. However, the prevalence of hypertension was higher in LeIKD (92.4%) than in the other trials and patients randomised to Look AHEAD (inclusion criteria: BMI ≥ 27 kg/m2) had a higher mean BMI of 35.9 ± 5.8 kg/m2. Regarding medication, LeIKD showed the lowest percentage of patients prescribed with sulfonylureas (5.2%). EXCADI was the only compared trial that also conducted CPET. Mean peak V̇O2 (24.7 ± 5.9 vs 18.6 ± 4.6 mL/min/kg) and VT1 (18.9 ± 4.1 vs 11.1 ± 2.3 mL/min/kg) were higher in EXCADI than in LeIKD.
Discussion
To date, LeIKD is one of the largest studies in patients with CIHD and T2DM providing a tremendous clinical and lifestyle-related dataset including CPET among 499 patients. Accordingly, this manuscript contributes to a better characterisation and understanding of this multi-morbid patient population and provides insights into the current healthcare and disease management of patients in Germany.
The LeIKD study population was characterised by advanced age, predominantly male gender, relatively high educational status and a high prevalence of comorbidities and risk factors. To reduce microvascular complications and CV events, a target HbA1c of < 7% is recommended in most T2DM patients, while in older patients less stringent targets of < 8% can be considered on an individual basis. 2 Despite the high prevalence of comorbidities and risk factors in patients included in the present trial, the mean HbA1c (6.9 ± 0.9%) was within target range and only 36% had an HbA1c ≥ 7% (11.5% with an HbA1c ≥ 8%). Compared to a subgroup of the EAV survey (2016-2017, across 27 countries in Europe) with both CIHD and T2DM (n=2452) and a mean HbA1c of 7.2 ± 1.7% (45% with an HbA1c ≥ 7%; 26% with an HbA1c ≥ 8%), 5 this suggests a predominately adequate glycaemic control within the present study population. This is also supported by the higher number of patients with anti-diabetic medication in LeIKD (87%) compared to EAV (75%), 5 including a similar proportion of prescribed insulin (EAV with 30% 5 vs LeIKD with 27.5%). This could, at least in part, be due to the fact that all patients were included in a DMP for CIHD or T2DM prior to the study inclusion. DMP consist of regular medical visits and offers for educational programmes.
In LeIKD, the use of glucose-lowering drugs with cardio-protective capacity, particularly SGLT-2 inhibitors (21.8%) and GLP-1 receptor antagonists (13.0%) was higher than in EAV (1% each), 5 indicating an accelerating implementation of these drug classes in clinical practice during the last years. Despite a high prevalence of hypertension (92.4%), the mean systolic blood pressure (137 ± 17 mmHg) was within the target values of 130–140 mmHg for older patients (>65 years). 6 However, this observation is biased by the requirement of an exercise training approval including adequate blood pressure behaviour. In contrast, LDL-C was rather poorly managed. According to current guideline recommendations, LDL-C should be less than 55 mg/dl in patients with very high CV risk. 2 In the present study, only 41 patients (8%) reached this target (24% with LDL-C < 70 mg/dl as previously recommended until 2019), with an average LDL-C of 92 ± 34 mg/dl. This replicates the results of the EAV survey with almost two-thirds of patients above the LDL-C target of less than 70 mg/dl. 5 In patients with T2DM and CIHD, lipid lowering drug therapy is indicated as a class IA recommendation, 2 however, 79 study patients were not prescribed any statins or alternative lipid lowering drugs at baseline.
Besides medical treatment, LeIKD baseline characteristics also indicate some potential for the improvement of lifestyle-related measures. Although obesity has long been recognised as a prevalent driver of several cardio-metabolic risk factors including T2DM, it is still not reasonably treated compared to other modifiable risk factors. 21 Similar to EAV (88.5% with BMI > 25 kg/m2 and 49% with BMI higher > 30 kg/m2), 5 89.2% and 43.5% of patients included in LeIKD were classified as overweight and obese, respectively. In the present study, obese patients had an increased prevalence of hypertension, higher levels of triglycerides and a lower QoL-PCS. Interestingly, mean HbA1c and severity of CIHD were not significantly different between obese and non-obese patients. This could in part be explained by a lower mean age of the obese patients, as HbA1c levels and severity of CIHD are expected to increase with age. Furthermore, T2DM and CIHD were diagnosed at a younger age in the obese subgroup. While peak V̇O2 was significantly lower when adjusted to body weight, absolute peak V̇O2 and %-predicted peak V̇O2 were similar between obese and non-obese patients. This highlights the common pitfall of adjusting peak V̇O2 to body weight (instead of lean body mass) and suggests that in the present study, obesity was not significantly associated with exercise capacity during non-weight-bearing cycling.
Compared to CPET reference values, 12 only one third of patients in LeIKD had an adequate exercise capacity (≥90% predicted). Low exercise capacity is one of the most relevant and at the same time one of the most poorly treated risk factors for the development and progression of T2DM and CIHD.22,23 Accordingly in the present study, patients with lower exercise capacity had more severe CIHD, significantly higher levels of HbA1c and a lower QoL-PCS. On the other hand, duration of CIHD or T2DM were not significantly different between subgroups of exercise capacity, although an impaired exercise capacity may already be observed in the early beginnings of diabetic pathology. 24 Nevertheless, it is concerning that the subgroup with the lowest exercise capacity still had the highest proportion of current smokers. Interestingly, we observed significantly higher values for total and LDL cholesterol with higher exercise capacity, which might be explained by a more intense treatment in the sicker patient population. There were no significant differences in the percentage of heart failure patients among the different exercise capacity groups. However, this is most likely be explained by an insufficient power, as only 120 patients had a confirmed diagnosis of heart failure. Similarly, with only 20% of patients being prescribed with SGLT2-inhibitors, the power may be too small to detect a possible difference between subgroups of exercise capacity. Furthermore, potential positive effects of SGLT2-inhibitors on exercise capacity 25 may be overlaid by the fact that SGLT2-inhibitors are more likely be prescribed in patients with higher disease severity and concomitant heart failure. 2 The latter could also be the reason for the significant differences observed for the prescription of DPP4-inhbitors between subgroups of peak V̇O2.
An appropriate management of modifiable lifestyle-related risk factors requires that patients have a certain understanding of their disease. Low HL as well as a low general and health-related education are associated with a higher prevalence of CV risk factors, T2DM and CV disease, low diabetes specific knowledge and a decreased access to healthcare screening or basic services.8,26,27 A limited HL has been shown to be associated with a lower participation rate in clinical trials 26 and programmes on weight reduction, healthy diet and physical activity. 28 In LeIKD, about 40% of the study population had an inadequate (15.9%) or problematic HL (26.6%), which is comparable to results of a German national health survey (GEDA2014/2015-EHIS) in patients with CV disease (44% with insufficient HL). 8 However, only 2.4% of the LeIKD participants (compared to 18% in EAV 5 ) had a low education level. One possible reason for this might be that historically, the TK health insurance has primarily insured individuals with technical professions or a technical academic education. Contrary to the GEDA2014/2015-EHIS survey, 8 education level, sex or BMI were not significantly different between subgroups of HL in the present study. We also found no significant differences between HL groups for CIHD and T2DM disease severity or established risk factors, except for alcohol consumption, with – surprisingly – a higher consumption in the group with sufficient HL. Nevertheless, consistent with the analyses in subgroups of BMI and peak V̇O2, patients with lower HL had a lower QoL-PCS, which emphasises the importance of nutritional, exercise and HL training in these patients. Furthermore, only for HL we found significant differences in the QoL-MCS. This could indicate that despite non-significant differences in disease severity and risk factor burden, patients with a low HL experience more impairment due to mental health related factors and require additional support and patient empowerment in the management of their health condition.
LeIKD study population in context of other studies on T2DM and CV risk
Compared to other clinical trials in CIHD and T2DM, there were no relevant differences in baseline characteristics that could limit the generalisability of the results of the present trial. However, in contrast to the trials selected for comparison, the LeIKD trial enrolled the oldest study population with the lowest baseline HbA1c and a low percentage of female participants (16%), the latter being comparable to the EXCADI trial (16% females), another lifestyle intervention trial in CIHD and T2DM. While female participation is typically low (around 27%) in clinical CIHD trials 29 and less women than men attend cardiac prevention and rehabilitation programmes, 30 the pharmacological trials CAROLINA (40%) and CARMELINA (31%) as well as the Look AHEAD lifestyle intervention in T2DM (59%) enrolled a considerably higher percentage of female participants. In EXCADI, the only compared trial applying CPET, mean peak V̇O2 was about ca. 30% higher, which could, at least in part, be explained by the younger mean age, a lower prevalence of heart failure and the fact that CPET was performed on treadmill compared to bicycle ergometers in LeIKD. 31
Recruitment strategy
By applying a novel recruitment strategy based on medical records (via ICD-10 codes) with initial contact through one of the largest public health insurances in Germany, a large number of potential participants could be identified and randomised within 13 months. The applied eligibility criteria were chosen in order to include as many suitable patients with CIHD and T2DM as possible. However, many initially selected patients had to be excluded during the screening process because of not meeting the target population (e.g. HbA1c < 6.5% without antidiabetic medication; documented exclusion of arteriosclerosis/obvious miscoding for ICD-10: I20-I25). Furthermore, a large number of patients were not clinically stable (e.g. uncontrolled hypertension, signs of acute ischaemia) or did not receive the required medical treatment to safely perform a home-based exercise intervention, or refused to participate due to concerns about the use of telemedical devices. While owning a smartphone was not a prerequisite for inclusion, participation required a basic willingness to use technical devices.
Limitations
This study has several limitations. All patients had to be insured at the TK health insurance fund, which may have influenced some patient characteristics. While the diagnosis of T2DM was confirmed during screening (HbA1c ≥ 6.5% or anti-diabetic medication), the diagnosis of CIHD was primarily based on the medical records (ICD-10: I20-I25) provided by TK health insurance, which may have led to the inclusion of some patients with incorrect coding. Based on the study inclusion criteria “able to perform physical exercise”, “clinically stable” and with “optimal medical cardiac treatment”, disease severity is likely to be lower than in pharmacological trials and the general population with T2DM and CIHD. Furthermore, sicker patients might be less willing to participate in lifestyle intervention trials and an additional self-selection may have occurred due to the required use of a smartphone and telemedical devices. Nevertheless, despite a reasonably well managed HbA1c, clinical characteristics were quite similar to other lifestyle and pharmacological trials in this patient population.
Conclusion
To date, LeIKD provides one of the largest clinical and lifestyle-related datasets including CPET from 499 patients with CIHD and T2DM, who are likely to be representative for this special patient population. Exercise capacity was confirmed to be linked to the severity of CIHD and T2DM, which reinforces the relevance of intensifying lifestyle intervention strategies in this multi-morbid, heterogeneous and understudied high-risk patient population.
Acknowledgement
The authors thank all patients and staff at study sites who participated in the trial.
Appendix.
Notation
- ACE
angiotensin-converting-enzyme
- AR
androgen receptor
- ASA
acetylsalicylic acid
- BMI
body mass index
- CARMELINA
CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin
- CAROLINA
CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes
- CCS
Canadian Cardiovascular Society angina grading scale
- CIHD
Chronic ischaemic heart disease
- COPD
chronical obstructive pulmonary disease
- COVID-19
coronavirus disease 2019
- CPET
cardiopulmonary exercise testing
- CV
cardiovascular
- DMP
Disease management programme
- DPP4
dipeptidyl peptidase-4
- EAV
EUROASPIRE V (European Action on Secondary and Primary Prevention by Intervention to Reduce Events)
- ECG
electrocardiogram
- ENHANCE
Enhancing Adherence in Type 2 Diabetes trial
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated haemoglobin
- HDL-C
high density lipoprotein cholesterol
- HFmrEF
heart failure with mildly reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HL
health literacy
- HLS-EU-Q16
Health literacy score
- ICD
International Statistical Classification of Diseases and Related Health Problems
- LDL-C
low density lipoprotein cholesterol
- LeIKD
Lifestyle Intervention in Chronic Ischaemic Heart Disease and Type 2 Diabetes
- LS
lifestyle intervention
- LVEF
left ventricular ejection fraction
- MCS
mental component score
- NOAC
novel oral anticoagulants
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- PCS
physical component score
- QoL
quality of life
- RER
respiratory exchange ratio
- SGLT-2
sodium/glucose cotransporter 2
- SHIP
Study of Health in Pomerania
- T2DM
Type 2 diabetes mellitus
- TK
Techniker Krankenkasse
- UC
usual care
- VT
ventilatory threshold
- V̇O2
oxygen consumption
Footnotes
Author Contributions: SMTD and JK wrote the manuscript with contribution of SM and MH. JK had full access to all data in the study and takes responsibility for the accuracy of the data analysis. All authors contributed to the study design or data acquisition and interpretation, critically revised the manuscript and gave their final approval.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FE reported receiving grants from DFG, BMBF, Servier, and personal fees from Bayer Healthcare, Merck, Novartis, Servier, Berlin Chemie, Boehringer Ingelheim, Vifor Pharma, AstraZeneca and PharmaCosmos outside the submitted work. JB reported grants from AstraZeneca and personal fees from Amgen outside the submitted work. EBW reported receiving personal fees from Novartis (honoraria for lectures and advisory board activities), Boehringer Ingelheim (honoraria for advisory board activities) and CVRX (honoraria for lectures) outside the submitted work. BH reported receiving personal fees from IDS Diagnostic Systems outside the submitted work. MH reported receiving institutional funding from Techniker Krankenkasse (Health Insurance Company, Hamburg, Germany), grants from Novartis (principal investigator of the Activity Study in HFrEF) and personal fees from Bristol-Myers Squibb, Berlin Chemie-Menarini, Novartis, Daiichi-Sankyo, AstraZeneca, Roche, Abbott (advisory board on exercise and diabetes), Sanofi, Pfizer, Boehringer Ingelheim and Bayer, and serves as an advisor for Medical Park SE, Germany, outside the submitted work. All other authors (SMTD, JK, FG, JT, IFW, MG, KE, PvK, AD, OW, BW, FF, SN, TN, VA, SM) reported no conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The LeIKD study was funded by the Federal Joint Committee (Innovationsfonds des Gemeinsamen Bundesausschusses, Germany), reference number 01NVF17015.
ORCID iDs
Sophia MT Dinges https://orcid.org/0000-0002-2897-4291
Stephan Mueller https://orcid.org/0000-0003-4890-3981
References
- 1.Global Health Estimates . 2020: deaths by cause, age, sex, by Country and by Region, 2000-2019. Geneva: World Health Organization, 2020. [Google Scholar]
- 2.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. DOI: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini G, Norhammar A, Gyberg V, et al. Is coronary artery disease inevitable in type 2 diabetes? From a glucocentric to a holistic view on patient management. Diabetes Care 2020; 43: 2001–2009. DOI: 10.2337/dci20-0002. [DOI] [PubMed] [Google Scholar]
- 4.Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 2016; 118: 535–546. DOI: 10.1161/circresaha.115.307611. [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini G, de Bacquer D, De Backer G, et al. Screening for glucose perturbations and risk factor management in dysglycemic patients with coronary artery disease—A persistent challenge in need of substantial improvement: a report from ESC EORP EUROASPIRE V. Diabetes Care 2020: 43: 733. DOI: 10.2337/dc19-2165. [DOI] [PubMed] [Google Scholar]
- 6.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2019; 41: 407–477. DOI: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 7.Arnold SV, Bhatt DL, Barsness GW, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American heart association. Circulation 2020; 141: e779–e806. DOI: 10.1161/cir.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diederichs C, Jordan S, Domanska O, et al. Health literacy in men and women with cardiovascular diseases and its association with the use of health care services - Results from the population-based GEDA2014/2015-EHIS survey in Germany. PLoS One 2018; 13: e0208303. DOI: 10.1371/journal.pone.0208303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Korn P, Sydow H, Neubauer S, et al. Lifestyle intervention in chronic ischaemic heart disease and type 2 diabetes (the LeIKD study): study protocol of a prospective, multicentre, randomised, controlled trial. BMJ Open 2021; 11: e042818. DOI: 10.1136/bmjopen-2020-042818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevick MA, Korytkowski M, Stone RA, et al. Biophysiologic outcomes of the enhancing adherence in type 2 diabetes (ENHANCE) trial. J Acad Nutr Diet 2012; 112: 1147–1157. DOI: 10.1016/j.jand.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gignac GE, Szodorai ET. Effect size guidelines for individual differences researchers. Pers Indiv Differ 2016; 102: 74–78. DOI: 10.1016/j.paid.2016.06.069. [DOI] [Google Scholar]
- 12.Gläser S, Ittermann T, Schäper C, et al. Referenzwerte für die Spiroergometrie – Ergebnisse der Study of Health in Pomerania (SHIP). Pneumologie 2013; 67: 58–63. [DOI] [PubMed] [Google Scholar]
- 13.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020–2027. DOI: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 14.Röthlin F, Pelikan J, Ganahl K. Die Gesundheitskompetenz der 15-jährigen Jugendlichen in Österreich. In Abschlussbericht der österreichischen Gesundheitskompetenz Jugendstudie im Auftrag des Hauptverbands der österreichischen Sozialversicherungsträger (HVSV), 2013. [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 16.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular outcome trial of LINAgliptin versus glimepiride in type 2 diabetes (CAROLINA®). Diab Vasc Dis Res 2015; 12: 164–174. DOI: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The CAROLINA randomized clinical trial. JAMA 2019; 322: 1155–1166. DOI: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstock J, Perkovic V, Alexander JH, et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA(®)): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol 2018; 17: 39. DOI: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrkjeland R, Njerve IU, Anderssen S, et al. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: a randomised clinical trial. Diab Vasc Dis Res 2015; 12: 325–333. DOI: 10.1177/1479164115590552. [DOI] [PubMed] [Google Scholar]
- 20.Bray G, Gregg E, Haffner S, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res 2006; 3: 202–215. DOI: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020; 126: 1477–1500. DOI: 10.1161/circresaha.120.316101. [DOI] [PubMed] [Google Scholar]
- 22.Fang ZY, Sharman J, Prins JB, et al. Determinants of exercise capacity in patients with type 2 diabetes. Diab Care 2005; 28: 1643–1648. DOI: 10.2337/diacare.28.7.1643. [DOI] [PubMed] [Google Scholar]
- 23.Winzer EB, Woitek F, Linke A. Physical activity in the prevention and treatment of coronary artery disease. J Am Heart Assoc 2018; 7: e007725. DOI: 10.1161/jaha.117.007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesti L, Pugliese NR, Sciuto P, et al. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol 2020; 19: 134. DOI: 10.1186/s12933-020-01109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar N, Garg A, Bhatt DL, et al. Empagliflozin improves cardiorespiratory fitness in type 2 diabetes: translational implications. Can J Physiol Pharmacol 2018; 96: 1184–1187. DOI: 10.1139/cjpp-2018-0359. [DOI] [PubMed] [Google Scholar]
- 26.Magnani JW, Mujahid MS, Aronow HD, et al. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation 2018; 138: e48–e74. DOI: 10.1161/cir.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruthans J, Mayer O, Jr, De Bacquer D, et al. Educational level and risk profile and risk control in patients with coronary heart disease. Eur J Prev Cardiol 2016; 23: 881–890. DOI: 10.1177/2047487315601078. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz R, Jordan S, Müters S, et al. Population-wide use of behavioural prevention and counselling programmes for lifestyle-related cardiovascular risk factors in Germany. Eur J Prev Cardiol 2012; 19: 849–856. DOI: 10.1177/1741826711410949. [DOI] [PubMed] [Google Scholar]
- 29.Jin X, Chandramouli C, Allocco B, et al. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020; 141: 540–548. DOI: 10.1161/circulationaha.119.043594. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini G, De Bacquer D, Vynckier P, et al. Gender differences in screening for glucose perturbations, cardiovascular risk factor management and prognosis in patients with dysglycaemia and coronary artery disease: results from the ESC-EORP EUROASPIRE surveys. Cardiovasc Diabetol 2021; 20: 38. DOI: 10.1186/s12933-021-01233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasserman K, Hansen J, Sue DY, et al. Principles of exercise testing and interpretation: Including pathophysiology and clinical applications. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2011, p. 1–592. [Google Scholar]