Abstract
The functional lumen imaging probe (FLIP)measures luminal dimensions using impedance planimetry, performed most often during sedated upper endoscopy. Mechanical properties of the esophageal wall and opening dynamics of the esophagogastric junction (EGJ) can be objectively evaluated in esophageal motor disorders, eosinophilic esophagitis, esophageal strictures, during esophageal surgery and in postsurgical symptomatic states. Distensibility index, the ratio of EGJ cross sectional area to intraballoon pressure, is the most useful FLIP metric. Secondary peristalsis from balloon distension can be displayed topographically as repetitive anterograde or retrograde contractile activity in the esophageal body, similar to high-resolution manometry. Real-time interpretation and postprocessing of FLIP metadata can complement the identification of esophageal outflow obstruction and achalasia, especially when findings are inconclusive from alternate esophageal tests in symptomatic patients. FLIP can complement the diagnosis of achalasia when manometry and barium studies are inconclusive or negative in patients with typical symptoms. FLIP can direct adequacy of disruption of the EGJ in achalasia when used during and immediately after myotomy and pneumatic dilation. Lumen diameter measured using FLIP in eosinophilic esophagitis and in complex strictures can potentially guide management. An abbreviated modification of the Grading of Recommendations Assessment, Development, and Evaluation was used to determine the quality of available evidence and recommendations regarding FLIP utilization. FLIP metrics that are diagnostic or suggestive of an abnormal motor pattern and metrics that define normal esophageal physiology were developed by consensus and are described in this review.
WHAT IS THE FUNCTIONAL LUMEN IMAGING PROBE?
The functional lumen imaging probe (FLIP) is a novel device that measures luminal cross sectional area (CSA) and pressure in the esophagus using impedance planimetry and serves as an adjunct to existing esophageal investigative tests (1–3). A distensible balloon encasing a catheter with multiple pairs of impedance electrodes is used, and the balloon is distended with fluid of known conductivity and volume (Figure 1). A mechanical pump in the FLIP module controls distension speed and volume, and simple touch screen buttons allow user input into the process of volumetric distension.
Figure 1.
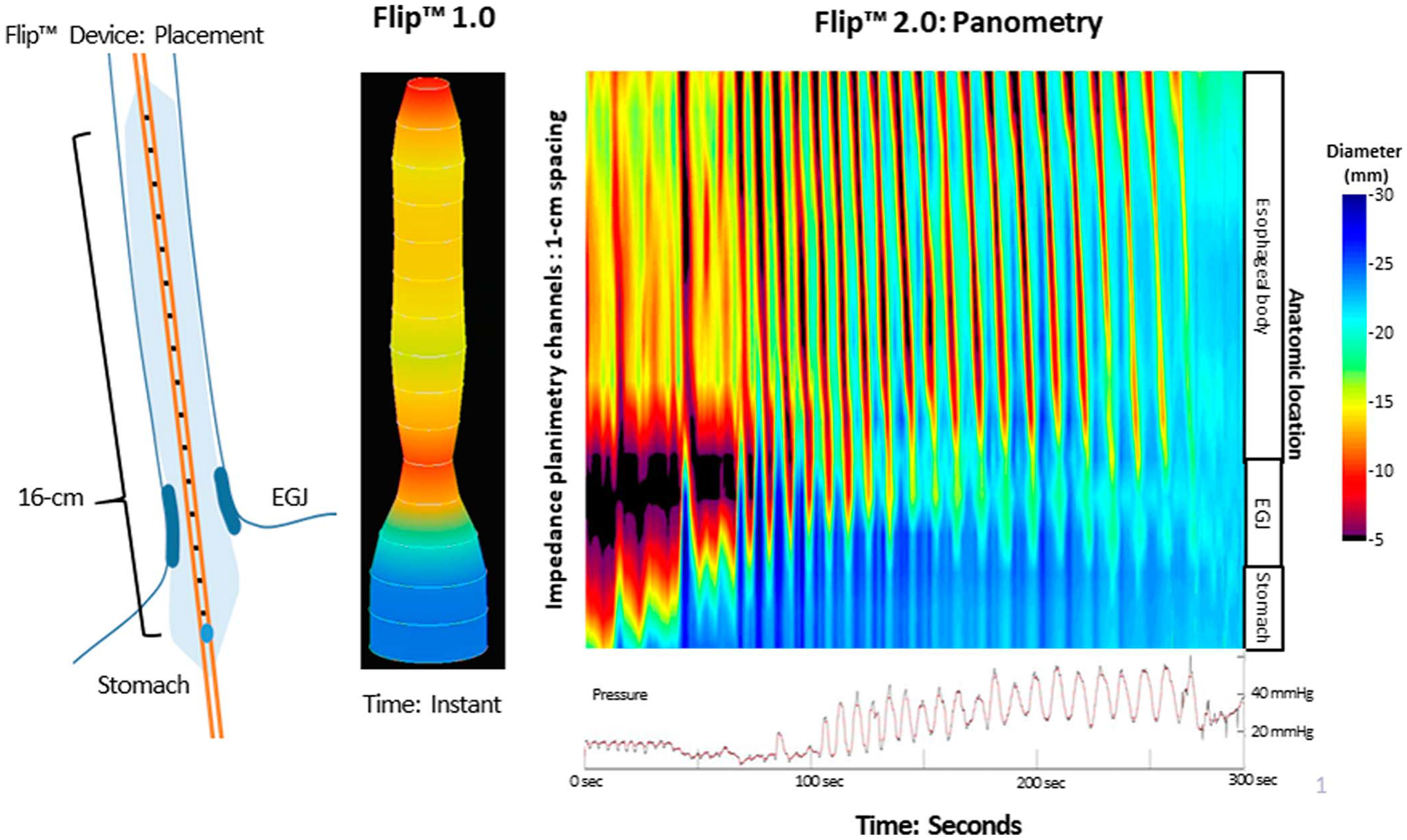
FLIP device placement through the EGJ and the correlating FLIP 1.0 images using a 3-dimensional rendering of the esophageal lumen created by cross-sectional area measurements at 1 cm intervals for the 16-cm catheter and 0.5 cm in the 8-cm catheter. The FLIP 2.0 panometry uses FLIP topography to provide a space-time domain representation of diameter changes that represent contraction and distention of the esophageal lumen. EGJ, esophagogastric junction; FLIP, functional lumen imaging probe.
WHAT EQUIPMENT IS NEEDED FOR PERFORMING THE FLIP PROCEDURE?
The FLIP catheter is available in 2 main configurations (EF 325: 8 cm catheter with 16 sensors spaced 0.5 cm apart, and EF-322: 16 cm catheter with 16 sensors spaced 1 cm apart) and uses 2 distinct data display and analysis platforms (Table 1). The shorter 8-cm catheter provides information regarding esophagogastric junction (EGJ) distensibility and CSA, whereas the 16-cm catheter can provide esophageal body secondary peristalsis patterns (contractility) in addition to EGJ metrics. The dedicated catheters used for FLIP are approved by the US Food and Drug Administration (FDA) for clinical use.
Table 1.
FLIP protocol by catheter type
| Catheter configuration | ||
| Balloon length | EF-325: 8 cm | EF-322: 16 cm |
| Sensors | 16 sensors spaced 0.5 cm apart | 16 sensors spaced 1 cm apart |
| Protocol | ||
| Baseline placement | Balloon should span the EGJ with the EGJ waist in the mid-balloon length | Balloon should span the EGJ with 2–3 sensors distal to the EGJ |
| Baseline fill volume | 20 mL | 30 mL |
| Baseline wait time | 15 s | 15 s |
| Balloon fill protocol | Fill to 30 mL, 40 mL, 50 mL | Fill to 40 mL, 50 mL, 60 mL, 70 mLa |
| Wait time at each fill | 30 s | 60 s |
| Measurements | ||
| EGJ-DIb Diameterb Intrabag pressureb | EGJ-DIb Diameterb Intrabag pressureb Presence of RACs Presence of RRCs Presence of any contractility | |
EGJ, esophagogastric junction; EGJ-DI, EGJ-distensibility index; FLIP, functional lumen imaging probe; RAC, repetitive anterograde contraction; RRC, repetitive retrograde contraction.
Fill to 70 mL if intrabag pressures do not reach greater than 15–20 mm Hg or outflow obstruction is suspected.
Measured when the narrowest luminal diameter achieves maximal diameter.
The FLIP 1.0 module converts impedance recordings to CSA measurements along the length of the catheter while simultaneously measuring pressure with a single sensor at the distal end within the distending balloon. Data are displayed as a 3-dimenisonal rendering of the esophageal lumen using either catheter configuration (Figure 2). The FLIP 2.0 module uses diameter topography to display the diameter-pressure changes across a space-time continuum, using the 16-cm catheter. This concept is not new; previous investigators have reported that a distending stimulus in the esophageal lumen will elicit a contractile response (4,5). Using topography to describe the diameter space-time continuum to assess contractility was a natural progression akin to the evolution of high-resolution manometry (HRM) (6). The change in the diameter/pressure relationship over time can be used to describe motility patterns across the distal esophagus through the EGJ (Figure 3).
Figure 2.
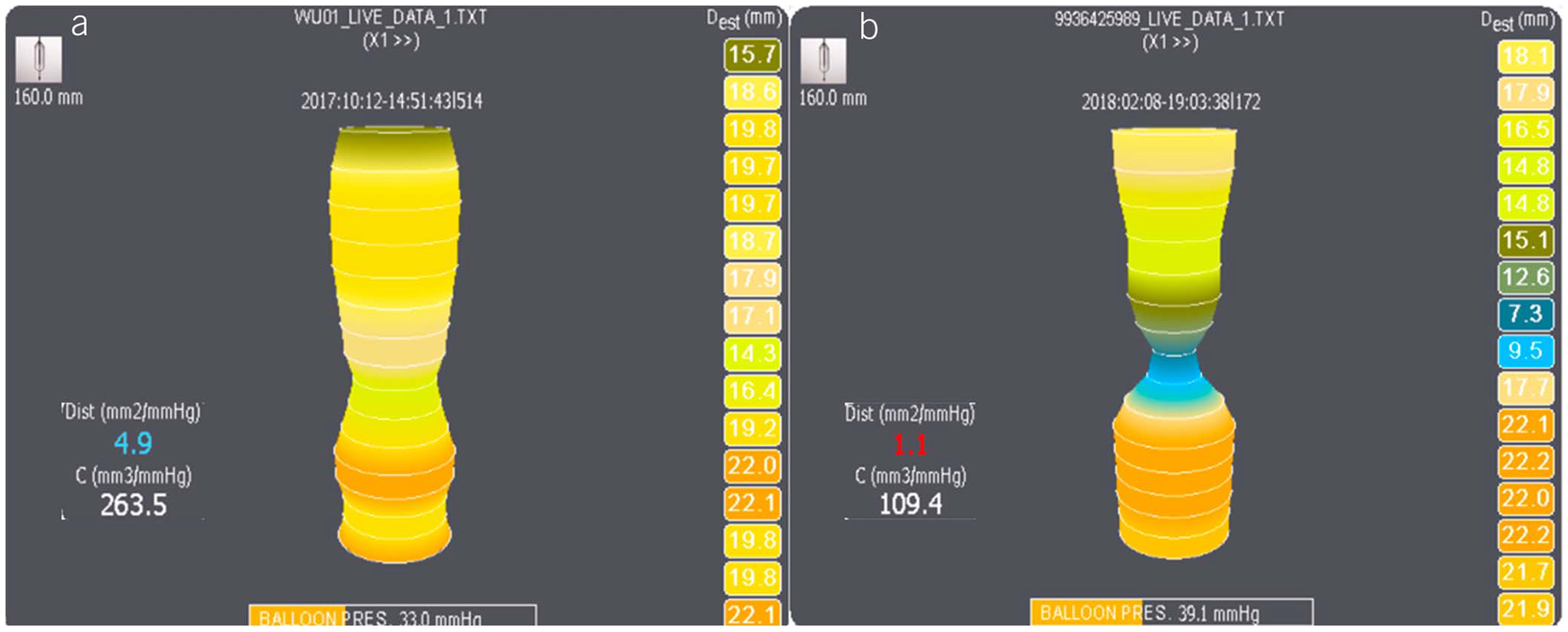
Examples of normal and abnormal EGJ distensibility on first generation FLIP (FLIP 1.0). (a) Normal EGJ distensibility, with an EGJ distensibility index (EGJ-DI) of 4.9 mm2/mm Hg. (b) Abnormal EGJ distensibility in a patient with achalasia, with an EGJ-DI of 1.1 mm2/mm Hg. EGJ-DI values <2 mm2/mm Hg are definitely abnormal and indicate an obstructive EGJ process. EGJ, esophagogastric junction; EGJ-DI, EGJ distensibility index; FLIP, functional lumen imaging probe.
Figure 3.
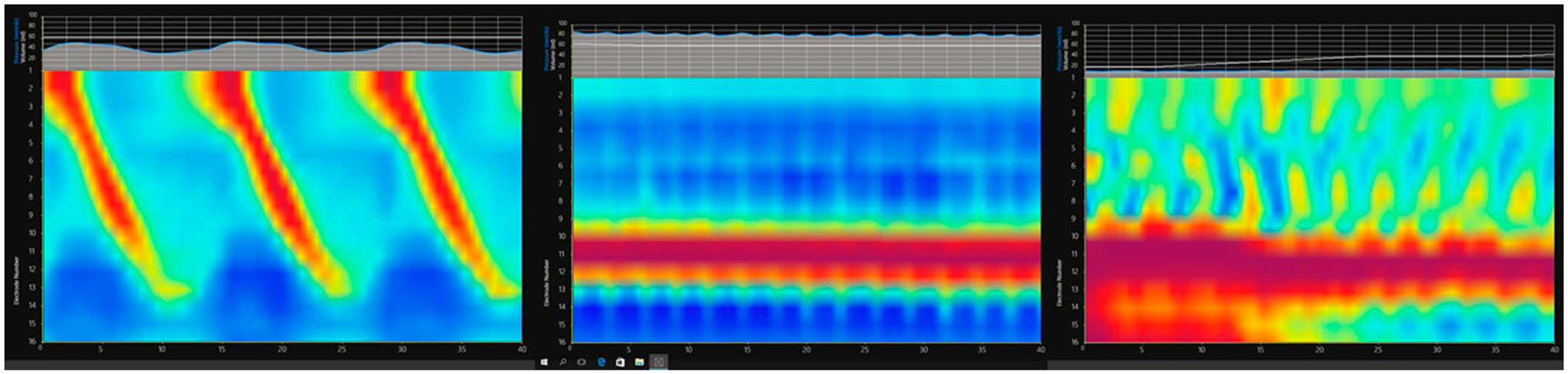
Examples of esophageal body contraction patterns on second generation FLIP (FLIP 2.0 or FLIP panometry). (a) Normal contraction pattern with repetitive antegrade contractions. (b) Absent esophageal body contractility in achalasia. (c) Repetitive antegrade and repetitive retrograde contractions in esophagogastric junction outflow obstruction. Note the closed EGJ, with the red band depicting lack of EGJ opening in images b and c. EGJ, esophagogastric junction; FLIP, functional lumen imaging probe.
HOW IS THE FLIP PROCEDURE PERFORMED?
The FLIP catheter is placed transorally immediately after but not during sedated upper endoscopy (7). Endoscopy serves to exclude mechanical obstructive processes and to estimate the distance from the incisors to the EGJ. Although transnasal placement is possible in the sedated patient, most available normative data are based on transoral placement. The catheter is inserted such that the EGJ is clearly identified, with a few sensors in the stomach and the remainder in the esophageal lumen (Figures 2 and 3). The catheter is held in place by the operator, who may need to adjust the position of catheter relative to the EGJ to maintain appropriate positioning of the impedance electrodes during volumetric distension because esophageal contractions can push the balloon distally.
WHAT IS THE STANDARD FLIP PROTOCOL?
Based on the current FDA labeling, standardized protocols were developed unique to catheter type and to location(s) of measurement in the foregut (EGJ alone, EGJ, and esophageal body) (Table 1). Regardless of catheter configuration, the FLIP protocol consists of volumetric distension of the FLIP balloon to predetermined volumes using a proprietary electrolyte solution with known conductance. Because endoscope presence can impact FLIP metrics, measurements are taken after the endoscope is removed (7). An initial volumetric distension to 20 mL when using the 8-cm catheter and to 30 mL with the 16-cm catheter is recommended because this triggers distension induced secondary peristalsis when present (Figure 3). An initial wait period of 15–30 seconds allows development of the contractile pattern. After this, the balloon is filled with 10 mL fluid aliquots till a target volume of 40 mL (8-cm catheter) or 60 mL (16-cm catheter) is reached. Fill to 70 mL is recommended when using the 16-cm catheter if intrabag pressures do not reach greater than 15–20 mm Hg or outflow obstruction is suspected because adequate balloon distension is necessary for accurate CSA assessment. Wait periods of 30–60 seconds are recommended at each distension volume to observe contractile patterns (Table 1). The best data on normative values of distensibility was obtained at the 60 mL fill volume using the EF-322 catheter when pressure measurements were documented to be above 20 mm Hg (8).
WHAT INFORMATION DOES FLIP PROVIDE?
The FLIP system assesses esophageal biophysical and motor responses to distention by measuring simultaneous CSA at the level of each pair of impedance electrodes and pressure within the distended balloon (9). The relationship between CSA and pressure provides a measure of EGJ distensibility, termed distensibility index (DI), a metric used for diagnosing disorders with impaired lower esophageal sphincter (LES) relaxation and/or mechanical obstruction at the EGJ (10–12). Moreover, esophageal luminal distension from the balloon induces secondary peristalsis, which in turn causes changes in luminal diameter in the esophageal body. These diameter changes can be plotted in topographic fashion akin to HRM (2,13), allowing display and identification of anterograde and retrograde contractile activity in the esophageal body.
WHAT METRICS ARE USEFUL IN FLIP INTERPRETETATION?
DI is the most investigated and, to date, most useful metric from FLIP testing (14). Normative values range from 3.1 to 9.0 mm2/mm Hg (8,10,15–18), with reduced EGJ opening corresponding to values between 0 and 2.0 mm2/mm Hg and borderline values ranging from 2.1 to 3.0 mm2/mm Hg (2,8,19) (Figure 2).
Esophageal contraction reduces lumen volume and diameter, and therefore, serial display of diameter changes indirectly depicts contraction, a smaller lumen diameter, or lumen closure corresponding to contraction (Figure 1). Using the process of interpolation, diameter changes can be displayed similar to esophageal pressure topography, and normal or abnormal propagation in the antegrade or retrograde direction can be assessed, providing an assessment of motor function that correlates with primary peristalsis on HRM (Figure 3) (2). Consequently, the contractile response to volumetric distention can be formalized around 4 distinct categories (Table 2): (i) repetitive anterograde contractions (RACs), with runs of ≥3 consecutive anterograde contractions considered normal; (ii) repetitive retrograde contractions (RRCs), an abnormal response associated often with spasm, achalasia, and postsurgical EGJ outflow obstruction; (iii) absent contractility, suggestive of aperistalsis, scleroderma, and significant ineffective esophageal motility; and (iv) other contractile patterns not fulfilling criteria for the above 3 categories, termed diminished or disordered contractile response, which likely represents a milder form of motor dysfunction.
Table 2.
FLIP Panometry diagnoses and disease states
| EGJ-DI (2.1–3.0 mm2/mm Hga) | |||||
|---|---|---|---|---|---|
| EGJ-DI (0–2 mm2/mm Hg) | Max D <12 mm Bag pressure >20 mm Hg | Max D >12 mm Bag pressure >20 mm Hg | EGJ-DI (3.1–9.0 mm2/mm Hg) | EGJ-DI (>9.0 mm2/mm Hg) | |
| RACs | EGJOO with normal contractile response | EGJOO with normal contractile response | Normal contractile response | Normal contractile response | Normal contractile response with increased EGJ distensibility |
| DDCR | EGJOO w/DDCR | EGJOO w/DDCR | DDCR | DDCR | DDCR with increased EGJ distensibility |
| Absent | EGJOO w/absent contractile response | EGJOO w/absent contractile response | Absent contractile response | Absent contractile response | Absent contractile response with increased EGJ distensibility |
| RRCs | EGJOO w/retrograde contractile response | EGJOO w/retrograde contractile response | Retrograde contractile response | Retrograde contractile response | Retrograde contractile response with increased EGJ distensibility |
For application to endoscopy-negative dysphagia patients without a hernia > 3 cm or mechanical obstruction/esophagitis. EGJ-distensibility index (DI) at EF-322 60 mL fill volume.
DDCR, disordered and/or diminished contractile response; EGJ, esophagogastric junction; EGJOO, EGJ outflow obstruction FLIP, functional lumen imaging probe; RAC, repetitive anterograde contraction; RRC, repetitive retrograde contraction.
Rule out mechanical stricture if Max diameter is less than 18 and there is a fixed plateau max diameter during volumetric distention-strongly consider obtaining a timed barium esophagram with barium tablet.
WHAT ARE THE LIMITATIONS OF THE FLIP PROCEDURE?
Despite accumulating evidence on the clinical utility of FLIP, limitations exist, and quality of available evidence is low. First, direct comparative studies with imaging modalities such as barium esophagography and scintigraphy are limited. Second, availability is limited, and costs are higher than other esophageal tests (i.e., barium swallow and HRM), especially because FLIP is performed during sedated endoscopy. The cost of equipment is approximately $25,000 for the original FLIP system, and $65,000 for FLIP 2.0 with panometry; each single-use catheter costs up to $350. Although many of the metrics are intuitive, training is needed for the operator and assistants, adding to the cost of adoption of the technology. Third, there is no satisfactory software solution, allowing real-time or immediate postprocessing of data, making data analysis for scientific purposes difficult and clinical reporting unsatisfactory. Refinements have not been forthcoming from the manufacturer. Finally, there is no storage solution for data within the FLIP module; external storage devices are currently used for both FLIP metadata and live FLIP 2.0 video.
This review article evaluates available evidence regarding the clinical value of FLIP in esophageal disorders, using an abbreviated modification of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system whenever possible, incorporating existing evidence with expert consensus opinion in areas with limited data.
METHODS
FLIP consensus meetings were held at 2 separate venues (Chicago, IL, and Tolochenaz, Switzerland), with participants selected by the chairs (J.E.P., E.S., and M.d.P.) based on their expertise and experience in esophageal and foregut disease and in the use of FLIP in clinical practice and research. The US consensus meeting focused on the development of protocols and establishment of best practice guidelines, conclusions from which were presented at and assimilated into the European Consensus Meeting. Each of the European Consensus participants performed additional focused literature searches of the MEDLINE database on preassigned topics to identify and review the existing medical literature on FLIP, using pertinent search terms including FLIP, endo-FLIP, eso-FLIP, impedance planimetry, DI, CSA, FLIP topography, FLIP panometry, RACs, and RRCs. The preassigned topics included technical aspects and methodology of the FLIP procedure; natural history and clinical diagnosis of foregut disorders where FLIP has been studied; FLIP metrics in normal subjects, esophageal outflow obstruction disorders, and achalasia, other esophageal motor disorders, eosinophilic esophagitis (EoE), gastroesophageal reflux disease (GERD), and complex strictures; and outcome data from FLIP utilization in these disorders. Consensus statements were graded on the quality of the supporting evidence according to the GRADE system (20). The strength of the individual statements is based on the aggregate evidence quality and a balance between benefits and harms. Nearly all the studies assessed were downgraded a point to very low quality for small sample size, for indirectness to the population, intervention or outcome at hand, or for inability to measure point estimates. GRADE category is indicated in the text within parenthesis. Consensus was achieved through careful evaluation and discussion of available literature and consensus agreement on recommendations that lacked supporting evidence. The study was critically reviewed and approved by all the participants.
FLIP in relationship to HRM in achalasia and esophageal outflow obstruction
An imperfect HRM definition of EGJ obstruction disorders, partly related to false positive (and sometimes false negative) median IRP values, leads to diagnostic confusion in patients with obstructive symptoms (21). Although HRM is used often for the evaluation of obstructive symptoms not explained by endoscopy and/or barium radiography, manometry has very low GRADE evidence and only a conditional recommendation based on objective review of the literature (21). Provocative tests performed during HRM (preferably combined with stationary impedance) and barium esophagography (timed upright, with or without a 13 mm barium pill) have been proposed to resolve these diagnostic difficulties but both carry very low to low GRADE evidence and conditional recommendations (21). Cross-sectional imaging and endoscopic ultrasonography are useful only in limited situations where neoplasia or a mass are suspected (22). In this diagnostic context where mechanical or motor obstruction of the EGJ is being considered, FLIP may have clinical value as an adjunctive maneuver that can be performed during initial or subsequent endoscopy.
FLIP has been compared with HRM in the diagnosis of achalasia spectrum disorders, other EGJ outflow obstruction disorders, and in predicting outcome from EGJ disruption. FLIP was demonstrated to identify abnormal EGJ distensibility in patients with typical achalasia symptoms but without classic HRM features of achalasia (12). EGJ distensibility measured using FLIP can diagnose achalasia despite normal IRP (12), which is a caveat in the use of IRP alone in excluding achalasia (23,24). In a study comparing FLIP with HRM in patients with dysphagia, FLIP accurately detected major motility disorders including achalasia (2). In a retrospective study of 34 patients with idiopathic EGJOO, all with a normal EGJ-DI (>3 mm2/mm Hg) who were treated conservatively had symptomatic improvement, although 78% with an abnormal EGJ-DI (<2 mm2/mm Hg) treated with achalasia-type therapy also improved (25). Values in the borderline range require further evaluation by assessing the maximal diameter attained when pressures are greater than 20 mm Hg, when diameter <12 mm could represent luminal narrowing (2,26,27). Although data are less robust with the 8-cmcatheter, the same values could be extrapolated during the 40 mL distention volume (14). Adjunct testing, such as a barium esophagram could provide further clarification. Thus, FLIP was able to identify patients with EGJ outflow obstruction disorders who were most likely to benefit from EGJ disruption.
The ability of FLIP 2.0 (FLIP panometry) to assess both EGJ distensibility and esophageal contractility could confirm the clinical relevance of isolated abnormal IRP on HRM or identify significant motility disorders missed with HRM (2). Studies assessing motor function in normal controls (8) revealed that the normal response to volumetric distention was antegrade contractions in a repetitive pattern with a frequency of 4–9 contractions per minute (RACs) (8). This pattern was noted in 95% of the subjects, and no normal controls were noted to have a completely absent response or retrograde contractions. FLIP has the potential to characterize achalasia by detecting nonocclusive esophageal contractions not observed with HRM (13). FLIP topography enhanced evaluation of esophageal function in nonobstructive dysphagia by detecting an abnormal response to esophageal distension in 50% of patients diagnosed with ineffective esophageal motility or a normal HRM study (28). Studies assessing various patient groups found that RRCs could be seen in patients with achalasia, spastic motor disorders, and postfundoplication dysphagia (29). In a prospective study of 40 consecutive patients, real-time FLIP panometry detected esophageal motility disorders, including achalasia, at the endoscopic encounter (30). In addition, normal motility on FLIP panometry was predictive of a benign HRM. Despite good correlation with manometry (2,30), further prospective studies are needed to determine whether FLIP metrics could improve outcomes as opposed to just being an adjunct to the current HRM approach.
We suggest that FLIP is complementary, and in some cases an alternative, to HRM in identifying EGJ outflow obstructive disorders including achalasia (conditional recommendation, very low quality of evidence).
FLIP in relationship to barium esophagram in achalasia and esophageal outflow obstruction
Most of the initial studies with FLIP used barium esophagography as an outcome measure in achalasia and EGJ outflow obstruction. FLIP can be conceptualized as a static esophagram with the added benefit of controlled volume distention and simultaneous pressure measurements. The high-resolution planimetry measurements reconstruct lumen anatomy while the simultaneous pressure measure provides assessment of esophageal wall distensibility and contractile response to volume distention. A recent study demonstrated significant correlation of EGJ-DI in predicting abnormal barium retention (R −0.54, area under the curve 0.9), outperforming HRM (31). More importantly, this study established that EGJ-DI is complementary to barium esophagography because it identified abnormal barium retention defined by esophagram in the context of adequate myotomy and abnormal anatomy (31). Three additional studies corroborate FLIP metrics with bolus retention on esophagography in achalasia, with an EGJ-DI threshold of approximately 3.0 mm2/mm Hg (10,25,32).
In addition, FLIP can define contractile patterns similar to barium esophagography while corroborating EGJ findings on HRM, making FLIP a hybrid of esophagography and manometry. Secondary peristalsis, tertiary peristalsis, and spastic or focal contractions seen on fluoroscopy in achalasia types 1 and 2 are also described during FLIP panometry, but not on HRM because these contractions are nonoccluding (13). Finally, FLIP accurately measures esophageal diameter, especially the maximal diameter attained during volumetric distention (distensibility plateau) (27). Thus, FLIP can accurately define diameter of lumen stenosis and patterns of motility similar to barium esophagography and can function as a dynamic esophagram.
We suggest that EGJ-DI on FLIP is complementary to assessment of esophageal emptying on barium esophagography (conditional recommendation, very low quality of evidence).
FLIP during and after treatment of achalasia and esophageal outflow obstruction
Contrary to EGJ basal pressure and even IRP, impaired EGJ distensibility after achalasia treatment is reliably associated with poor esophageal emptying and poor clinical response, measured using Eckardt score <3 (10,32). In this context, EGJ-DI has been successfully used in segregating incomplete symptomatic response (manifesting as low EGJ-DI, >2 mm2/mm Hg) from adequate symptomatic response (manifesting as normal EGJ-DI, >3 mm2/mm Hg) after achalasia management, making FLIP a useful tool for the follow-up of patients with symptomatic achalasia after invasive LES disruption (10,32,33). Improvement in EGJ-DI has been demonstrated after successful laparoscopic (Heller) myotomy (10), per oral endoscopic myotomy (POEM) (34), and pneumatic dilation (PD) (35). Thus, efficacy of LES disruption is determined by improvement in LES distensibility, a factor that is optimally assessed using endo-FLIP (14).
Because EGJ-DI correlates with symptom response from achalasia management, this metric has been used during achalasia management to confirm adequate LES disruption. The final EGJ DI and CSA during POEM (36,37), surgical myotomy (38,39), and PD (40) correlate well with clinical response in studies using intraprocedure FLIP. On real-time intraoperative FLIP monitoring during laparoscopic Heller myotomy, actual incision of circular muscle fibers was identified as the point when EGJ-DI increased dramatically; there was a minor decline after partial fundoplication, and further increase when pneumoperitoneum was deflated (38). Similarly, during POEM, creation of the submucosal tunnel and myotomy resulted in EGJ-DI increment (38). The change in EGJDI with POEM (mean change: 7.0 ± 3.1 mm2/mm Hg) was higher than that seen with myotomy and partial fundoplication (mean change: 5.1 ± 3.4 mm2/mm Hg); increase in EGJ-DI correlated with symptom improvement in both settings, with optimal symptom improvement recorded when postprocedure EGJ-DI was between 4.5 and 8.5 mm2/mm Hg (39). Similar benefits of intraoperative FLIP during myotomy and POEM have been reported by other investigators (36,37,41). Intraoperative FLIP may allow tailoring of the length of myotomy, and incision of circular muscle fibers can be discontinued when adequate increment in EGJ-DI is documented (42,43).
We suggest that measurement of EGJ distensibilty on FLIP after LES-directed therapy for achalasia can be used to assess degree of post-therapy EGJ disruption (conditional recommendation, very low quality of evidence) and symptom response to therapy (conditional recommendation, very low quality of evidence).
FLIP in GERD and fundoplication
The pathophysiology of GERD includes several mechanisms (44), spanning from transient LES relaxations (45) to impairment of chemical clearance (46–48), esophageal body hypomotility (49–51), and abnormal EGJ barrier function (52,53). Impairment in the EGJ barrier could be associated with increasing compliance and distensibility. Consequently, because FLIP measures EGJ distensibility, it has been hypothesized that FLIP could improve understanding of GERD pathophysiology and potentially aid in diagnosis. Although EGJ-DI values greater than 9.0 mm2/mm Hg are abnormal and could conceivably be associated with an incompetent antireflux barrier (18,54), there are little outcome data to support using this threshold as a diagnostic cutoff for GERD. One preliminary study demonstrated that the EGJ-DI in 20 patients with typical reflux symptoms was significantly higher than that in 20 asymptomatic volunteers (16). By contrast, a lower EGJDI was reported in 18 patients with heartburn compared with 21 asymptomatic control subjects, with no difference in EGJ-DI between patients with and without abnormal acid exposure, suggesting that EGJ-DI may not be discriminative for GERD (18). FLIP can identify a hiatus hernia and can distinguish the LES component as distinct from the crural diaphragm. Distensibility of the LES was reported to be higher in the presence of a hiatus hernia, compared with asymptomatic control subjects (55). Finally, using FLIP 2.0 panometry, Carlson et al. (56) observed that abnormal esophageal acid exposure was associated with an impaired contractile response to volume distension of the esophagus, corroborating the well-known concept that acid exposure is dependent on acid clearance mechanisms. Nevertheless, data on the diagnostic utility of FLIP in GERD remains incomplete, especially because FLIP metrics in GERD show considerable overlap with that seen in healthy controls. Further studies with a well-defined GERD population will be necessary to evaluate the role of FLIP in evaluating GERD pathophysiology and diagnosis of GERD. The role of FLIP for improving our understanding of GERD pathophysiology and management remains intriguing.
We suggest against use of FLIP to diagnose GERD in routine clinical practice (conditional recommendation, very low quality of evidence).
FLIP has also been studied in measuring surgical outcomes after fundoplication or endoscopic antireflux procedures (57). Reduction in EGJ-DI after fundoplication is greater when a hiatus hernia is present, reflecting higher initial EGJ distensibility when the EGJ is disrupted in GERD (58); however, the final EGJ-DI after fundoplication is reported to be similar, regardless of the presence of hiatus hernia. More importantly, an abnormally low EGJ-DI after fundoplication correlated with a need for reoperation for persistent dysphagia in small series, indicating that EGJ-DI can predict symptom outcome and need for further intervention (58).
Because EGJ distensibility reduces after antireflux procedures compared with control subjects, FLIP has been proposed as a real-time tool to tailor antireflux interventions (16). Intraoperative FLIP use is feasible, and there is reduction in EGJ distensibility immediately after antireflux procedures (58–61). In a series of 75 patients who underwent Toupet or Nissen fundoplication, EGJ-DI consistently decreased after fundoplication during intraoperative FLIP. EGJ-DI values were higher when Toupet fundoplication is performed rather than a 360 degree Nissen fundoplication (62), whereas EGJ-DI was similar between primary unoperated GERD and disrupted EGJ in need of redo antireflux surgery (62). In a series of 40 patients, FLIP was used to tailor the degree of crural closure and size of the fundoplication; the change in EGJ-DI paralleled symptom improvement and absence of dysphagia (63). In a series of 175 patients, final intraoperative EGJ-DI <2 mm Hg/mm2 was associated with dysphagia and gas bloat more often, whereas values 2–3.5 mm Hg/mm2 provided optimal postfundoplication outcomes (64). In another study of 42 GERD patients undergoing transoral incisionless fundoplication, preoperative EGJ distensibility was an independent predictor for objective treatment outcome (normalization of esophageal acid exposure time) but not for clinical outcomes (symptom scores) after transoral incisionless fundoplication (65).
Although FLIP has potential in tailoring fundoplication and identifying tight wraps, findings in these studies are not conclusive, but there is enough preliminary evidence to warrant prospective studies evaluating the utility of FLIP in fundoplication and endoscopic antireflux procedures.
We suggest that intraoperative FLIP measurements can be used to facilitate tailoring of fundoplication (conditional recommendation, very low quality of evidence).
FLIP in EoE
Longstanding inflammation in EoE leads to remodeling and stricturing of the esophagus, which can be associated with food bolus impactions. Prevention and reversal of remodeling is an important treatment target. FLIP allows the measurement of effects of EoE on the biomechanics of the esophageal wall, particularly esophageal luminal diameter. With diffuse esophageal narrowing, increase in balloon volume and pressure do not result in a corresponding increase in cross-sectional area, suggesting fibrosis and a fixed lumen—this phenomenon is termed the distensibility plateau and reflects the maximum diameter of the most narrow point in the tubular esophagus (17). Narrow luminal diameters thus correlate with lower distensibility plateau values (27), which in turn correlate with lamina propria fibrosis and occurrence of food impactions. Although impaired distensibility can be seen in patients with EoE of all ages, distensibility decreases with increasing age and disease duration (66).
Impaired distensibility is partially reversible, especially after successful treatment with steroids or diet; conversely, distensibility does not change in patients with failing therapy (26). In the setting of persisting symptoms despite optimal therapy, serial assessment of distensibility and luminal diameter using FLIP could provide an objective outcome metric that can indicate the need for escalation of therapy. Similarly, in the presence of histologic remission with persisting symptoms, FLIP may reveal other mechanisms underlying clinical symptoms such as an increased esophageal stiffness or reduced distensibility because of chronic esophageal fibrosis, which might indicate the need for esophageal dilation. Because this can be reliably measured with FLIP, FLIP distensibility measurements are gaining importance as secondary endpoints in EoE trials and mechanistic studies. FLIP measurements are therefore an easy adjunct for the evaluation of esophageal diameter and distensibility in EoE. However, the role of FLIP for routine EoE clinical care is not yet clear, and further prospective studies are required.
We suggest that in patients with EoE, FLIP can be used to assess fibrostenotic remodeling of the esophagus (conditional recommendation, very low quality of evidence).
FLIP in stricture dilation
When dealing with suspected or confirmed stenoses of the esophagus, the clinical decision-making process needs to address 4 issues: whether dilation is needed, assessment of complex stricture diameter when endoscopic inspection is suboptimal, the optimal end-dilation diameter, and the optimal dilation technique.
Most esophageal strictures can be fully evaluated endoscopically, where symptoms generally guide the therapeutic decision. Open endoscopic biopsy forceps can determine the initial lumen diameter and help determine the optimal end diameter of dilation. However, with complex strictures that cannot be traversed with an endoscope, uncertainty may persist regarding esophageal morphology distal to stricture. In these cases, fluoroscopy is usually recommended to define stricture anatomy and to facilitate guide wire placement without kinks (67). This is an area where FLIP could provide an alternative to fluoroscopy in providing accurate diameter assessments during index endoscopy, where dilation could also be simultaneously performed.
An area that is particularly difficult to assess endoscopically is the pharyngoesophageal junction (PEJ), where video fluoroscopic assessment is typically performed. However, adequacy of the upper esophageal sphincter opening can be influenced not only by mechanical factors but also by the pharyngeal propulsive deficiency and reduced hyolaryngeal traction (68), making video fluoroscopic assessment of suspected PEJ strictures potentially unreliable. Wu and collaborators used FLIP technology to functionally image the PEJ in 34 patients, who underwent oncological treatment for head and neck cancer and presented with dysphagia, together with 20 controls without dysphagia (69). Narrow CSA in patients with PEJ strictures was significantly smaller than controls and cancer patients without stricture (P < 0.001). Using a cutoff of 114 mm2 (at 60 mL volumetric distension), the accuracy of CSA for diagnosing PEJ strictures was 100%. After dilatation, CSA increased with a mean difference of 29 mm2 (P < 0.001). There was a moderate correlation between CSA postdilatation and the largest dilatator used to induce tear, with FLIP slightly underestimating the size by approximately 2.1 mm. Thus, FLIP can safely be used to assess the stricture size and allow selection of appropriate dilators, which is particularly relevant when using through-the-scope balloon dilators and in the case of PEJ or complex strictures. An area that needs further investigation is the utility of FLIP in directing the choice of type of dilator (through the scope balloon vs bougie). Although there are no comparative or cost-effectiveness studies, FLIP can be useful in such settings in providing diameter and CSA information to define stricture diameter, FLIP dilating balloons (esoFLIP) can be used to perform therapeutic hydraulic dilatation without need for fluoroscopy or barium esophagography (70). In a small single center study on 19 pediatric patients, use of FLIP hydraulic dilation provided a larger diameter increase compared with standard balloon dilation directed by diagnostic FLIP (4 mm vs 2.2 mm), but this was not statistically significant likely because of the small cohort size (71). It remains unclear whether FLIP can direct the choice of dilation technique because no comparative or cost-effectiveness studies exist. Three existing randomized controlled trials comparing wire-guided bougies to through the scope balloon dilators showed no difference in terms of outcomes, including safety and efficacy (72–74). Thus, eventual choice of dilation technique is typically based on physician choice and availability. The FLIP hydraulic balloon dilator (esoFLIP) has been evaluated for dilatation of achalasia in a small feasibility study of 10 patients (type 1 = 4 patients; type 2 = 4 patients; and type 3 = 2 patients) and Eckardt score >3. An 8-cm FLIP balloon dilator with 30 mm maximum diameter was placed over a guidewire and alongside the endoscope. Dilation to 28–30 mm was performed successfully under endoscopic vision in all patients, with no serious adverse events. The median EGJ-DI increased from 1.1 mm2/mm Hg at baseline to 7.0 mm2/mm Hg after the second dilatation (P = 0.005). At 1-month follow-up, the median Eckardt score decreased from 7.5 to 1 (P = 0.005), with 8 patients maintaining clinical remission; 2 patients with persistent dysphagia were treated with surgical myotomy, and a third patient required PD at 3 months. Symptom recurrence in 30% of this small cohort is likely attributable to the fact that dilation was only performed to 30 mm, whereas dilation to higher diameters (35 mm, 40mm) is sometimes required to achieve optimal results. A second study reported short-term efficacy, both objective (improvement in barium column) and subjective (improvement in Eckardt score), in 28 patients managed using the FLIP hydraulic balloon dilator (75). Development of a 35-mm FLIP hydraulic balloon dilator may improve outcomes in patients who have an incomplete response to the 30-mm dilation.
We suggest that FLIP can be used to measure the cross-sectional area of PEJ and esophageal strictures to guide endoscopic dilation (conditional recommendation, very low quality of evidence).
Clinical utilization of FLIP: recommendations based on consensus
The most useful metric in the clinical utilization of FLIP is the EGJ-DI. Based on available normative data in healthy asymptomatic individuals, and data from studies evaluating motor esophageal outflow obstruction (achalasia spectrum disorders) and structural obstruction (strictures, EoE), EGJ-DI values <2 mm2/mm Hg are consistently abnormal (Table 2 and Figure 5). EGJ-DI values between 2 and 3 mm2/mm Hg are likely abnormal but need additional abnormal metrics to support this (14). This can include low EGJ diameter (<13 mm) (27,76) or RRCs (2). Although absent contractility is also likely abnormal, there are instances when contractility declines when higher volumes are used for volumetric distension, and longer periods of observation at the baseline distension volume may be necessary to confirm the absence of contractility, especially with a normal EGJ-DI. Nonrepetitive contractility and EGJ diameter of 13–18 mm are also considered indeterminate because these may not necessarily be conclusive for an abnormal motor or structural pattern in isolation.
Figure 5.

A scheme for interpretation of FLIP metrics. EGJ-DI <2 mm2/mm Hg indicates the presence of an obstructive EGJ process, whereas values >3 mm2/Hg indicate normal esophageal biomechanics and physiology, especially in the setting of EGJ diameter >18 mm, presence of RACs, and normal endoscopy with biopsy. When EGJ-DI is not conclusive, EGJ diameter <13 mm, absent contractility and RRCs in isolation are likely abnormal. All other findings are indeterminate and need alternate evidence for designation of obstructive pathophysiology. Nonrepetitive contractile response is also termed disorganized and/or DDCR not fulfilling criteria for RACs or RRCs. DDCR, diminished contractile response; EGJ, esophagogastric junction; EGJ-DI, EGJ distensibility index; FLIP, functional lumen imaging probe; RAC, repetitive anterograde contraction; RRC, repetitive retrograde contraction.
The finding of RACs with EGJ-DI >3 mm2/mm Hg and an EGJ diameter of .18 mm in the context of a normal endoscopy establishes the absence of motor or structural obstruction in the esophageal body or EGJ (Figure 4).
Figure 4.

Volumetric distension protocol for FLIP testing. EGJ-DI is recorded at 40 mL volumetric distension when using an 8-cm balloon, and at 60 mL volumetric distension when using a 16-cm balloon. The following 4 metrics are reported: (a) maximum CSA or diameter at the EGJ or lower esophageal sphincter (narrowest CSA), (b) intraballoon pressure at maximum CSA, (c) EGJ-DI at maximum CSA (median of 3 values), and (d) contractility pattern with FLIP 2.0. CSA, cross sectional area; EGJ, esophagogastric junction; EGJ-DI, EGJ distensibility index; FLIP, functional lumen imaging probe.
Therefore, in patients with esophageal transit symptoms (dysphagia with or without chest pain and esophageal-type regurgitation) and a normal upper endoscopy, FLIP findings could potentially direct further management. Although FLIP could be performed at the index endoscopy in these patients, it is more likely that FLIP will be performed for further definition of incomplete findings on alternate esophageal evaluation, including HRM and barium esophagography. Prospective studies are needed on the use of FLIP at baseline endoscopy in patients referred with dysphagia to evaluate the utility of FLIP early in the diagnostic algorithm.
If EGJ-DI is less than 2 mm2/mm Hg, probability of achalasia, esophageal outflow obstruction, or other structural processes are likely and specific management targeting the primary etiology of outflow obstruction may be warranted (Table 2 and Figure 5). If RRCs are identified with EGJ-DI >3.0, esophageal body spastic processes may be the mechanism. Although GERD cannot be reliably identified based on FLIP alone, normal or high EGJ-DI are both compatible with GERD.
CONCLUSIONS
This review and expert consensus describing the FLIP technique and reviewing currently recognized indications in achalasia, EoE, and GERD using GRADE provides the basis for utilization of this technique in routine clinical practice. Although FLIP has value in assessing patients with dysphagia, it is less clear how it may affect management of GERD, and thus, future research should focus on GERD and intraoperative assessment of the EGJ in fundoplication (Table 3). Given the widespread diffusion of this technology in the past couple of years, guidance and ongoing research regarding protocols for FLIP use, accepted indications, and emerging applications of FLIP in the evaluation and management of foregut disorders is timely and clinically needed at this time.
Table 3.
Priorities for future research
| Normative data in a broad spectrum of asymptomatic patients |
| Use of FLIP as a first-line diagnostic tool in patients with dysphagia during index endoscopy |
| Intraoperative assessment of EGJ (surgery and endoscopy) |
| Phenotyping patients with EoE |
| Adjunct to dilatation/use of FLIP based hydraulic balloon dilation |
| Use of FLIP in other sphincters: pylorus, anal sphincter, and upper esophageal sphincter |
| EGJ, esophagogastric junction; EoE, eosinophilic esophagitis; FLIP, functional lumen imaging probe. |
Potential competing interests:
E.S. has served as a consultant for AbbVie, Allergan, MSD, Takeda, Sofar, and Janssen, he is a speaker for Medtronic, Reckitt-Benckiser, Malesci, and Zambon. M.d.P. has served as a consultant for Medtronic. A.J.B. received research funding from Nutricia, Norgine and Bayer and received speaker and/or consulting fees from Laborie, EsoCap, Diversatek, Medtronic, Dr Falk Pharma, Calypso Biotech, Thelial, Robarts, Reckett Benkiser, Regeneron, Celgene, Bayer, Norgine, AstraZeneca, Almirall and Allergan. D.A.C. has served as a consultant and speaker for Medtronic; he also has a licensing agreement with Medtronic. J.O.C. has served as a consultant for Isothrive, Medtronic and Sanofi. A.K. has served as a consultant for Medtronic. M.F.V. has served as a consultant for Medtronic and received research support from Diversatek. R.Y. has served as a consultant for Medtronic, Ironwood Pharmaceuticals, and Diversatek. D.P. has served as a consultant for Medtronic and Sanofi, and received a travel grant from Vifor. J.E.P. has served as a consultant for Medtronic, Diversatek, Torax, Ironwood, Takeda, and Astra Zeneca, he has received research support from Impleo and has stock options with Crospon. S.R. has served as a speaker for Medtronic, Mayoly Spindler, and has received research support from Diversatek, and Crospon, she has received a travel grant from Biocodex. C.P.G. has served as a consultant for Medtronic, Diversatek, Torax, Ironwood and Quintiles, he is a speaker for Medtronic and Diversatek. Logistics and funding for consensus meetings was provided by Medtronic, Inc. Medtronic had no role in the discussion of content, evaluation of quality of data, formulation of consensus opinions or in the preparation, review and submission of the manuscript.
Footnotes
Guarantor of the article: C. Prakash Gyawali, MD, MRCP
REFERENCES
- 1.Carlson DA. Functional lumen imaging probe: The FLIP side of esophageal disease. Curr Opin Gastroenterol 2016;32:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol 2016; 111:1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SS, Gregersen H, Hayek B, et al. Unexplained chest pain: The hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med 1996;124:950–8. [DOI] [PubMed] [Google Scholar]
- 4.Penagini R, Picone A, Bianchi PA. Effect of morphine and naloxone on motor response of the human esophagus to swallowing and distension. Am J Physiol 1996;271:G675–80. [DOI] [PubMed] [Google Scholar]
- 5.Paterson WG, Rattan S, Goyal RK. Esophageal responses to transient and sustained esophageal distension. Am J Physiol 1988;255:G587–95. [DOI] [PubMed] [Google Scholar]
- 6.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP). Neurogastroenterol Motil 2013;25:e765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianca A, Schindler V, Schnurre L, et al. Endoscope presence during endoluminal functional lumen imaging probe (FLIP) influences FLIP metrics in the evaluation of esophageal dysmotility. Neurogastroenterol Motil 2020:e13823. [DOI] [PubMed] [Google Scholar]
- 8.Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol 2019;17:674–81.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon BP, Frokjaer JB, Kunwald P, et al. The functional lumen imaging probe (FLIP) for evaluation of the esophagogastric junction. Am J Physiol Gastrointest Liver Physiol 2007;292:G377–84. [DOI] [PubMed] [Google Scholar]
- 10.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328–35. [DOI] [PubMed] [Google Scholar]
- 11.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013;11:1101–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil 2017;29: e12908. [DOI] [PubMed] [Google Scholar]
- 13.Carlson DA, Lin Z, Kahrilas PJ, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology 2015;149:1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional lumen imaging probe for the management of esophageal disorders: Expert review from the clinical practice updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatek MA, Kahrilas K, Soper NJ, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg 2010;14:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011;140:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker E, Sweis R, Anggiansah A, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil 2013;25:904–10. [DOI] [PubMed] [Google Scholar]
- 19.Rieder E, Swanstrom LL, Perretta S, et al. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc 2013; 27:400–5. [DOI] [PubMed] [Google Scholar]
- 20.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyawali CP, Carlson DA, Chen J, et al. ACG clinical guideline: Clinical use of esophageal physiologic testing. Am J Gastroenterol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beveridge CA, Falk GW, Ahuja NK, et al. Low yield of cross-sectional imaging in patients with esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2020;18:1643–4. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Gawron AJ. Achalasia: A systematic review. JAMA 2015; 313:1841–52. [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27: 160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Triggs JR, Carlson DA, Beveridge C, et al. Functional luminal imaging probe panometry identifies achalasia-type esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson DA, Hirano I, Zalewski A, et al. Improvement in esophageal distensibility in response to medical and diet therapy in eosinophilic esophagitis. Clin Transl Gastroenterol 2017;8:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016;48:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson DA, Crowell MD, Kimmel JN, et al. Loss of peristaltic reserve, determined by multiple rapid swallows, is the most frequent esophageal motility abnormality in patients with systemic sclerosis. Clin Gastroenterol Hepatol 2016;14:1502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson DA, Kahrilas PJ, Ritter K, et al. Mechanisms of repetitive retrograde contractions in response to sustained esophageal distension: A study evaluating patients with postfundoplication dysphagia. Am J Physiol Gastrointest Liver Physiol 2018;314:G334–G340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson DA, Gyawali CP, Kahrilas PJ, et al. Esophageal motility classification can be established at the time of endoscopy: A study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc 2019;90:915–23 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain AS, Carlson DA, Triggs J, et al. Esophagogastric junction distensibility on functional lumen imaging probe topography predicts treatment response in achalasia-anatomy matters!. Am J Gastroenterol 2019;114:1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su B, Callahan ZM, Novak S, et al. Using impedance planimetry (EndoFLIP) to evaluate myotomy and predict outcomes after surgery for achalasia. J Gastrointest Surg 2020;24:964–71. [DOI] [PubMed] [Google Scholar]
- 34.Verlaan T, Rohof WO, Bredenoord AJ, et al. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc 2013;78:39–44. [DOI] [PubMed] [Google Scholar]
- 35.Smeets FG, Masclee AA, Keszthelyi D, et al. Esophagogastric junction distensibility in the management of achalasia patients: Relation to treatment outcome. Neurogastroenterol Motil 2015;27:1495–503. [DOI] [PubMed] [Google Scholar]
- 36.Ngamruengphong S, von Rahden BH, Filser J, et al. Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: A multicenter study. Surg Endosc 2016;30:2886–94. [DOI] [PubMed] [Google Scholar]
- 37.Yoo IK, Choi SA, Kim WH, et al. Assessment of clinical outcomes after peroral endoscopic myotomy via esophageal distensibility measurements with the endoluminal functional lumen imaging probe. Gut Liver 2019;13: 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitelbaum EN, Boris L, Arafat FO, et al. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc 2013;27:4547–55. [DOI] [PubMed] [Google Scholar]
- 39.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc 2015;29:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu PI, Szczesniak MM, Craig PI, et al. Novel intra-procedural distensibility measurement accurately predicts immediate outcome of pneumatic dilatation for idiopathic achalasia. Am J Gastroenterol 2018; 113:205–12. [DOI] [PubMed] [Google Scholar]
- 41.DeHaan RK, Frelich MJ, Gould JC. Intraoperative assessment of esophagogastric junction distensibility during laparoscopic heller myotomy. Surg Laparosc Endosc Percutan Tech 2016;26:137–40. [DOI] [PubMed] [Google Scholar]
- 42.Ilczyszyn A, Hamaoui K, Cartwright J, et al. Intraoperative distensibility measurement during laparoscopic Heller’s myotomy for achalasia may reduce the myotomy length without compromising patient outcome. Dis Esophagus 2016;29:455–62. [DOI] [PubMed] [Google Scholar]
- 43.Teitelbaum EN, Sternbach JM, El Khoury R, et al. The effect of incremental distal gastric myotomy lengths on EGJ distensibility during POEM for achalasia. Surg Endosc 2016;30:745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol Motil 2017;29: e13104. [DOI] [PubMed] [Google Scholar]
- 45.Roman S, Holloway R, Keller J, et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol Motil 2017;29:e13067. [DOI] [PubMed] [Google Scholar]
- 46.Frazzoni L, Frazzoni M, de Bortoli N, et al. Postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance can link PPI-responsive heartburn to reflux better than acid exposure time. Neurogastroenterol Motil 2017;29:e13116. [DOI] [PubMed] [Google Scholar]
- 47.Frazzoni M, Frazzoni L, Tolone S, et al. Lack of improvement of impaired chemical clearance characterizes PPI-refractory reflux-related heartburn. Am J Gastroenterol 2018;113:670–6. [DOI] [PubMed] [Google Scholar]
- 48.Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol 2016;14:40–6. [DOI] [PubMed] [Google Scholar]
- 49.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastrooesophageal reflux disease. Aliment Pharmacol Ther 2011;34:476–86. [DOI] [PubMed] [Google Scholar]
- 50.Kahrilas PJ, Dodds WJ, Hogan WJ, et al. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology 1986;91:897–904. [DOI] [PubMed] [Google Scholar]
- 51.Simren M, Silny J, Holloway R, et al. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut 2003;52:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: A real added value? Neurogastroenterol Motil 2015;27:1423–31. [DOI] [PubMed] [Google Scholar]
- 53.Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil 2015;27:1175–82. [DOI] [PubMed] [Google Scholar]
- 54.Pandolfino JE, Shi G, Curry J, et al. Esophagogastric junction distensibility: A factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol 2002;282:G1052–8. [DOI] [PubMed] [Google Scholar]
- 55.Lottrup C, McMahon BP, Ejstrud P, et al. Esophagogastric junction distensibility in hiatus hernia. Dis Esophagus 2016;29:463–71. [DOI] [PubMed] [Google Scholar]
- 56.Carlson DA, Kathpalia P, Craft J, et al. The relationship between esophageal acid exposure and the esophageal response to volumetric distention. Neurogastroenterol Motil 2018;30:e13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su B, Callahan ZM, Kuchta K, et al. Use of impedance planimetry (Endoflip) in foregut surgery practice: Experience of more than 400 cases. J Am Coll Surg 2020;231:160–71. [DOI] [PubMed] [Google Scholar]
- 58.Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus 2014;27:637–44. [DOI] [PubMed] [Google Scholar]
- 59.Hoppo T, McMahon BP, Witteman BP, et al. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg 2011;15: 1112–20. [DOI] [PubMed] [Google Scholar]
- 60.Rinsma NF, Smeets FG, Bruls DW, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc 2014;28:941–9. [DOI] [PubMed] [Google Scholar]
- 61.Perretta S, McAnena O, Botha A, et al. Acta from the EndoFLIP(R) symposium. Surg Innov 2013;20:545–52. [DOI] [PubMed] [Google Scholar]
- 62.DeHaan RK, Davila D, Frelich MJ, et al. Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg Endosc 2017;31:193–8. [DOI] [PubMed] [Google Scholar]
- 63.Kim MP, Meisenbach LM, Chan EY. Tailored fundoplication with endoluminal functional lumen imaging probe allows for successful minimally invasive hiatal hernia repair. Surg Laparosc Endosc Percutan Tech 2018;28:178–82. [DOI] [PubMed] [Google Scholar]
- 64.Su B, Novak S, Callahan ZM, et al. Using impedance planimetry (EndoFLIP) in the operating room to assess gastroesophageal junction distensibility and predict patient outcomes following fundoplication. Surg Endosc 2020;34:1761–8. [DOI] [PubMed] [Google Scholar]
- 65.Smeets FG, Keszthelyi D, Bouvy ND, et al. Does measurement of esophagogastric junction distensibility by EndoFLIP predict therapy-responsiveness to endoluminal fundoplication in patients with gastroesophageal reflux disease? J Neurogastroenterol Motil 2015;21: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menard-Katcher C, Benitez AJ, Pan Z, et al. Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am J Gastroenterol 2017;112:1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sami SS, Haboubi HN, Ang Y, et al. UK guidelines on oesophageal dilatation in clinical practice. Gut 2018;67:1000–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y, McCullough GH. Maximal hyoid excursion in poststroke patients. Dysphagia 2010;25:20–5. [DOI] [PubMed] [Google Scholar]
- 69.Wu PI, Szczesniak MM, Maclean J, et al. Clinical utility of a functional lumen imaging probe in management of dysphagia following head and neck cancer therapies. Endoscopy 2017;49:848–54. [DOI] [PubMed] [Google Scholar]
- 70.Kappelle WF, Bogte A, Siersema PD. Hydraulic dilation with a shapemeasuring balloon in idiopathic achalasia: A feasibility study. Endoscopy 2015;47:1028–34. [DOI] [PubMed] [Google Scholar]
- 71.Ng K, Mogul D, Hollier J, et al. Utility of functional lumen imaging probe in esophageal measurements and dilations: A single pediatric center experience. Surg Endosc 2020;34:1294–9. [DOI] [PubMed] [Google Scholar]
- 72.Cox JG, Winter RK, Maslin SC, et al. Balloon or bougie for dilatation of benign esophageal stricture? Dig Dis Sci 1994;39:776–81. [DOI] [PubMed] [Google Scholar]
- 73.Saeed ZA, Winchester CB, Ferro PS, et al. Prospective randomized comparison of polyvinyl bougies and through-the-scope balloons for dilation of peptic strictures of the esophagus. Gastrointest Endosc 1995; 41:189–95. [DOI] [PubMed] [Google Scholar]
- 74.Scolapio JS, Pasha TM, Gostout CJ, et al. A randomized prospective study comparing rigid to balloon dilators for benign esophageal strictures and rings. Gastrointest Endosc 1999;50:13–7. [DOI] [PubMed] [Google Scholar]
- 75.Schnurre L, Murray F, Schindler V, et al. Short-term outcome after singular hydraulic eso-FLIP dilation in achalasia: A feasibility study. Neurogastroenterol Motil 2020;00:e13864. [DOI] [PubMed] [Google Scholar]
- 76.Carlson DA, Lin Z, Hirano I, et al. Evaluation of esophageal distensibility in eosinophilic esophagitis: An update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil 2016; 28:1844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


