Abstract
Objective
To investigate biomarkers of disease progression in cerebrospinal fluid (CSF) and serum in adult patients with spinal muscular atrophy (SMA). Furthermore, we assess the clinical response to nusinersen treatment in adults with SMA over a longer follow‐up period than the previously reported 6–14 months.
Methods
We included 16 adults with SMA type 3–4 for nusinersen treatment over 22 months in this prospective study. We evaluated chitotriosidase‐1 (CHIT1) and chitinase‐3‐like protein 1 (YKL‐40) as neuroinflammatory biomarkers in CSF, and neurofilament light chain (NfL) and heavy chain (pNfH) as neurodegenerative markers in CSF and serum at baseline, month 6, 14 and 22, together with a wide range of clinical outcome measures.
Results
Levels of CHIT1 increased significantly (p = 0.048) throughout the 22‐month treatment period and pNfH decreased significantly (p = 0.022) in CSF, but both did not correlate with clinical outcome measures. YKL‐40 correlated strongly with neurofilaments in CSF (rho = 0.76) and decreased significantly (p = 0.037) in patients with improvements in the revised upper limb module (RULM). Finally, patients showed significant improvements in hand grip strength, hand motor function, medical research council (MRC) sum score, and peak expiratory flow (PEF) after 22 months of treatment.
Interpretation
YKL‐40 in CSF correlated with clinical improvements during nusinersen treatment. In contrast, CHIT1 and pNfH in CSF changed significantly during treatment but did not correlate with clinical outcomes. Finally, we demonstrated a sustained clinical effect of nusinersen treatment in adults after 22 months.
Introduction
Spinal muscular atrophy (SMA) is a rare, autosomal‐recessive neuromuscular disease, most often caused by a homozygous deletion of the SMN1 gene. 1 The resulting lack of survival of motor neuron (SMN) protein leads to progressive muscle weakness and often respiratory insufficiency. SMA is classified into clinical subtypes 1 through 4, according to the age of onset and achievement of motor milestones. 2 Although SMA is generally considered as a neurodegenerative disorder, some evidence suggests that neuroinflammation may take part to the pathology of SMA, with microglia and astrocytes playing an important role in motor neuron damage. 3
Nusinersen is an antisense oligonucleotide (ASO) treatment that leads to an increased SMN protein production by the SMN2 genes and has recently been shown to be effective in both children and adults with SMA. 4 , 5 , 6 , 7 , 8 However, long‐term data of sustained therapeutic effect are lacking in adults with SMA as most studies are limited to 10–14 months of follow‐up. Additionally, there are currently no biomarkers in serum or cerebrospinal fluid (CSF) that can serve as outcome measures of disease progression and consistently correlate with therapeutic response in adults with SMA. Furthermore, despite some recent advances, there are still no validated clinical or biochemical biomarkers available that can predict individual clinical improvement, which will become increasingly relevant due to the advent of more therapeutic options. 9 , 10 , 11 , 12
Chitotriosidase 1 (CHIT1) and chitinase‐3‐like protein 1 (YKL‐40) are (neuro‐)inflammatory biomarkers that are expressed in the central nervous system by glial cells like microglia and astrocytes. 13 In amyotrophic lateral sclerosis (ALS), another motor neuron disease, studies showed that both CHIT1 and YKL‐40 were elevated in CSF and correlated with disease progression rate and a shorter survival. 14 , 15 No such data are currently available for individuals with SMA, except for a single recent study that concluded that baseline CHIT1 concentrations in CSF are elevated compared to controls and significantly increase over 14 months of treatment with nusinersen. 16
Other potentially interesting biomarkers for SMA patients are the neurodegenerative markers neurofilament light chain (NfL) and phosphorylated neurofilament heavy chain (pNfH), which were also found to be elevated in ALS and correlated with disease progression. 17 A recent study in infants with SMA type 1 showed that pNfH was elevated in plasma and CSF, correlated with multiple indicators of disease severity, and rapidly declined after treatment initiation with nusinersen. 18 In contrast, a few studies measuring neurofilaments in adults with SMA type 2 and 3 have shown contradictory results over a short period of follow‐up (6–14 months), making it difficult to interpret the relevance of these biomarkers. 19 , 20 , 21 , 22
In this study, we evaluated both neuroinflammatory and neurodegenerative biomarkers in CSF and serum to assess if they could be used to measure disease progression or even predict therapeutic response as a prognostic marker, as well as how they correlate with each other and to clinical outcome measures in adults with SMA. Additionally, we investigated the effect of nusinersen treatment on a wide range of clinical outcome measures in adults with SMA type 3–4 over a follow‐up period of 22 months.
Patients and Methods
Study design
Adults with SMA type 3–4 were included in the study and received intrathecal nusinersen injections every 4 months, after an initial loading phase. Inclusion criteria were a genetically confirmed 5q SMA, SMA types 3 or 4 and adult age at the time of enrollment (≥18 years). A relative contra‐indication for lumbar puncture, such as a history of scoliosis surgery, was an exclusion criterium. Out of 18 potential participants, two were excluded for medical reasons. As all 16 eligible participants preferred treatment with nusinersen, we could not include an untreated control group in the design of this study due to ethical reasons.
Intrathecal injection of nusinersen was performed after collection of 5 mL of CSF by standard lumbar puncture at each visit. In two patients with severe scoliosis, lumbar puncture was performed under imaging control (lumbar spine CT scan), followed by radioscopy‐guided intrathecal injection of nusinersen. CSF samples and concurrently drawn blood samples were immediately centrifuged (1995g for 10 min at 4°C for CSF and room temperature for blood) and stored into aliquots of 1 mL (Nunc CryoTube Vials, Thermo scientific, 375353) at −80°C until analysis. We measured the following clinical outcome measures at baseline, month 6, 14, and 22: bilateral hand grip strength, 60‐point Medical Research Council (MRC) sum score, 6‐minute walk distance (6MWD), Hammersmith Functional Motor Scale Expanded (HFMSE), Revised Upper Limb Module (RULM), Forced Vital Capacity (FVC), Peak Expiratory Flow (PEF) and the patient‐reported Activity Limitations scale (ActivLim). 23 , 24 , 25 , 26 , 27 , 28 , 29 Additionally, we calculated a 9‐point sub‐score of the RULM as a measure of hand motor function out of the following five tests: drawing a path, picking up tokens, pushing a button light, tearing folded paper, and opening a Ziploc container (tests C, D, G, H, and I), as described in De Wel et al. 8 The results of these outcome measures at month 14 have been described in detail previously. 8 At each visit, a standard blood sample was taken to measure serum creatine kinase (CK) levels.
Written informed consent was obtained from all participants and both the Ethics Committee Research UZ/KU Leuven (S62874) and the Belgian Federal Agency for Medicines and Health Products (FAGG) approved the study (EudraCT‐nr: 2019‐005007‐40). The study conforms with the World Medical Association Declaration of Helsinki.
Biomarkers in serum and CSF
We measured CHIT1 and YKL‐40 as markers of neuroinflammation in CSF, and NfL and pNfH as markers of neuronal damage in both CSF and serum at baseline, month 6, 14, and 22. All biomarkers were assessed at the same time points as the clinical outcome measures.
To assess CHIT1 in CSF, we used a commercial enzyme‐linked immunosorbent assay (ELISA) (cat # CY‐8074, CircuLex, distributed by MBL International, Woburn, MA). Samples were diluted 1:10 in dilution buffer, the limit of detection (LOD) of the assay was 48.3 pg/mL and the lower limit of quantification (LLOQ) was 56.3 pg/mL. Intra‐assay coefficient of variation (CV) of all samples was 4.6%.
CSF levels of YKL‐40 were assessed with an ELISA (cat # 8020, MicroVue, San Diego, CA; LOD: 5400 pg/mL; LLOQ: 15600 pg/ mL), after 1:2 dilution in dilution buffer. Intra‐assay CV of all samples was 4.2%.
NfL in CSF and serum samples were analyzed by Quanterix® (Lexington, MA) and pNfH samples in serum and CSF by BioAgilityx® (Durham, NC). NfL concentrations were assessed with the single‐molecule array (SiMoA®) NF‐light Advantage Kit by Quanterix® (LOD: 0.038 pg/mL, LLOQ: 0.174 pg/mL), which uses anti‐NfL monoclonal antibodies produced by Uman Diagnostics (Umeå, Sweden). CSF samples were diluted 1:100 and serum samples 1:4. Intra‐assay CV of all samples was 5.7% and inter‐assay CV was 8% (this was the only analysis in which for some patients (5/16) not all samples were tested on the same plate).
Concentrations of pNfH were assessed with Simple Plex® enzyme‐linked lectin assay (ELLA) (LOD: 1.17 pg/mL, LLOQ: 7.47 pg/mL). Both CSF and serum samples were each diluted 1:4. Intra‐assay CV of all samples was 2.1%. CHIT1, YKL‐40 and NfL measurements were performed in duplicate, and pNfH was analyzed in triplicate.
Statistical analysis
We used RStudio® Desktop (Open Source License, version 1.2.5001) for statistical analyses and creation of figures. We applied paired t‐tests for comparison of clinical outcome measures between visits at baseline and month 22, or the Wilcoxon signed‐rank test if criteria for normality were not met, as assessed by the Shapiro–Wilk test. Pearson and Spearman correlation coefficients were used to assess the associations between biomarkers and the following clinical variables of interest at baseline: height, age, sex, ambulatory status, disease duration, SMN2 copy number, SMA type, MRC sum score, hand grip strength, HFMSE, RULM, FVC, PEF, and ActivLim score.
Linear Mixed Models were used to analyze longitudinal biomarker data, with time‐point as a fixed effect and patient identification as a random effect to avoid pseudo‐replication in all standard models. For each biomarker, we created individual models with each abovementioned variable of interest by adding them as a fixed effect. If one of these additional variables was significant, they were added to the final model as fixed variables. This led to the addition of age and disease duration at baseline to the standard mixed effect models of pNfH and NfL in CSF and serum, as well as YKL‐40 in CSF. Additionally, the RULM score was added to the YKL‐40 model, and SMA type to the CHIT1 model. Significance level was determined at α = 0.05.
Results
Sixteen adults with SMA were included in this study. One patient missed the 22‐month visit because he experienced a pulmonary embolism. This was reported as a serious adverse event, without a suspected relation with nusinersen treatment. One other patient was lost to follow‐up after 18 months of treatment. Thus, all results at 22 months are derived from 14/16 patients (and for MRC sum score 13/16 patients due to missing data). Additionally, because 9/16 patients were non‐ambulatory in this study, we excluded the 6MWD from statistical analysis.
Patient characteristics are detailed in Table 1. The median age at loss of ambulation was 25 years (range 10–48) for non‐ambulatory SMA 3 patients. No patients needed non‐invasive ventilation (NIV) for respiratory failure, but three used continuous positive airway pressure (CPAP) for obstructive sleep apnea syndrome (OSAS). None of the patients had had scoliosis surgery, as this was a relative contra‐indication to participate in this study.
Table 1.
Study group demographic, genetic, and clinical characteristics.
| Characteristics | SMA patients (n = 16) |
|---|---|
| Gender | |
| Male [n (%)] | 10 (62.5%) |
| Female [n (%)] | 6 (37.5%) |
| Height [cm] | 172.5 (range: 148–187) |
| Weight [kg] | 63.5 (range: 45–82) |
| BMI [kg/m2] | 22.7 (range: 16–35) |
| SMA type [n (%)] | |
| 3 | 14 (87.5%) |
| 4 | 2 (12.5%) |
| *SMN2 copy number [n (%)] | |
| 3 | 13 (81.3%) |
| 4 | 2 (12.5%) |
| 5 | 1 (6.3%) |
| Age at symptom onset [y] | 6.5 (range: 1–30) |
| Disease duration [y] | 30.5 (range: 8–60) |
| Age at study inclusion [y] | 37.5 (range: 22–66) |
| Functional motor state | |
| Ambulatory | 7 (43.7%) |
| Non‐ambulatory | 9 (56.3%) |
Values shown as n (%) or median (range). *SMN2 quantification was done by quantitative polymerase chain reaction (qPCR) in eight patients, and by Multiplex Ligation Probe Amplification (MLPA) in the other eight patients. BMI, body mass index; SMA, spinal muscular atrophy; SMN2, survival of motor neuron 2 gene.
Neuroinflammatory biomarkers: CHIT1 & YKL‐40
CHIT1 concentration in CSF at baseline was median 1183 pg/mL (range: 294–7083) and levels of CHIT1 increased significantly throughout the study period (p = 0.048, +86 pg/mL/month; Fig. 1A). This increase was observed in all 16 individual participants. There were no significant correlations between CHIT1 concentrations in untreated patients at baseline and a range of clinical characteristic including height, age, sex, ambulatory status, disease duration, SMN2 copy number, SMA type, MRC sum score, hand grip strength, HFMSE, RULM, FVC, PEF, and ActivLim score. Additionally, none of these variables were associated with the significant increase in CHIT1 levels during the 22 months of treatment, except for the SMA type, as SMA type 4 patients experienced a faster increase in CHIT1 than SMA type 3 patients (p = 0.035).
Figure 1.
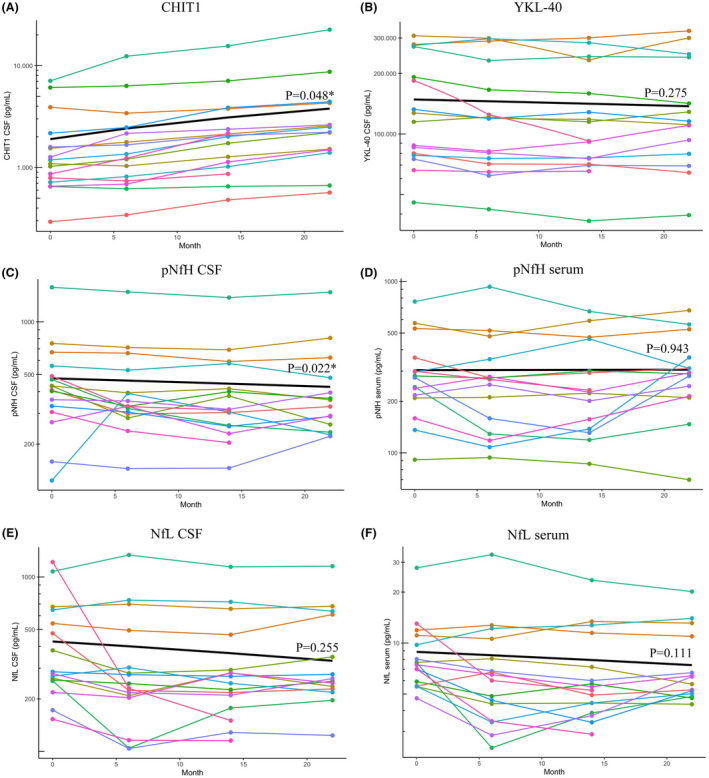
Evolution of CSF and serum biomarkers over 22 months of nusinersen treatment in adults with SMA. All figures have a log10 transformed y‐axis, with values from the measurement range of each specific biomarker added on the y‐axis for optimal readability. Each patient is represented by an individual colored line. For 2/16 patients, biomarker measurements at month 22 were not available. (A) CHIT1 concentrations increase significantly over 22 months of treatment with nusinersen. (B) YKL‐40 levels decline during the study, but this change is not significant. (C and D) pNfH in CSF decreases significantly throughout the study, but pNfH levels in serum remain unchanged. For one patient, serum pNfH levels were unmeasurable low. (E and F) Both NfL in CSF and in serum show decreasing trends that are not significant. *Denotes a significant p‐value. Abbreviations: CHIT1, chitotriosidase‐1; YKL‐40, chitinase‐3‐like protein 1; CSF, cerebrospinal fluid; pNfH, phosphorylated neurofilament heavy chain; NfL, neurofilament light chain.
YKL‐40 concentrations in CSF showed a decreasing trend during treatment with nusinersen but this was not significant (p = 0.275; Fig. 1B). However, an improvement in the RULM score was significantly associated with a decrease in YKL‐40 concentrations during the treatment period (p = 0.037, −1887 pg/mL per 1‐point increase in RULM score, Fig. 2). The median YKL‐40 concentration in untreated patients at baseline was 120,978 pg/mL (range: 45,638–307,828) and correlated strongly with age (rho = 0.76, p < 0.01; Fig. 3). A moderate correlation with disease duration was also found, but this became insignificant after the age of patients was taken into account.
Figure 2.
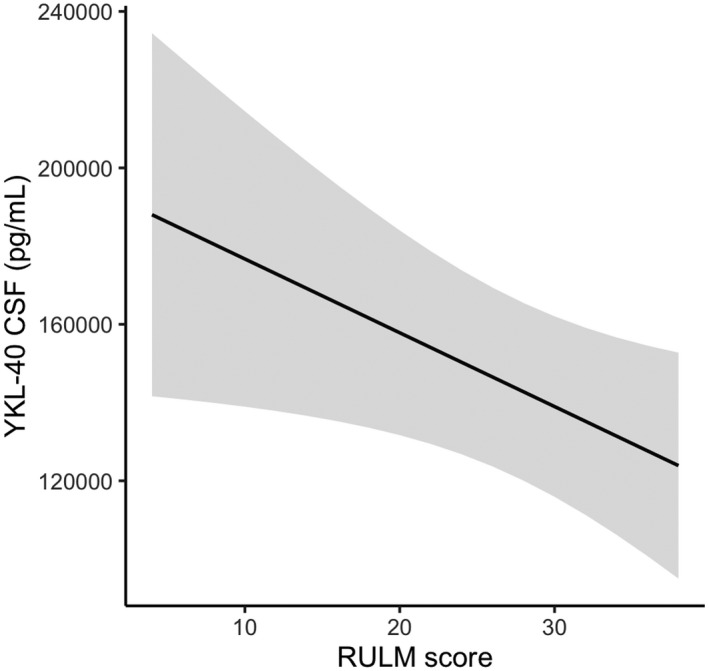
Association between decrease in YKL‐40 CSF levels and improvements in RULM score during treatment with nusinersen. YKL‐40 levels decreased more in patients showing improvements in the RULM score during treatment with nusinersen. The gray zone surrounding the regression line represents the confidence interval. Abbreviations: YKL‐40, chitinase‐3‐like protein 1; CSF, cerebrospinal fluid; RULM, Revised Upper Limb Module.
Figure 3.
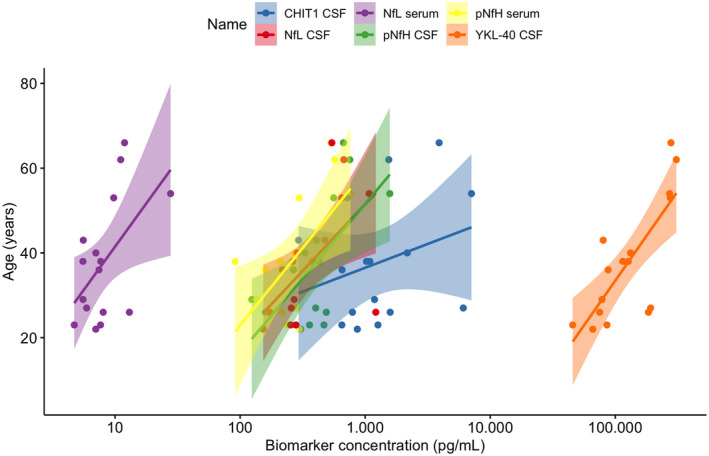
Correlation of biomarkers in CSF and serum with age in untreated adults with SMA at baseline. All biomarkers, except for CHIT1 in CSF and NfL in serum, significantly correlated with age, meaning that higher levels were observed with increasing age: YKL‐40 (rho = 0.76, p < 0.001), pNfH CSF (rho = 0.59, p = 0.016), pNfH serum (r = 0.71, p = 0.002), NfL CSF (rho = 0.66, p = 0.006), NfL serum (rho = 0.43, p = 0.099) and CHIT1 (rho = 0.28, p = 0.288). Abbreviations: CHIT1, chitotriosidase‐1; YKL‐40, chitinase‐3‐like protein 1; CSF, cerebrospinal fluid; pNfH, phosphorylated neurofilament heavy chain; NfL, neurofilament light chain.
Neurodegenerative biomarkers: pNfH & NfL
Concentrations of pNfH in CSF declined significantly during treatment with nusinersen (p = 0.022, −2.3 pg/mL/month), whereas pNfH concentrations in serum did not change from baseline (p = 0.943; Fig. 1C and D). Baseline pNfH concentration was median 419 pg/mL (range: 124–1574) in CSF, and median 275 pg/mL (range: 91–763) in serum. For one patient, serum pNfH concentrations were unmeasurable low and samples were retested in a 1:2 dilution but remained below the limit of quantification. Baseline median NfL was 283 pg/mL (range: 153–1216) in CSF, and 8 pg/mL (range: 5–28) in serum, and both showed a decreasing trend under treatment with nusinersen that was not significant (p = 0.255 and p = 0.111, respectively; Fig. 1E and F). For both pNfH and NfL in CSF and serum none of the variables of interest mentioned in the previous section were significantly associated with the evolution of these biomarkers during treatment with nusinersen. Baseline pNfH levels did correlate with age in both CSF (rho = 0.59, p = 0.016) and serum (r = 0.69, p = 0.004; Fig. 3). Levels of pNfH in serum also correlated with disease duration at baseline (r = 0.59, p = 0.012). For NfL, baseline levels in CSF correlated positively with age (rho = 0.66, p = 0.006; Fig. 3) and disease duration (rho = 0.50, p = 0.047), and inversely with patients' MRC sum scores (rho = −0.56, p = 0.031), with lower muscle strength scores associated with higher NfL CSF concentrations.
Because some patients experienced a rapid decrease in neurofilament levels during the loading phase (Fig. 1C–F), we performed a specific post hoc analysis of the evolution of neurofilaments between baseline and month 6. For this purpose, we analyzed CSF and serum samples at baseline, 2, 4, 8 weeks and 6 months. This analysis did not show different results compared to the changes observed over 22 months of treatment, with only pNfH in CSF decreasing significantly (p = 0.010). Next, we evaluated if a relevant decrease in neurofilaments (defined as >2× the intra‐assay CV%, as 95% of data lie within 2× standard deviation (SD) from the mean) in the first 6 months of nusinersen treatment was a prognostic indicator of improvements in clinical outcome measures after 6, 14 or 22 months of treatment. However, no association between early neurofilament decreases and early or long‐term clinical effects of nusinersen was observed.
Correlations between the different biomarkers in CSF and serum
The correlations between the four biomarkers in CSF of untreated patients with SMA at baseline are shown in Figure 4. We observed a strong correlation at baseline between the neurodegenerative biomarkers pNfH and NfL in CSF. In contrast, there was no significant correlation between the neuroinflammatory biomarkers CHIT1 and YKL‐40 in CSF at baseline. YKL‐40 significantly correlated with both pNfH and NfL levels in CSF at baseline, but CHIT1 did not.
Figure 4.
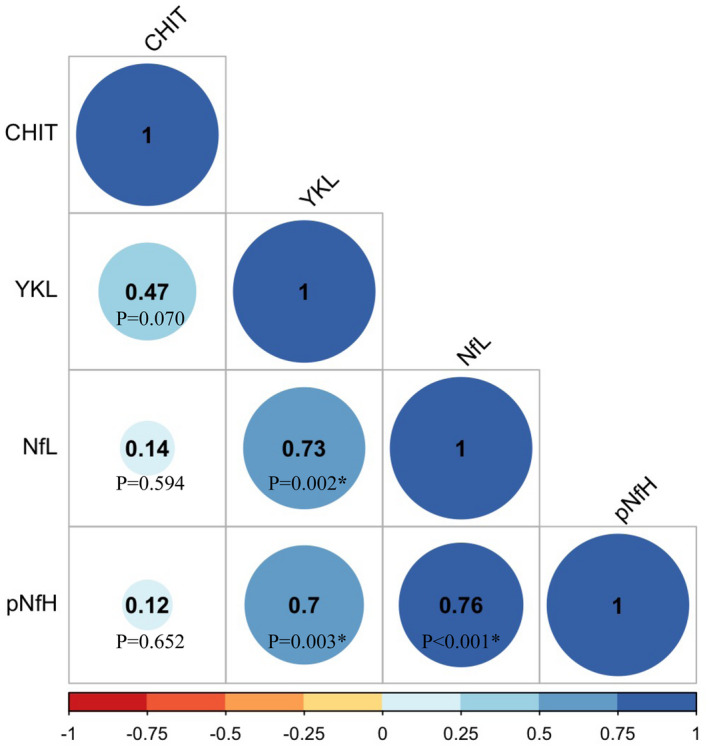
Correlogram of biomarkers in CSF of untreated adults with SMA at baseline. The correlogram shows the spearman's rho correlation coefficient and accompanying p‐values between all four biomarkers in CSF of untreated adults with SMA at baseline. We observed a strong correlation between YKL‐40 and neurofilaments in CSF, as well as between the neurofilaments in CSF. CHIT1 did not correlate with any of the other biomarkers. *Denotes a significant p‐value. Abbreviations: CHIT1, chitotriosidase‐1; YKL‐40, chitinase‐3‐like protein 1; CSF, cerebrospinal fluid; pNfH, phosphorylated neurofilament heavy chain; NfL, neurofilament light chain.
Finally, there was no correlation between the increase in CHIT1 levels and the decrease in pNfH levels throughout the 22 months of treatment in the patients with SMA.
In serum, we observed a moderately strong correlation at baseline between pNfH and NfL (rho = 0.57, p = 0.029). Baseline pNfH levels in serum also correlated with pNfH levels in CSF (rho = 0.62, p = 0.013), but this was not the case for NfL levels in serum and CSF (rho = 0.47, p = 0.070).
Clinical outcome measures after 22 months of nusinersen treatment
Hand grip strength significantly improved on average by 54% (p = 0.002) and 50% (p = 0.010) in the right and left hand at month 22 compared to baseline, respectively (Table 2). Additionally, we noted significant improvements in the 60‐point MRC sum score (+7%, p = 0.039) and PEF (+6%, p = 0.011) at month 22 compared to baseline. The RULM hand score, a 9‐point subscore of the RULM pertaining to hand motor function, also improved significantly at month 22 compared to baseline (+8%, p = 0.027). This increase in hand motor function was significantly associated with the measured increase in hand grip strength over 22 months (p = 0.022). We did not observe further significant improvements in these outcome measures at month 22 when compared to month 14 evaluations instead of baseline.
Table 2.
Clinical outcome measures at baseline and after 22 months of nusinersen treatment.
| Outcome measures | Baseline | 22‐month analysis | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p‐values | |
| Hand grip strength (right) [Kg] | 6.94 ± 8.84 | 10.70 ± 9.54 | 0.002 |
| Hand grip strength (left) [Kg] | 6.25 ± 9.31 | 9.36 ± 11.4 | 0.010 |
| MRC sum score | 36.9 ± 10.3 | 39.5 ± 8.96 | 0.039 |
| HFMSE [score] | 27.3 ± 19.8 | 28.1 ± 20.8 | 0.916 |
|
RULM [score] |
27.1 ± 8.10 | 27.8 ± 9.45 | 0.262 |
| RULM hand score | 7.44 ± 2.1 | 8.07 ± 1.62 | 0.027 |
| FVC [L] | 4.03 ± 1.16 | 4.17 ± 1.32 | 0.087 |
| PEF [L/sec] | 7.19 ± 1.91 | 7.60 ± 2.05 | 0.011 |
| ActivLim [logits] | −1.80 ± 2.68 | −1.28 ± 2.77 | 0.094 |
p‐values in bold are significant at α = 0.05. SD, standard deviation; MRC, medical research council; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; FVC, Forced Vital Capacity; PEF, Peak Expiratory Flow; ActivLim, Activity Limitations scale.
The classic RULM, HFMSE, FVC and the patient‐reported ActivLim score all showed improvements at month 22 compared to baseline that was not significant. Serum CK levels remained stable over 22 months of nusinersen treatment (p = 0.218).
We could not detect a difference in clinical improvement based on sex, although both FVC and PEF were higher at baseline in males (p = 0.002 and 0.035, respectively). When evaluating the ambulatory and non‐ambulatory subgroups separately, we found that ambulatory patients only improved in hand grip strength and PEF, and non‐ambulatory patients only improved in MRC sum score.
Discussion
We showed that during treatment with nusinersen a decrease in YKL‐40 CSF levels was associated with improvements in the RULM score. The neuroinflammatory biomarker CHIT1 increased and pNfH decreased significantly in CSF over 22 months of follow‐up, but this did not correlate with clinical outcome measures. Furthermore, we observed a strong correlation between YKL‐40, pNfH, and NfL in CSF of untreated patients at baseline. The neurofilament decreases in some individuals in the first 6 months after treatment initiation were not predictive of early or late clinical improvements. Finally, this study demonstrated the prolonged therapeutic effect of nusinersen treatment in adults with SMA 3–4, as evidenced by a sustained significant improvement of hand grip strength, hand motor function, MRC sum score, and PEF after 22 months of treatment.
CHIT1 levels increase significantly during treatment, but do not correlate with clinical outcome
CHIT1 concentrations in CSF increased significantly throughout the 22‐month treatment period in all 16 individual participants. However, because CHIT1 is a microglial marker, and microglial activity decreases in SMA mice treated with SMN enhancing ASOs, we would have expected a decrease in CHIT1 levels, just as we saw a decreasing trend for YKL‐40 following nusinersen treatment in the SMA patient group, rather than an increase. 30 The increase in CHIT1 is thus probably not related to a therapeutic effect but might be caused by the repeated intrathecal administrations of an ASO leading to a mild inflammatory response, as suggested by Freigang et al. (and supported by increased total protein levels in multiple studies). 16 , 31 , 32 After completion of the study, we analyzed CSF samples of 11/16 patients at month 30 of the treatment period, and found that four patients showed increased protein levels of which three had the highest CHIT1 levels in the study. No patients showed a pleocytosis. A connection with the recently discovered “nusinophages” (macrophages with unidentified inclusions that are only found in CSF of nusinersen‐treated patients) is possible and should be investigated in the future, as well as what can be expected of the natural evolution of CHIT1 in untreated adults with SMA. 33 Nevertheless, considering that CHIT1 did not correlate with any clinical outcome measures, either during treatment with nusinersen or at baseline, we conclude that it does not appear to be a relevant outcome measure to assess therapeutic efficacy.
Interestingly, the correlation between CHIT1 and YKL‐40 in untreated patients at baseline just failed to reach significance, despite the fact that both are expressed by glial cells that are known to play a role in neuroinflammation in SMA. 3 However, this could simply be due to the sample size of the study. The observed faster increase in CHIT1 in SMA type 4 (n = 2) versus type 3 (n = 14) patients might have a similar explanation.
Lastly, we did not evaluate patients for the presence of a common gene polymorphism that leads to a CHIT1 enzyme deficiency, but as none of the patients had CHIT1 values below the LLOQ, and CHIT1 levels increased in every patient during the study, this probably did not have a meaningful impact on our findings. 34
The association between clinical improvements in the RULM score and decreasing levels of YKL‐40 suggests that this neuroinflammatory biomarker could play a role in the follow‐up of adult SMA patients treated with nusinersen. Furthermore, it could be hypothesized that the non‐significant decrease in YKL‐40 over time is an effect of nusinersen treatment, as normally concentrations increase significantly during the aging process. Indeed, YKL‐40 strongly correlated with age in untreated patients at baseline in this study, which is in line with observations in other neurological diseases. 35 However, due to the lack of an untreated control group (which would pose an ethical dilemma), this could not be evaluated in this study. This is the first study to assess YKL‐40 in adult SMA patients, and even though the role of this biomarker has not yet been fully elucidated, our findings warrant further research in a larger group of patients.
In contrast with neurofilaments, we only measured CHIT1 and YKL‐40 in CSF and not in serum, because both biomarkers are highly susceptible to change in serum due to a myriad of non‐neurological diseases such as atherosclerosis, diabetes mellitus, asthma, infectious and inflammatory diseases, and various types of cancers, among others. 34 , 36 It is very difficult to correct for all these different factors, which makes these (neuro‐) inflammatory markers in serum less interesting as potential biomarkers of disease progression, as opposed to in CSF.
Neurofilament heavy chain levels decrease significantly in nusinersen‐treated adult SMA patients
Only pNfH in CSF declined significantly during the treatment period, but this did not correlate with clinical improvements. Nevertheless, this decrease is probably a therapeutic effect of nusinersen treatment, as we know from ALS studies that pNfH levels in CSF remain stable in untreated patients and even increase in serum in early stages of that disease. 17 , 37 NfL in CSF correlated with one marker of disease severity at baseline (MRC sum score) but did not change significantly during treatment or correlate with clinical improvements. We also showed that early decreases of neurofilament levels in CSF or serum of individual patients with SMA were not predictive of improvements in clinical outcome measures after 6, 14 or 22 months of treatment. These results indicate that neurofilaments are probably not relevant outcome measures for disease progression in adults with SMA, as opposed to in infants with SMA type 1 or other neurological diseases such as ALS. 18 One possible explanation is that both SMA type 1, and ALS are considerably more rapidly progressing diseases with more fulminant neuronal damage. Another reason could be that nusinersen treatment in adults produces an overall stabilization, as opposed to more pronounced improvements in infants. Elevation of pNfH levels and subsequent decline after nusinersen treatment initiation in infants with SMA in the NURTURE trial were also most pronounced in those with 2 SMN2 copies rather than three copies, such as most patients in the present study had. 38
Finally, the finding that NfL in CSF was not correlated to NfL in serum is surprising, especially considering CSF and serum levels of pNfH did correlate.
Nusinersen leads to further clinical improvements after 22 months of treatment
We observed a further improvement in hand grip strength, hand motor function, MRC sum score, and PEF after 22 months of treatment with nusinersen, thus providing evidence for a sustained therapeutic effect beyond previous reports at 6, 10, or 14 months. 6 , 7 , 8 We previously showed that even the sometimes small numeric increases in the outcome measures observed here can be clinically relevant to patients. 8 The continuous improvement in PEF became significant at the 22‐month evaluation compared to baseline, which suggests that spirometry should be included in the follow‐up of nusinersen‐treated patients with SMA. In contrast, previous reports have suggested the opposite, based on the fact that natural history studies did not show a further deterioration of pulmonary function in untreated adult patients, and two studies reported only transient early increases in pulmonary function with nusinersen. 7 , 8 , 39 Interestingly, even though both PEF and FVC showed numeric improvements compared to baseline, only PEF improved significantly. This is not an incongruous finding, as these tests measure different aspects of respiration. Indeed, previous research has shown that measures of expiratory muscle strength (such as PEF) correlate with increased muscle size in a pulmonary rehabilitation program, whereas volume measures of pulmonary function (such as FVC) do not. 40
The main limitations of this study are the rather small sample size and the lack of an untreated control group as explained above. Comparison of subgroups of patients was therefore purely exploratory. Furthermore, considering that SMA is generally slowly progressive in adults, a longer follow‐up period than 22 months could be necessary to detect significant changes in biomarkers and/or clinical outcome measures. Nevertheless, we feel that these results offer important insights that should be explored in further studies.
We conclude that YKL‐40 in CSF correlated with clinical improvements during treatment and warrants further research. Concentrations of CHIT1 and pNfH in CSF changed significantly during nusinersen treatment but did not correlate with clinical outcome, at least for the study duration of 22 months. An early decline of neurofilaments in CSF or serum after treatment initiation was not predictive of early or late clinical improvements. Finally, adults with SMA 3–4 treated with nusinersen showed an overall clinical stabilization as well as improvements in hand grip strength, hand motor function, MRC sum score, and PEF after 22 months.
Author Contributions
All authors were involved in the design of the study and editing of the manuscript. BDW and KGC were responsible for writing the manuscript, and BDW and MDS performed all data analyses.
Acknowledgments
KGC is Chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. KGC is member of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD) and of the European Reference Network for Rare Neurological Diseases (ERN‐RND).
Annals of Clinical and Translational Neurology 2022;9(8): 1241–1251
Funding Information This study was partially funded by Biogen. BDW and MDS are supported by the Research Foundation—Flanders (FWO, PhD fellowship fundamental research grant numbers 1159121N and 11E6319N, respectively). KP is a senior clinical investigator of the Research Foundation—Flanders (FWO, Fonds voor Wetenschappelijk Onderzoek Flanders, Belgium) (grant number 18B2622N).
Funding Statement
This work was funded by Biogen ; FWO (Research Foundation ‐ Flanders) grants 1159121N, 11E6319N, and 18B2622N.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33:831‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zerres K, Schöneborn SR. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52:518‐523. [DOI] [PubMed] [Google Scholar]
- 3. Abati E, Citterio G, Bresolin N, Comi GP, Corti S. Glial cells involvement in spinal muscular atrophy: could SMA be a neuroinflammatory disease? Neurobiol Dis. 2020;140:104870. [DOI] [PubMed] [Google Scholar]
- 4. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. N Engl J Med. 2017;377:1723‐1732. [DOI] [PubMed] [Google Scholar]
- 5. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later‐onset spinal muscular atrophy. N Engl J Med. 2018;378:625‐635. [DOI] [PubMed] [Google Scholar]
- 6. Hagenacker T, Wurster CD, Günther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non‐interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19:317‐325. [DOI] [PubMed] [Google Scholar]
- 7. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q‐SMA type 3 – a prospective observational study. J Neuromuscul Dis. 2019;6:453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Wel B, Goosens V, Sobota A, et al. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J Neurol. 2021;268:923‐935. [DOI] [PubMed] [Google Scholar]
- 9. Baranello G, Darras BT, Day JW, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384:915‐923. [DOI] [PubMed] [Google Scholar]
- 10. Mendell JR, Al‐Zaidy S, Shell R, et al. Single‐dose gene‐replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713‐1722. [DOI] [PubMed] [Google Scholar]
- 11. Gavriilaki M, Moschou M, Papaliagkas V, et al. Biomarkers of disease progression in adolescents and adults with 5q spinal muscular atrophy: a systematic review and meta‐analysis. Neuromuscul Disord. 2022;32(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 12. Schorling DC, Kölbel H, Hentschel A, et al. Cathepsin D as biomarker in cerebrospinal fluid of nusinersen‐treated patients with spinal muscular atrophy Eur J Neurol. 2022;29(7):2084–2096. [DOI] [PubMed] [Google Scholar]
- 13. Andrés‐Benito P, Domínguez R, Colomina MJ, Llorens F, Povedano M, Ferrer I. YKL40 in sporadic amyotrophic lateral sclerosis: cerebrospinal fluid levels as a prognosis marker of disease progression. Aging. 2018;10:2367‐2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson AG, Gray E, Thézénas ML, et al. Cerebrospinal fluid macrophage biomarkers in amyotrophic lateral sclerosis. Ann Neurol. 2018;83:258‐268. [DOI] [PubMed] [Google Scholar]
- 15. Gille B, De Schaepdryver M, Dedeene L, et al. Inflammatory markers in cerebrospinal fluid: independent prognostic biomarkers in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2019;90:1338‐1346. [DOI] [PubMed] [Google Scholar]
- 16. Freigang M, Steinacker P, Wurster CD, et al. Increased chitotriosidase 1 concentration following nusinersen treatment in spinal muscular atrophy. Orphanet J Rare Dis. 2021;16:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poesen K, De Schaepdryver M, Stubendorff B, et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology. 2017;88:2302‐2309. [DOI] [PubMed] [Google Scholar]
- 18. Darras BT, Crawford TO, Finkel RS, et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6:932‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faravelli I, Meneri M, Saccomanno D, et al. Nusinersen treatment and cerebrospinal fluid neurofilaments: an explorative study on spinal muscular atrophy type 3 patients. J Cell Mol Med. 2020;24:3034‐3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wurster CD, Günther R, Steinacker P, et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther Adv Neurol Disord. 2019;12:756286419846058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wurster CD, Steinacker P, Günther R, et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol. 2020;267:36‐44. [DOI] [PubMed] [Google Scholar]
- 22. Rich KA, Fox A, Yalvac M, et al. Neurofilament levels in CSF and serum in an adult SMA cohort treated with Nusinersen. J Neuromuscul Dis. 2022;9:111‐119. [DOI] [PubMed] [Google Scholar]
- 23. Merlini L, Mazzone ES, Solari A, Morandi L. Reliability of hand‐held dynamometry in spinal muscular atrophy. Muscle Nerve. 2002;26:64‐70. [DOI] [PubMed] [Google Scholar]
- 24. Seferian AM, Moraux A, Canal A, et al. Upper limb evaluation and one‐year follow up of non‐ambulant patients with spinal muscular atrophy: an observational multicenter trial. PLoS One. 2015;10:e0121799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kleyweg RP, van der Meché FGA, Schmitz PIM. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barré syndrome. Muscle Nerve. 1991;14:1103‐1109. [DOI] [PubMed] [Google Scholar]
- 26. Dunaway Young S, Montes J, Kramer SS, et al. Six‐minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. 2016;54:836‐842. [DOI] [PubMed] [Google Scholar]
- 27. O'Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the hammersmith functional motor scale for SMA II and III patients. Neuromuscul Disord. 2007;17:693‐697. [DOI] [PubMed] [Google Scholar]
- 28. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2017;55:869‐874. [DOI] [PubMed] [Google Scholar]
- 29. Batcho CS, van den Bergh P, van Damme P, et al. How robust is ACTIVLIM for the follow‐up of activity limitations in patients with neuromuscular diseases? Neuromuscul Disord. 2016;26:211‐220. [DOI] [PubMed] [Google Scholar]
- 30. Ando S, Osanai D, Takahashi K, Nakamura S, Shimazawa M, Hara H. Survival motor neuron protein regulates oxidative stress and inflammatory response in microglia of the spinal cord in spinal muscular atrophy. J Pharmacol Sci. 2020;144:204‐211. [DOI] [PubMed] [Google Scholar]
- 31. Wurster CD, Koch JC, Cordts I, et al. Routine cerebrospinal fluid (CSF) parameters in patients with spinal muscular atrophy (SMA) treated with Nusinersen. Front Neurol. 2019;10:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1–2 trial of antisense oligonucleotide Tofersen for SOD1 ALS. N Engl J Med. 2020;383:109‐119. [DOI] [PubMed] [Google Scholar]
- 33. Gingele S, Hümmert MW, Alvermann S, et al. Routine cerebrospinal fluid cytology reveals unique inclusions in macrophages during treatment with Nusinersen. Front Neurol. 2019;10:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithel Biol Pharmacol. 2012;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Craig‐Schapiro R, Perrin RJ, Roe CM, et al. YKL‐40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng Y, Li G, Chang D, Su X. YKL‐40 as a novel biomarker in cardio‐metabolic disorders and inflammatory diseases. Clin Chim Acta. 2020;511:40‐46. [DOI] [PubMed] [Google Scholar]
- 37. De Schaepdryver M, Goossens J, De Meyer S, et al. Serum neurofilament heavy chains as early marker of motor neuron degeneration. Ann Clin Transl Neurol. 2019;6:1971‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wijngaarde CA, Veldhoen ES, van Eijk RPA, et al. Natural history of lung function in spinal muscular atrophy. Orphanet J Rare Dis. 2020;15:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kyeongbong L. Correlation between respiratory muscle strength and pulmonary function with respiratory muscle length increase in healthy adults. Phys Ther Rehabil Sci. 2021;10:398‐405. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


