Abstract
Objective
To examine associations of antidepressant, anxiolytic and hypnotic use amongst older women (≥65 years) with incident Parkinson's Disease (PD), using data from Women's Health Initiative linked to Medicare claims.
Methods
PD was defined using self‐report, first diagnosis, medications and/or death certificates and psychotropic medications were ascertained at baseline and 3‐year follow‐up. Cox regression models were constructed to calculate adjusted hazard ratios (aHR) with 95% confidence intervals (CI), controlling for socio‐demographic, lifestyle and health characteristics, overall and amongst women diagnosed with depression, anxiety and/or sleep disorders (DASD).
Results
A total of 53,996 WHI participants (1,756 PD cases)—including 27,631 women diagnosed with DASD (1,137 PD cases)—were followed up for ~14 years. Use of hypnotics was not significantly associated with PD risk (aHR = 0.98, 95% CI: 0.82, 1.16), whereas PD risk was increased amongst users of antidepressants (aHR = 1.75, 95% CI: 1.56, 1.96) and anxiolytics (aHR = 1.48, 95% CI: 1.25, 1.73). Compared to non‐users of psychotropic medications, those who used 1 type had ~50% higher PD risk, whereas those who used ≥2 types had ~150% higher PD risk. Women who experienced transitions in psychotropic medication use (‘use to non‐use’ or ‘non‐use to use’) between baseline and 3‐year follow‐up had higher PD risk than those who did not. We obtained similar results with propensity scoring and amongst DASD‐diagnosed women.
Interpretation
The use of antidepressants, anxiolytics or multiple psychotropic medication types and transitions in psychotropic medication use was associated with increased PD risk, whereas the use of hypnotics was not associated with PD risk amongst older women.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder and the most frequently diagnosed movement disorder affecting nearly 1 million individuals in the United States. 1 , 2 , 3 PD is the outcome of progressive degeneration of dopaminergic neurons in the nigrostriatal pathway, which leads to dopamine deficiency in the substantia nigra pars compacta located in the midbrain. 2 , 4 , 5 PD dementia is likely related to Lewy bodies which are cytoplasmic aggregates of insoluble α‐synuclein. 2 , 4 , 5 Whereas 30% of PD is heritable, the majority of PD cases are sporadic in nature or have been attributed to environmental influences. 5 , 6 , 7 According to the biopsychosocial model, stressful life events and chronic stress can lead to a maladaptive state that can adversely impact an individual's physical and mental health through neuroendocrine, immune and behavioural mechanisms and potentially lead to neurodegeneration. 8 , 9 , 10 Depression, anxiety and sleep disorders are frequently co‐morbid non‐motor symptoms (NMS) of PD, which may precede or co‐occur with PD motor symptoms, are highly prevalent but often unrecognized or under‐treated and may be detrimental to a PD patient's quality of life. 2 , 3 , 11 There is growing evidence from case–control and retrospective cohort studies that widely used psychotropic medications—including antidepressants, anxiolytics and hypnotics for the treatment of depression, anxiety and sleep disorders—can impact PD risk, progression and outcomes with implications for public health and preventive medicine. 4 , 12 , 13 , 14 , 15 Previous studies have also suggested that psychiatric disorders, such as depression and anxiety, as well as psychotropic medications used to treat these disorders, may be prodromal to the onset of PD motor symptoms. 3 , 13 , 16 Despite the fact that sleep disorders may be linked to neurodegeneration, 3 few studies have evaluated the role of hypnotics in PD. 17 , 18 In a case–control study of 959 prevalent cases of parkinsonism (767 with PD) and 1989 controls, Dick and colleagues 4 found that antidepressant (OR = 1.92, 95% CI: 1.49, 2.49), anxiolytic (OR = 1.95, 95% CI: 1.54, 2.47) and hypnotic (OR = 1.33, 95% CI: 1.07, 1.65) medication use for >1 year were independent predictors of PD/parkinsonism. 4 Available studies have separately examined distinct types of psychotropic medications but have not examined concurrent use or trajectories in the use of distinct psychotropic medication types in relation to PD risk. 13 , 19 More importantly, the extant literature often failed to establish a temporal relationship between psychotropic medication use and PD risk by the use of longitudinal study designs or the exclusion of PD cases that occur within a few years of follow‐up. In addition, the extant literature often failed to deal with issues of confounding by indication through propensity score analysis or by restricting their samples to individuals affected by depression, anxiety and/or sleep disorders.
Women are considered a high‐risk group for depression, anxiety and/or sleep disorders as well as psychotropic medication use for the treatment of these conditions particularly after menopause. 20 , 21 To date, no studies have adequately targeted older women ≥65 years of age who account for a large proportion of late‐onset PD cases having characteristically more severe motor symptoms, more frequent NMS, a faster course and shorter survival than early‐onset PD. 22 , 23 We performed a secondary analysis of longitudinal data from the Women's Health Initiative (WHI) study to evaluate whether antidepressant, anxiolytic and hypnotic medication use was associated with incident PD in women ≥65 years of age, overall, and amongst those diagnosed with depression, anxiety and/or sleep disorders, by applying risk adjustment and propensity score analysis. We hypothesized that psychotropic medication use, at baseline and over time, is causally or non‐causally related to PD, by being a modifiable risk factor for PD, a marker of disease severity or a prodromal feature of PD and can, therefore, identify high‐risk groups for future PD diagnosis amongst women ≥65 years of age, which may benefit from monitoring for the development of PD motor and non‐motor symptoms within healthcare settings.
Methods
Women's Health Initiative
WHI collected longitudinal data on a multiethnic sample of postmenopausal women who were recruited and enrolled between 1993 and 1998 at 40 geographically diverse clinical centres (24 states and the District of Columbia) in the United States. The WHI study received institutional review board approval with informed consent from all participating clinical centres. The WHI study design, eligibility criteria, recruitment methods and measurement protocols are described elsewhere. 24 , 25 Briefly, the WHI clinical trials (WHI‐CTs) (n = 68,132) and the WHI observational study (WHI‐OS) (n = 93,676) are two components of the WHI (n = 161,808). Within the clinical trial component, four overlapping WHI‐CTs evaluated outcomes of menopausal hormone therapy (hormone therapy [HT] trials), calcium and vitamin D supplementation ([CaD] Trial) and a low‐fat eating pattern (Dietary Modification Trial). The WHI‐OS evaluated causes of morbidity and mortality outcomes. The main WHI studies ended in 2005 and of 150,076 participants who were actively followed up at the end of these studies, 76.9% participated in Extension Study 1 (2005–2010) and 86.9% of those eligible participated in Extension Study 2 (2010–2020). 26 , 27 At baseline, WHI participants, 50–79 years of age, completed the same self‐administered questionnaire covering demographics, general health, clinical and anthropometric characteristics, medical history, personal habits as well as medication use and several of these characteristics were assessed at later follow‐up times. In this study, we followed WHI participants using linked WHI‐Medicare data. The research was conducted in accordance with the Helsinki Declaration as revised in 1989. This study received an exempt determination from the institutional review board at Walter Reed National Military Medical Center.
Design and participants
In this retrospective cohort study, WHI‐CT and WHI‐OS participants, ≥65 years of age at baseline, were included in the study if they had available WHI‐Medicare (Part A and/or Part B) linked data. Furthermore, we excluded women with self‐reported PD or International Classification of Diseases (9th Revision, Clinical Modification) [ICD‐9‐CM] primary or secondary codes from Medicare data that reflect a PD diagnosis at WHI baseline. We also excluded WHI participants with ICD‐9‐CM Clinical Classification Software (CCS) codes indicating other mental health problems besides depression and/or anxiety disorders, with the exception of those having ICD‐9‐CM codes for sleep disorders. We further excluded WHI participants who reported using medications at baseline or during the follow‐up visits with Assigned Therapeutic Classes of ‘Antipsychotic/Antimanic’, ‘Attention deficit hyperactivity disorder (ADHD)/Anti‐narcoplepsy/Anti‐obesity/Anorexiants’ and ‘psychotherapeutic and neurological agents (miscellaneous)’ as well as those with a comorbidity of delirium, dementia and amnestic and other cognitive disorders based on Medicare data. These subjects were excluded because healthcare‐seeking behaviours linked to these medications and diagnoses can result in differential misclassification of a PD diagnosis. Finally, we excluded women who had <12 months of WHI‐Medicare linked data after the baseline visit and those with missing data on key variables. A sub‐analysis was performed which excluded women without a history of depression, anxiety and/or sleep disorders at baseline (Tables S1‐S2).
Linkage of Women's Health Initiative to medicare data
The U.S. Centers for Medicare & Medicaid Services (CMS) Medicare is the federal health insurance program for individuals ≥65 years and those <65 years who have disabilities or end‐stage renal disease. The WHI and CMS Medicare data were linked together amongst consenting participants using their social security number, date of birth and, if needed, date of death and residential zip code. Nearly 97% of Medicare‐eligible participants were successfully linked. Data linkage was made for participation in traditional fee‐for‐service (80%) but not Medicare Health Maintenance Organization programs (20%). The linkage between WHI and Medicare data was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Details regarding Medicare files can be obtained from the Research Data Assistance Center (http://www.resdac.org/cms‐data). 28 , 29 , 30 , 31 , 32 , 33 Study analyses were restricted to WHI participants who were ≥65 years and enrolled in fee‐for‐service Medicare Parts A and B at baseline.
Study variables
Depression, anxiety and/or sleep disorders
Diagnoses of mental health problems were based on twelve ICD‐9‐CM CCS codes. 34 We used available Medicare (Medicare Provider Analysis and Review [MEDPAR], outpatient and carrier) data to define depression (CCS codes for ‘mood disorders’) and anxiety (CCS codes for ‘anxiety disorders’) disorders at baseline as dichotomous (‘yes’ or ‘no’) variables. Additionally, ICD‐9‐CM primary and secondary diagnostic codes classified sleep disorders as insomnia, sleep‐related breathing disorders, hypersomnia, circadian rhythm sleep disorder, parasomnia, sleep‐related movement disorder and other sleep disorders. 35 We used available Medicare (MEDPAR, outpatient and carrier) data to define sleep disorders at baseline as a dichotomous (‘yes’ or ‘no’) variable. Depression, anxiety and sleep disorders at baseline were examined in relation to psychotropic medication use in bivariate and propensity score analyses. Furthermore, a sub‐sample of study‐eligible WHI participants was defined using ICD‐9‐CM/CCS codes to include those diagnosed with depression, anxiety and/or sleep disorders at baseline (Table S1).
Psychotropic medication use
Antidepressant, anxiolytic and hypnotic use
WHI participants were instructed to bring prescription and non‐prescription medication containers to the baseline and follow‐up visits. For medications used for >2 weeks, drug names and doses were entered into a medications database and assigned therapeutic class codes using the Master Drug Data Base (MDDB: Medi‐Span, Indianapolis, IN; Medi‐Span software: First DataBank, Inc., San Bruno, CA) (Table S2). Dichotomous (‘yes’ or ‘no’) antidepressant, anxiolytic and hypnotic use were ascertained at baseline (form 44) and 3 years of follow‐up (form 153). Amongst study‐eligible participants who completed both visits, slight agreement existed between baseline and 3‐year follow‐up visits on psychotropic medication use in the overall sample (antidepressant (κ = 0.36, 95% CI: 0.34, 0.37), anxiolytic (κ = 0.26, 95% CI: 0.24, 0.28), hypnotic (κ = 0.19, 95% CI: 0.17, 0.21)) and a sub‐sample of women diagnosed with depression, anxiety and/or sleep disorders (antidepressant (κ = 0.33, 95% CI: 0.31, 0.35), anxiolytic (κ = 0.27, 95% CI: 0.25, 0.30) and hypnotic (κ = 0.19, 95% CI: 0.17, 0.22)). As such, psychotropic medication use was carried forward for women with no follow‐up data, and a sensitivity analysis that relied on baseline data alone was performed.
Patterns of psychotropic medication use
Antidepressants, anxiolytics and hypnotics were considered three distinct psychotropic medication types. Amongst subjects having medication use data at baseline and 3 years of follow‐up, patterns of psychotropic medication use were defined as a categorical variable (‘no psychotropic medication [baseline and follow‐up]’; ‘1 type of psychotropic medication [baseline and/or follow‐up]’; ‘≥2 types of psychotropic medications [baseline and/or follow‐up]’).
Transitions in psychotropic medication use
Amongst subjects having medication use data at baseline and on 3 years of follow‐up, transitions in any type of psychotropic medication (antidepressant, anxiolytic and/or hypnotic) between baseline and 3‐year follow‐up were evaluated as follows: Increase (‘non‐use to use’) for at least one type of psychotropic medication; Decrease (‘use to non‐use’) for at least one type of psychotropic medication without an increase in other types; No change in use of psychotropic medication types. A more detailed definition that relies on patterns of psychotropic medication use was evaluated in relation to PD risk for exploratory purposes.
Parkinson's disease
Incident PD was defined at each available year of follow‐up, including self‐reported PD from the WHI medical update form, Medicare (MEDPAR, outpatient and carrier) codes that reflect the first occurrence of PD primary or secondary diagnosis using ICD‐9‐CM and International Classification of Diseases (10th Revision [ICD‐10]) diagnostic codes (as appropriate), use of medications consistent with PD diagnosis (forms 44 and 153) and/or deaths attributed to PD (Tables S1‐S2). 36 The level of agreement between PD definitions with and without ICD‐9‐CM/ICD‐10 diagnostic codes was estimated amongst eligible subjects (κ = 0.44, 95% CI: 0.42, 0.47). Time‐to‐event, whether PD onset, loss to follow‐up or death, were calculated from the baseline questionnaire until December 31, 2018, whichever came first, whilst ensuring the exclusion of PD cases and follow‐up time between baseline and 3 years of follow‐up to account for immortal time bias.
Socio‐demographic, lifestyle and health characteristics
Besides WHI component, socio‐demographic (age, race/ethnicity, marital status, education, household income, lifestyle (smoking status, alcohol use, physical activity) and health (body mass index (BMI), history of cardiovascular disease, history of hypertension, history of diabetes, history of hyperlipidemia, self‐rated health) characteristics were collected at baseline. Frequency and duration of walking, mild, moderate and strenuous physical activities over the past week were assessed and total weekly kilocalories of energy expenditure were calculated in metabolic equivalents. History of cardiovascular disease was defined in terms of previous coronary heart disease, angina, aortic aneurysm, carotid endarterectomy or angioplasty, atrial fibrillation, congestive heart failure, cardiac arrest, stroke or transient ischemic attack. History of diabetes was defined as physician‐diagnosed diabetes or use of diabetes medications. History of hyperlipidemia was defined as using lipid‐lowering medications or having been told of high cholesterol by a physician. Self‐rated health was assessed using one item: ‘In general would you say your health is excellent, very good, good, fair or poor? 26 , 37
Statistical analysis
All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Summary statistics included means (±standard deviations) for continuous variables and frequencies with percentages for categorical variables. Bivariate associations were examined using Chi‐square, independent samples t‐tests or one‐way analysis of variance, as appropriate. We also fit Cox proportional hazards models to examine associations of psychotropic medication use with incident PD, after controlling for socio‐demographic, lifestyle and health characteristics through risk adjustment and propensity scoring. These two methods were applied in order to examine hypothesized associations, before and after taking into account confounding by indication. The proportional hazards assumptions were assessed using Schoenfeld residuals. Hypothesized relationships were examined in the overall sample and amongst a sub‐sample of women diagnosed with depression, anxiety and/or sleep disorders at baseline. Propensity score analysis is an alternative method to multivariable regression that ensures treatment groups have equal distribution on characteristics affecting outcome and that differences in outcomes between various treatments can be attributed to treatment. 38 , 39 We calculated propensity scores or predicted probabilities of antidepressant, anxiolytic and hypnotic use for each subject based on a set of relevant characteristics using a two‐step procedure: (1) Use of antidepressants, anxiolytics and hypnotics were modelled as outcome variables in a priori stepwise logistic regression models whereby the disorder of concern along with a subset of WHI component, socio‐demographic, lifestyle and health characteristics were selected as predictors; (2) predicted probabilities of antidepressant, anxiolytic or hypnotic use (‘propensity scores’) were calculated and used as weights within Cox regression models that examined these exposures as predictors of PD risk. We further applied the CAUSALTRT procedure with the inverse probability of treatment weighting (IPTW) option to graphically examine and verify the balance in propensity scores as well as socio‐demographic, lifestyle and health characteristics according to treatment selection. Furthermore, we stratified risk‐adjusted models by (1) time‐to‐event (<5 years vs. ≥5 years), (2) age at baseline (<70 vs. ≥ 70) and (3) WHI component (CT vs. OS) to account for the potential violation of the proportional hazards assumption as well as temporality and design differences between WHI components. Finally, we performed sensitivity analyses in fully risk‐adjusted models, to assess: (1) validity of PD definition by repeating key analyses without ICD‐9‐CM/ICD‐10 codes; (2) validity of antidepressant, anxiolytic and hypnotic use definitions by repeating key analyses with only baseline exposure data; (3) association of antidepressant and anxiolytic medication use with PD amongst individuals with sleep disorders only; (4) interaction effects of psychotropic medication and self‐rated health in relation to PD. Since <5% of potentially eligible subjects had missing data on covariates, complete subject analyses were performed. Two‐tailed statistical tests were assessed at α = 0.05.
Results
As shown in Figure 1, 71,010 of 161,808 WHI participants reported age ≥ 65 years at baseline and of those 53,996 remained after applying all eligibility criteria; these women were followed for an average of 14.39 (±6.18) years, yielding 1,756 (3.25%) PD cases. Of those, 17,266 subjects had psychotropic medication data available at baseline and follow‐up visits. A sub‐sample of 27,631 subjects was diagnosed with depression, anxiety and/or sleep disorders at baseline and followed for an average of 14.29 (±5.93) years, yielding 1,137 (4.11%) PD cases. Of those, 14,803 subjects had psychotropic medication data available at baseline and follow‐up visits.
Figure 1.
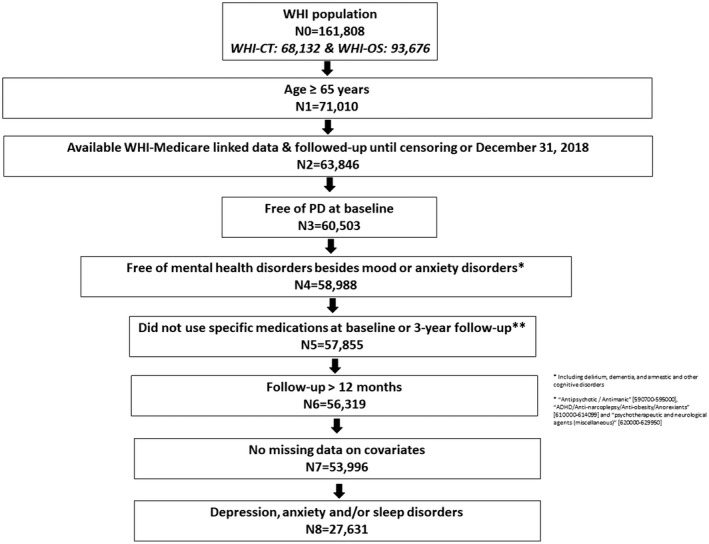
Study Flowchart.
Psychotropic medication use by socio‐demographic, lifestyle and health characteristics of the overall analytic sample are displayed in Tables 1, 2, 3, 4. The estimated prevalence rates of antidepressant, anxiolytic and hypnotic medication use were ~15%, ~7% and ~8%, respectively. Whereas ~23% used one type of psychotropic medication, 5% used two or three types of psychotropic medications simultaneously. Between baseline and follow‐up visits, approximately 23% experienced a change in psychotropic medication use including ~13% who switched from non‐use to use of any medication type [Increase] and 10% who switched from use to non‐use of any medication type without an increase in other types [Decrease]. Psychotropic medication use generally varied significantly according to baseline characteristics. White, Asian, younger and obese women (with a history of cardio‐metabolic risk factors) and those with worse self‐rated health were more likely to use anti‐depressants, anxiolytics and/or hypnotics. Furthermore, psychotropic medication use differed significantly amongst those with and without a diagnosis of depression, anxiety and/or sleep disorders.
Table 1.
Antidepressant, anxiolytic, hypnotic and patterns of psychotropic medication use by socio‐demographic characteristics of study sample—Women's Health Initiative (n = 53,996) a .
| Overall | N = 53,996 |
Patterns of psychotropic medication use (N = 29,537) |
|||||
|---|---|---|---|---|---|---|---|
| N (%) | Antidepressant | Anxiolytic | Hypnotic | 0 | 1 | ≥2 | |
| % | % | % | % | % | % | ||
| Overall | 53,996 (100) | 14.97 | 6.77 | 7.86 | 72.55 | 22.96 | 4.50 |
| WHI component | p < 0.0001 | p = 0.22 | p < 0.0001 | p < 0.0001 | |||
| CT | 20,576 (38.11) | 17.34 | 6.94 | 9.14 | 71.13 | 23.77 | 5.10 |
| OS | 33,420 (61.89) | 13.52 | 6.67 | 7.07 | 73.56 | 22.37 | 4.07 |
| Age (years) | p < 0.0001 | p = 0.09 | p = 0.03 | p = 0.24 | |||
| 65–69 | 27,021 (50.04) | 15.97 | 6.81 | 7.90 | 72.31 | 23.12 | 4.57 |
| 70–74 | 18,950 (35.10) | 14.67 | 6.94 | 8.09 | 72.53 | 22.89 | 4.58 |
| >74 | 8,025 (14.86) | 12.31 | 6.23 | 7.17 | 74.01 | 22.20 | 3.80 |
| Race/Ethnicity | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| White | 41,558 (76.96) | 16.02 | 6.98 | 8.65 | 71.97 | 23.35 | 4.68 |
| Black | 2,431 (4.50) | 10.16 | 5.92 | 4.81 | 79.16 | 18.11 | 2.73 |
| Asian | 8,707 (16.13) | 12.60 | 6.56 | 5.72 | 70.84 | 25.31 | 3.85 |
| Other | 1,300 (2.41) | 6.31 | 3.00 | 2.62 | 87.83 | 11.02 | 1.15 |
| Marital status | p < 0.0001 | p = 0.002 | p < 0.0001 | p < 0.0001 | |||
| Married/Partnered | 30,330 (56.17) | 15.68 | 7.08 | 8.30 | 71.76 | 23.50 | 4.74 |
| Single | 2,122 (3.93) | 11.69 | 5.37 | 5.89 | 77.55 | 19.12 | 3.33 |
| Divorced | 6,526 (12.09) | 15.89 | 6.67 | 7.26 | 71.40 | 23.87 | 4.73 |
| Widowed | 15,018 (27.81) | 13.60 | 6.39 | 7.51 | 74.31 | 21.73 | 3.95 |
| Education | p = 0.0005 | p < 0.0001 | p = 0.16 | p < 0.0001 | |||
| Less than high school | 3,172 (5.87) | 14.50 | 9.17 | 6.90 | 71.17 | 23.66 | 5.17 |
| High school graduate | 10,395 (19.25) | 15.97 | 7.46 | 8.08 | 70.82 | 24.06 | 5.12 |
| Some college | 20,794 (38.51) | 15.23 | 6.97 | 7.95 | 71.54 | 23.84 | 4.62 |
| College graduate | 19,635 (36.36) | 14.24 | 5.80 | 7.79 | 74.38 | 21.57 | 4.04 |
| Household income | p = 0.046 | p < 0.0001 | p = 0.0003 | p = 0.07 | |||
| < $20,000 | 10,978 (20.33) | 15.07 | 7.68 | 7.45 | 71.63 | 23.65 | 4.72 |
| $20,000–$49,999 | 25,784 (47.75) | 15.33 | 6.77 | 8.04 | 71.95 | 23.45 | 4.60 |
| $50,000–$99,999 | 10,861 (20.11) | 14.64 | 6.16 | 8.33 | 73.32 | 22.29 | 4.39 |
| ≥$100,000 | 2,685 (4.97) | 14.38 | 5.66 | 8.16 | 74.79 | 21.22 | 3.99 |
| Unknown | 3,688 (6.83) | 13.61 | 6.64 | 6.21 | 74.51 | 21.49 | 4.00 |
Abbreviations: CT, clinical trial; OS, observational study; WHI, Women's Health Initiative.
p values were the outcome of Pearson's Chi‐square tests, independent samples t‐tests or one‐way analysis of variance tests, as appropriate.
Table 2.
Antidepressant, anxiolytic, hypnotic and patterns of psychotropic medication use by lifestyle and health characteristics of study sample—Women's Health Initiative (n = 53,996). a
| Overall | N = 53,996 |
Patterns of psychotropic medication use (N = 29,537) |
|||||
|---|---|---|---|---|---|---|---|
| N (%) | Antidepressant | Anxiolytic | Hypnotic | 0 | 1 | ≥2 | |
| % | % | % | % | % | % | ||
| Overall | 53,996 (100) | 14.97 | 6.77 | 7.86 | 72.55 | 22.96 | 4.50 |
| Smoking status | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Never | 28,686 (53.13) | 13.92 | 6.22 | 7.07 | 74.68 | 21.28 | 4.04 |
| Past | 22,622 (41.90) | 16.11 | 7.27 | 8.75 | 70.32 | 24.71 | 4.97 |
| Current | 2,688 (4.98) | 16.56 | 8.44 | 8.74 | 65.81 | 28.21 | 5.98 |
| Alcohol use | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Non‐drinker | 6,671 (12.35) | 13.22 | 6.24 | 5.80 | 75.66 | 21.06 | 3.27 |
| Former drinker | 10,299 (19.07) | 18.03 | 8.00 | 7.24 | 68.62 | 26.01 | 5.37 |
| <1 drink / week | 16,995 (31.47) | 14.77 | 6.40 | 7.43 | 73.46 | 22.25 | 4.30 |
| ≥1 drink / week | 20,031 (37.10) | 14.18 | 6.63 | 9.23 | 72.54 | 22.82 | 4.63 |
|
Physical activity (Met‐hours/week) |
p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Mean (SD) | 12.62 (13.39) |
11.47 (12.91) 12.83 (13.47) |
11.36 (13.11) 12.72 (13.40) |
11.74 (12.74) 12.70 (13.44) |
13.87 (13.79) | 12.57 (12.86) | 11.25 (13.37) |
| Body Mass Index (kg/m2) | p < 0.0001 | p = 0.05 | p = 0.18 | p < 0.0001 | |||
| <25 | 19,607 (36.31) | 13.12 | 6.95 | 7.58 | 74.30 | 21.44 | 4.27 |
| 25–29.9 | 19,692 (36.47) | 15.31 | 6.91 | 7.98 | 72.24 | 23.13 | 4.62 |
| ≥30 | 14,697 (27.22) | 16.99 | 6.33 | 8.07 | 70.40 | 24.95 | 4.65 |
| Medical history | |||||||
| Cardiovascular disease | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Yes | 14,267 (26.42) | 18.01 | 8.66 | 8.73 | 66.74 | 27.43 | 5.83 |
| No | 39,729 (73.58) | 13.88 | 6.09 | 7.54 | 74.23 | 21.66 | 4.11 |
| Hypertension | p < 0.0001 | p < 0.0001 | p = 0.007 | p < 0.0001 | |||
| Yes | 27,774 (51.44) | 19.74 | 7.47 | 8.16 | 70.14 | 24.94 | 4.92 |
| No | 26,222 (28.56) | 14.32 | 6.03 | 7.54 | 74.70 | 21.18 | 4.12 |
| Diabetes | p < 0.0001 | p < 0.0001 | p = 0.76 | p < 0.0001 | |||
| Yes | 6,515 (12.07) | 16.42 | 7.98 | 7.95 | 68.01 | 26.87 | 5.12 |
| No | 47,481 (87.93) | 13.44 | 6.60 | 7.85 | 73.18 | 22.41 | 4.41 |
| Hyperlipidemia | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Yes | 9,672 (17.91) | 17.38 | 8.09 | 8.89 | 68.81 | 25.43 | 5.76 |
| No | 44,324 (82.09) | 14.45 | 6.48 | 7.63 | 73.30 | 22.46 | 4.24 |
| Self‐rated health | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Excellent | 7,778 (14.40) | 10.23 | 4.37 | 6.83 | 79.60 | 17.79 | 2.61 |
| Very good | 22,249 (41.20) | 12.98 | 5.47 | 7.52 | 74.98 | 21.30 | 3.72 |
| Good | 19,073 (35.32) | 17.10 | 7.86 | 8.23 | 67.78 | 26.46 | 5.76 |
| Fair | 4,580 (8.48) | 22.79 | 12.05 | 9.69 | 56.70 | 33.03 | 10.11 |
| Poor | 316 (0.59) | 29.75 | 15.19 | 7.91 | 52.70 | 41.89 | 4.51 |
| Depression, Anxiety and/or sleep disorders | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Yes | 27,631 (51.17) | 6.23 | 9.75 | 10.55 | 59.27 | 33.11 | 7.62 |
| No | 26,365 (48.32) | 23.31 | 3.64 | 5.04 | 85.90 | 12.75 | 1.36 |
Abbreviations: CT, clinical trial; OS, observational study; WHI, Women's Health Initiative.
p values were the outcome of Pearson's Chi‐square tests, independent samples t‐tests or one‐way analysis of variance tests, as appropriate.
Table 3.
Transitions in psychotropic medication use by socio‐demographic characteristics of study sample—Women's Health Initiative (n = 29,537). a
| Overall |
Transitions in psychotropic medication use (N = 29,537) |
|||
|---|---|---|---|---|
| N (%) | Decrease | Same | Increase | |
| % | % | % | ||
| Overall | 29,537 (100) | 9.48 | 77.25 | 13.26 |
| WHI component | p < 0.0001 | |||
| CT | 12,271 (41.54) | 11.72 | 76.27 | 12.01 |
| OS | 17,266 (58.46) | 7.89 | 77.95 | 14.15 |
| Age (years) | p = 0.012 | |||
| 65–69 | 16,948 (57.38) | 9.82 | 8.77 | 9.88 |
| 70–74 | 9,665 (32.72) | 77.07 | 77.39 | 77.87 |
| >74 | 2,924 (9.90) | 13.12 | 13.83 | 12.24 |
| Race/Ethnicity | p < 0.0001 | |||
| White | 26,578 (89.98) | 9.67 | 76.75 | 13.59 |
| Black | 1,209 (4.09) | 8.11 | 82.55 | 9.35 |
| Asian | 1,142 (3.87) | 9.46 | 76.53 | 14.01 |
| Other | 608 (2.06) | 4.28 | 90.13 | 5.59 |
| Marital status | p < 0.0001 | |||
| Married/Partnered | 17,977 (60.86) | 9.21 | 76.81 | 13.98 |
| Single | 1,051 (3.56) | 8.75 | 81.16 | 10.09 |
| Divorced | 3,276 (11.09) | 10.01 | 76.13 | 13.86 |
| Widowed | 7,233 (24.49) | 10.02 | 78.29 | 11.68 |
| Education | p < 0.0001 | |||
| Less than high school | 1,103 (3.73) | 10.43 | 77.15 | 12.42 |
| High school graduate | 5,316 (18.00) | 11.14 | 75.34 | 13.53 |
| Some college | 11,150 (37.75) | 10.01 | 76.54 | 13.45 |
| College graduate | 11,968 (40.52) | 8.17 | 78.78 | 13.05 |
| Household income | p < 0.0001 | |||
| <$20,000 | 4,618 (15.63) | 11.00 | 76.68 | 12.32 |
| $20,000–$49,999 | 14,419 (48.82) | 9.86 | 76.38 | 13.77 |
| $50,000–$99,999 | 6,946 (23.52) | 8.45 | 78.55 | 13.00 |
| ≥$100,000 | 1,781 (6.03) | 7.58 | 79.23 | 13.19 |
| Unknown | 1,773 (6.00) | 8.46 | 78.79 | 12.75 |
Abbreviations: CT, clinical trial; OS, observational study; WHI, Women's Health Initiative.
p values were the outcome of Pearson's Chi‐square tests or one‐way analysis of variance tests, as appropriate.
Table 4.
Transitions in psychotropic medication use by lifestyle and health characteristics of study sample—Women's Health Initiative (n = 29,537). a
| Overall |
Transitions in psychotropic medication use (N = 29,537) |
|||
|---|---|---|---|---|
| N (%) | Decrease | Same | Increase | |
| % | % | % | ||
| Overall | 29,537 (100) | 9.48 | 77.25 | 13.26 |
| Smoking status | p < 0.0001 | |||
| Never | 16,196 (54.83) | 8.83 | 78.87 | 12.30 |
| Past | 12,288 (41.60) | 10.15 | 75.59 | 14.26 |
| Current | 1,053 (3.57) | 11.78 | 71.70 | 16.52 |
| Alcohol use | p < 0.0001 | |||
| Non‐drinker | 3,176 (10.75) | 8.53 | 80.67 | 10.80 |
| Former drinker | 4,694 (15.89) | 11.48 | 74.58 | 13.93 |
| <1 drink / week | 9,520 (32.23) | 9.47 | 77.93 | 12.59 |
| ≥1 drink / week | 12,147 (41.12) | 8.97 | 76.86 | 14.18 |
| Physical activity (Met‐hours/week) | p < 0.0001 | |||
| Mean (SD) | 13.47 (13.56) | 11.33 (12.07) | 13.78 (13.74) | 13.19 (13.34) |
| Body Mass Index (kg/m2) | p < 0.0001 | |||
| <25 | 11,061 (37.45) | 8.44 | 78.45 | 13.12 |
| 25–29.9 | 11,053 (37.42) | 9.70 | 76.99 | 13.31 |
| ≥30 | 7,423 (25.13) | 10.72 | 75.86 | 13.42 |
| Medical history | ||||
| Cardiovascular disease | p < 0.0001 | |||
| Yes | 6,635 (22.46) | 12.01 | 73.22 | 14.77 |
| No | 22,902 (77.54) | 8.75 | 78.42 | 12.83 |
| Hypertension | p < 0.0001 | |||
| Yes | 13,923 (47.14) | 10.23 | 75.52 | 14.26 |
| No | 15,614 (52.86) | 8.82 | 78.80 | 12.38 |
| Diabetes | p < 0.0001 | |||
| Yes | 3,610 (12.22) | 11.75 | 74.54 | 13.71 |
| No | 25,927 (87.78) | 9.17 | 77.63 | 13.20 |
| Hyperlipidemia | p < 0.0001 | |||
| Yes | 4,951 (16.76) | 10.81 | 74.77 | 14.42 |
| No | 24,586 (83.24) | 9.22 | 77.75 | 13.03 |
| Self‐rated health | p < 0.0001 | |||
| Excellent | 5,255 (17.79) | 6.72 | 82.06 | 11.23 |
| Very good | 13,389 (45.33) | 8.35 | 79.02 | 12.63 |
| Good | 9,305 (31.50) | 11.40 | 73.99 | 14.61 |
| Fair | 1,514 (5.13) | 16.91 | 65.59 | 17.50 |
| Poor | 74 (0.25) | 17.57 | 64.86 | 17.57 |
| Depression, Anxiety and/or sleep disorders | p < 0.0001 | |||
| Yes | 14,803 (50.12) | 13.09 | 66.93 | 19.99 |
| No | 14,734 (49.88) | 5.86 | 87.63 | 6.51 |
Abbreviations: CT, clinical trial; OS, observational study; WHI, Women's Health Initiative.
p values were the outcome of Pearson's Chi‐square tests or one‐way analysis of variance tests, as appropriate.
Psychotropic medication use as risk or protective factors for PD is presented in the overall analytic sample (Table 5) and amongst a sub‐sample of WHI participants diagnosed with depression, anxiety and/or sleep disorders (Table 6). In fully adjusted Cox regression models, the use of hypnotics was not significantly associated with PD risk in the overall sample (HR = 0.98, 95% CI: 0.82, 1.16, p = 0.80) or the sub‐sample (HR = 0.93, 95% CI: 0.77, 1.13, p = 0.47). By contrast, PD risk was increased amongst users of antidepressants in the overall sample (HR = 1.75, 95% CI: 1.56, 1.96, p < 0.0001) and the sub‐sample (HR = 1.62, 95% CI: 1.43, 1.84, p < 0.0001). Similarly, PD risk was increased amongst users of anxiolytics in the overall sample (HR = 1.48, 95% CI: 1.25, 1.73, p < 0.0001) and the sub‐sample (HR = 1.36, 95% CI: 1.14, 1.62, p < 0.0001). Compared to non‐users of psychotropic medications, those who used one type of psychotropic medications had ~50% increased risk of PD in the overall sample (HR = 1.49, 95% CI: 1.29, 1.72, p < 0.0001) and the sub‐sample (HR = 1.58, 95% CI: 1.32, 1.88, p < 0.0001) and those who used ≥2 types of psychotropic medications had ~150% increased PD risk in the overall sample (HR = 2.43, 95% CI: 1.92, 3.06, p < 0.0001) and the sub‐sample (HR = 2.25, 95% CI: 1.74, 2.93, p < 0.0001). In the overall sample, women who experienced an increase (HR = 1.62, 95% CI: 1.37, 1.92, p < 0.0001) or a decrease (HR = 1.30, 95% CI: 1.05, 1.61, p = 0.0145) in psychotropic medication use between baseline and follow‐up were at elevated risk for PD compared to those without medication change. Similar results were obtained amongst sub‐sample women with an increase (HR = 1.57, 95% CI: 1.29, 1.89, p < 0.0001) or a decrease (HR = 1.28, 95% CI: 1.00, 1.64, p = 0.0491) in psychotropic medication use. Besides WHI participants censored within 5 years from baseline, sub‐group analyses based on time‐to‐event, age and WHI component revealed similar results to the main analyses with respect to the relationship between psychotropic medication use and PD risk (Tables S3‐S4). We observed similar results after using a PD definition that did not cover ICD‐9‐CM/ICD‐10 codes or, defining antidepressant, anxiolytic and hypnotic medication use according to baseline data alone (Tables S5‐S6). A detailed definition for transitions suggested distinct levels of PD risk according to baseline and 3‐year follow‐up psychotropic medication use, with the highest risk amongst those having ≥2 psychotropic medication types at baseline and 1 psychotropic medication type at 3‐year follow‐up visit (Table S7). Moreover, individuals with sleep disorders only who used antidepressants or anxiolytics were not at significantly higher risk of PD than non‐users (Table S8) and the relationship between psychotropic medication use and PD risk did not vary according to self‐rated health (Table S9).
Table 5.
Psychotropic medication use as a risk factor for Parkinson's disease—Women's Health Initiative (n = 52,700).
| Unadjusted | Model I a | Model II b | Model III c | |
|---|---|---|---|---|
| HR (95% CI) | ||||
| Antidepressants | n = 52,700 | |||
| Yes | 1.81 (1.62, 2.03) | 1.85 (1.66, 2.07) | 1.86 (1.63, 2.05) | 1.75 (1.56, 1.96) |
| No | Ref. | Ref. | Ref. | Ref. |
| Anxiolytic | n = 52,700 | |||
| Yes | 1.56 (1.33, 1.83) | 1.56 (1.33, 1.83) | 1.51 (1.32, 1.82) | 1.48 (1.25, 1.73) |
| No | Ref. | Ref. | Ref. | Ref. |
| Hypnotic | n = 52,700 | |||
| Yes | 0.98 (0.82, 1.16) | 0.99 (0.84, 1.18) | 1.00 (0.84, 1.19) | 0.98 (0.82, 1.16) |
| No | Ref. | Ref. | Ref. | Ref. |
| Patterns | n = 29,500 | |||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 1.53 (1.32, 1.77) | 1.53 (1.33, 1.77) | 1.53 (1.32, 1.77) | 1.49 (1.29, 1.72) |
| ≥2 | 2.51 (1.99, 3.16) | 2.53 (2.00, 3.19) | 2.51 (1.99, 3.16) | 2.43 (1.92, 3.06) |
| Transitions | n = 29,500 | |||
| Decrease | 1.33 (1.08, 1.64) | 1.46 (1.09, 1.67) | 1.34 (1.08, 1.66) | 1.30 (1.05, 1.61) |
| No change | Ref. | Ref. | Ref. | Ref. |
| Increase | 1.67 (1.42, 1.97) | 1.66 (1.41, 1.96) | 1.66 (1.40, 1.96) | 1.62 (1.37, 1.92) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Model I controls for WHI component and socio‐demographic (age, race/ethnicity, marital status, education, household income) factors only.
Model II controls the Model I list of covariates + lifestyle (smoking status, alcohol use, physical activity) factors.
Model III controls for Model II covariates + other clinical characteristics (body mass index, history of cardiovascular disease, history of hypertension, history of diabetes, history of hyperlipidemia, self‐rated health).
Table 6.
Psychotropic medication use as a risk factor for Parkinson's disease amongst individuals with depression, anxiety and/or sleep disorder diagnoses at baseline—Women's Health Initiative (n = 27,162).
| Unadjusted | Model I a | Model II b | Model III c | |
|---|---|---|---|---|
| HR (95% CI) | ||||
| Antidepressants | n = 27,162 | |||
| Yes | 1.63 (1.44, 1.85) | 1.67 (1.47, 1.89) | 1.66 (1.46, 1.88) | 1.62 (1.43, 1.84) |
| No | Ref. | Ref. | Ref. | Ref. |
| Anxiolytic | n = 27,162 | |||
| Yes | 1.42 (1.19, 1.68) | 1.41 (1.18, 1.68) | 1.42 (1.19, 1.68) | 1.36 (1.14, 1.62) |
| No | Ref. | Ref. | Ref. | Ref. |
| Hypnotic | n = 27,162 | |||
| Yes | 0.91 (0.75, 1.09) | 0.93 (0.77, 1.12) | 0.94 (0.78, 1.13) | 0.93 (0.77, 1.13) |
| No | Ref. | Ref. | Ref. | Ref. |
| Patterns | n = 14,803 | |||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 1.61 (1.35, 1.92) | 1.60 (1.34, 1.92) | 1.61 (1.35, 1.92) | 1.58 (1.32, 1.88) |
| ≥2 | 2.32 (1.79, 3.00) | 2.32 (1.78, 3.00) | 2.32 (1.79, 3.01) | 2.25 (1.74, 2.93) |
| Transitions | n = 14,803 | |||
| Decrease | 1.30 (1.02, 1.66) | 1.30 (1.02, 1.67) | 1.31 (1.02, 1.67) | 1.28 (1.00, 1.64) |
| No change | Ref. | Ref. | Ref. | Ref. |
| Increase | 1.59 (1.32, 1.93) | 1.58 (1.30, 1.91) | 1.58 (1.31, 1.92) | 1.57 (1.29, 1.89) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Model I controls for WHI component and socio‐demographic (age, race/ethnicity, marital status, education, household income) factors only.
Model II controls the Model I list of covariates + lifestyle (smoking status, alcohol use, physical activity) factors.
Model III controls for Model II covariates + other clinical characteristics (body mass index, history of cardiovascular disease, history of hypertension, history of diabetes, history of hyperlipidemia, self‐rated health).
Propensity score analyses indicated that the use of antidepressants (HR = 1.34, 95% CI: 1.23, 1.46, p < 0.0001) and anxiolytics (HR = 1.29, 95% CI: 1.18, 1.40, p < 0.0001) but not hypnotics (HR = 0.97, 95% CI: 0.89, 1.07, p = 0.56) were positively associated with PD risk in the overall sample. Similar results were obtained for antidepressants (HR = 1.39, 95% CI: 1.25, 1.55, p < 0.0001), anxiolytics (HR = 1.26, 95% CI: 1.13, 1.41, p < 0.0001) and hypnotics (HR = 0.98, 95% CI: 0.87, 1.19 = 0, p = 0.73) in the sub‐sample of women diagnosed with depression, anxiety and/or sleep disorders (Table 7).
Table 7.
Propensity score weighting analyses for use of antidepressants, anxiolytics and hypnotics as risk factors for Parkinson's disease overall and amongst individuals with depression, anxiety and/or sleep disorder diagnoses at baseline—Women's Health Initiative.
|
Overall (N = 57,200) |
Depression, anxiety and/or sleep disorders (N = 27,162) |
|
|---|---|---|
| HR (95% CI) | ||
| Antidepressant | ||
| Yes | 1.34 (1.23, 1.46) a | 1.39 (1.25, 1.55) d |
| No | Ref. | Ref. |
| Anxiolytic | ||
| Yes | 1.29 (1.18, 1.40) b | 1.26 (1.13, 1.41) e |
| No | Ref. | Ref. |
| Hypnotic | ||
| Yes | 0.97 (0.89, 1.07) c | 0.98 (0.87, 1.10) f |
| No | Ref. | Ref. |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of antidepressant use which includes depression disorders, WHI component, age group, race/ethnicity, marital status, education, smoking status, alcohol use, cardiovascular disease, hypertension, diabetes, hyperlipidemia and self‐rated health.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of anxiolytic use which include anxiety disorders, WHI component, age group, race/ethnicity, marital status, education, smoking status, physical activity, body mass index, cardiovascular disease, hypertension, hyperlipidemia and self‐rated health.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of hypnotic use which includes sleep disorder, age group, race/ethnicity, marital status, education, household income, smoking status, alcohol use, physical activity, body mass index, cardiovascular disease, hypertension, diabetes, hyperlipidemia and self‐rated health.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of antidepressant use which includes depression disorders, WHI component, age group, race/ethnicity, marital status, education, smoking status, alcohol use, physical activity, cardiovascular disease, hypertension, diabetes, hyperlipidemia and self‐rated health.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of anxiolytic use which include anxiety disorders, WHI component, age group, race/ethnicity, marital status, education, household income, smoking status, body mass index, cardiovascular disease, hypertension and self‐rated health.
Propensity score weights generated using stepwise logistic regression (SLENTRY = 0.5 and SLSTAY = 0.5) for predictors of hypnotic use which include sleep disorder, WHI component, race/ethnicity, household income, smoking status, alcohol use, physical activity, hyperlipidemia and self‐rated health.
Discussion
Longitudinal studies of psychotropic medications, including those used to treat depression [antidepressants], anxiety [anxiolytics] and sleep disorders [hypnotics], remain scarce in the context of PD. 40 , 41 , 42 Several studies have implicated dysfunctional immunity, psychological stress, anxiety, depression and sleep disorders in PD onset, progression and altered outcomes, 8 , 43 , 44 , 45 , 46 highlighting the importance of investigating psychotropic medications often used to treat these disorders in relation to PD risk.
In this longitudinal study, we performed secondary analyses of existing data from the WHI‐CTs and WHI‐OS to evaluate antidepressant, anxiolytic and hypnotic use and their associations with PD risk, overall and amongst women with depression, anxiety and/or sleep disorders. In contrast with a previously published cohort study evaluating a specific hypnotic medication [zolpidem], 18 we found that the use of hypnotics was not significantly related to incident PD after controlling for confounders. In the National Health Insurance System of Taiwan cohort, first‐time users of zolpidem for >3 months (1998–2000) had greater PD incidence (HR = 1.88, 95% CI: 1.45, 2.45) as compared to a randomly selected frequency‐matched (age, sex and index date) sample of individuals without a history of zolpidem use. 18 However, after 5 years of follow‐up there was no difference in PD incidence, and zolpidem use with concurrent depression (HR = 4.79) increased the risk of PD compared to zolpidem use without concurrent depression. 18
This study found that the use of antidepressants and/or anxiolytics may be prodromal to future PD onset, after controlling for socio‐demographic, lifestyle and health characteristics using risk adjustment or propensity score analysis. The use of antidepressants or anxiolytics may be interpreted as an indication of depression/anxiety severity; as such, disentangling diagnosis and treatment are critical. In this study, we found a persistently strong and positive association between antidepressant/anxiolytic use and PD risk even after restricting the study sample to older women with a baseline diagnosis of depression, anxiety and/or sleep disorders as well as propensity score analysis. This suggests that these psychotropic medications may be associated with PD risk, independent of the presence or absence of these psychiatric disorders. An alternative explanation is that the use of psychotropic medications is a maker of disease severity and that the underlying psychiatric disorders, namely depression/anxiety, are the actual predisposing factors for PD.
The study also found positive associations of PD risk with concurrent use of psychotropic medications as well as change in the use of psychotropic medications over time. Patients treated for PD motor symptoms are prone to polypharmacy if they also use medication to treat NMS, including antidepressants, anxiolytics and hypnotics. 47 , 48 In addition, psychotropic medication use may add to the risk of other PD‐related effects such as higher all‐cause mortality, cognitive deficits and falls. 14 , 49 The finding that change (increase and decrease) in psychotropic medications over time also hastens PD onset is likely due to the fact that PD degeneration is ongoing and that depression/anxiety are part of a changing, progressing disease, with changing manifestations that can result in changing treatments. Possibly PD‐related morbidities resulted in a change in the prescription of psychotropic medications, a hypothesis that may be explored with a more granular examination of the time‐course of PD‐related issues such as physical function, affect and cognition. Alternatively, PD and mental health problems may be progressing over time and early medications are likely no longer useful, whilst others are being trialled. In addition, PD is a cause of progressive dementia, which complicates the clinical picture over time if it supervenes.
This study has several strengths. First, the WHI population is large with excellent retention during a lengthy follow‐up period. Second, the variable set is comprehensive and was rigorously collected allowing evaluation of many hypotheses whilst adjusting for confounders. Third, the longitudinal design enabled the establishment of a temporal relationship between exposure and outcome measurements. However, there are important limitations to this study. First, selection bias may be a concern given that only fee‐for‐service (not Health Maintenance Organization) Medicare claims diagnoses can be linked to study data as well as censoring of WHI participants over time which may or may not be informative in nature. Second, several study variables relied on self‐report, ICD‐9‐CM/ICD‐10 codes or assigned therapeutic classes potentially causing information bias. Moreover, PD was not amongst the physician‐adjudicated health outcomes in WHI, although it was a physician‐diagnosed condition available to us through Medicare claims. Sensitivity analyses whereby all data sources except for ICD‐9‐CM/ICD‐10 codes were used to define PD yielded similar results with respect to hypothesized relationships, with the exception of hypnotics which became associated with a 30% increased PD risk. Third, residual confounding due to unmeasured or inadequately measured confounders and confounding by indication in the context of propensity score analysis remain a concern given the observational nature of this study and the inability to control for access to care, patterns of care providers, severity of depression, anxiety and sleep‐related symptoms and treatment‐resistant psychiatric conditions. Fourth, temporality may still be an issue given the long latency period between the use of psychotropic medications and PD onset. Finally, given that the WHI focuses on postmenopausal women and that this study further restricted eligibility to Medicare‐eligible women 65 years and older, findings cannot be generalized to men or women who were less than 65 years of age at baseline.
In conclusion, the use of antidepressants and/or anxiolytics as well as the use of multiple psychotropic medications and change in use of psychotropic medications over time were associated with risk of PD, whereas the use of hypnotics was not clearly associated with PD risk amongst older women. Future PD studies should further explore the issue of confounding by indication by controlling for disease severity in an attempt to disentangle the respective roles of antidepressants and/or anxiolytics from those of underlying psychiatric conditions. In‐depth analyses are also needed to understand the role of polypharmacy, transitions in psychotropic medication use and symptomatology over time. Finally, future studies are needed that can examine hypothesized relationships in the context of a broader population of older adults using national and international databases such as the National Health and Nutrition Examination Surveys linked to Medicare data and the UK Biobank. Nevertheless, study findings provide sufficient evidence in favour of monitoring psychotropic medication users, especially those using antidepressants and anxiolytics, amongst women 65 years and older, for PD motor and non‐motor symptoms within healthcare settings.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy of Fort Belvoir Community Hospital, the Defense Health Agency, Department of Defense or the U.S. Government. Any discussion or mention of commercial products or brand names does not imply or support any endorsement by the Federal Government.
Author Contributions
Conception and design of the study: HAB, MJN, MAB, RLB, ABZ. Acquisition and analysis of data: HAB, NS, RW, JJC, AHS, RLB. Drafting a significant portion of the manuscript or figures: HA, NS, RW, JJC, MC, MJN, MAB, AHS, ABZ, RLB.
Conflicts of Interest
Nothing to report.
Supporting information
Table S1 International Classification of Diseases Codes.
Table S2. Assigned Therapeutic Classes.
Table S3. Psychotropic medication use as a risk factor for Parkinson's disease according to time‐to‐event, age and Women's Health Initiative components—Women's Health Initiative.
Table S4. Psychotropic medication use as a risk factor for Parkinson's disease amongst individuals with depression, anxiety and/or sleep disorder diagnoses at baseline according to time‐to‐event, age and Women's Health Initiative components—Women's Health Initiative.
Table S5. Psychotropic medication use as a risk factor for Parkinson's disease (PD) using PD definition that does not cover ICD‐9‐CM/ICD‐10 diagnostic codes—Women's Health Initiative.
Table S6. Antidepressant, anxiolytic and hypnotic medication use as a risk factor for Parkinson's disease (PD) using baseline medications data only—Women's Health Initiative.
Table S7. Detailed definition of transitions in psychotropic medication use as risk factors for Parkinson's disease – Women's Health Initiative.
Table S8. Antidepressant and anxiolytic use as a risk factor for Parkinson's disease (PD) amongst individuals with sleep disorders only—Women's Health Initiative.
Table S9. Psychotropic medication use, self‐rated health and their interactions in relation to Parkinson's disease (PD) in the overall sample—Women's Health Initiative.
Acknowledgements
The manuscript was supported in part by the Intramural Research Program of the National Institute on Aging in Baltimore, Maryland. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The authors thank the WHI investigators and staff for their dedication and the study participants for making the program possible. A listing of WHI investigators can be found at https://www‐whi‐org.s3.us‐west‐2.amazonaws.com/wp‐content/uploads/WHI‐Investigator‐Short‐List.pdf.
Annals of Clinical and Translational Neurology 2022;9(8): 1163–1176
References
- 1. Bomasang‐Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson's disease: a systematic review and meta‐analysis. Parkinsonism Relat Disord. 2015;21:833‐842. discussion 833. [DOI] [PubMed] [Google Scholar]
- 2. Dalle E, Mabandla MV. Early life stress, depression and Parkinson's disease: a new approach. Mol Brain. 2018;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shamim SA, Warriach ZI, Tariq MA, Rana KF, Malik BH. Insomnia: risk factor for neurodegenerative diseases. Cureus. 2019;11:e6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson's disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldman SM. Environmental toxins and Parkinson's disease. Annu Rev Pharmacol Toxicol. 2014;54:141‐164. [DOI] [PubMed] [Google Scholar]
- 7. Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S16‐S23. discussion S23‐15. [DOI] [PubMed] [Google Scholar]
- 8. Correia AS, Cardoso A, Vale N. Highlighting immune system and stress in major depressive disorder, Parkinson's, and Alzheimer's diseases, with a connection with serotonin. Int J Mol Sci. 2021;22:8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin KW, Ameringer SW, Cloud LJ. An integrated review of psychological stress in Parkinson's disease: biological mechanisms and symptom and health outcomes. Parkinsons Dis. 2016;2016:9869712‐9869715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein DS, Kopin IJ. Linking stress, catecholamine autotoxicity, and allostatic load with neurodegenerative diseases: a focused review in memory of Richard Kvetnansky. Cell Mol Neurobiol. 2018;38:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dissanayaka NNW, Au TR, Angwin AJ, et al. Depression symptomatology correlates with event‐related potentials in Parkinson's disease: an affective priming study. J Affect Disord. 2019;245:897‐904. [DOI] [PubMed] [Google Scholar]
- 12. Jacob EL, Gatto NM, Thompson A, Bordelon Y, Ritz B. Occurrence of depression and anxiety prior to Parkinson's disease. Parkinsonism Relat Disord. 2010;16:576‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zenesini C, Baldin E, Vignatelli L, et al. Use of antidepressants and the risk of Parkinson's disease in the local health Trust of Bologna: a historical cohort study. J Neurol Sci. 2019;405:116421. [DOI] [PubMed] [Google Scholar]
- 14. Martinez‐Ramirez D, Giugni JC, Almeida L, et al. Association between antidepressants and falls in Parkinson's disease. J Neurol. 2016;263:76‐82. [DOI] [PubMed] [Google Scholar]
- 15. Yang YW, Hsieh TF, Yu CH, Huang YS, Lee CC, Tsai TH. Zolpidem and the risk of Parkinson's disease: a nationwide population‐based study. J Psychiatr Res. 2014;58:84‐88. [DOI] [PubMed] [Google Scholar]
- 16. Shadfar S, Kim YG, Katila N, et al. Neuroprotective effects of antidepressants via upregulation of neurotrophic factors in the MPTP model of Parkinson's disease. Mol Neurobiol. 2018;55:554‐566. [DOI] [PubMed] [Google Scholar]
- 17. Andrade C. Zolpidem use as a predictor of Parkinson's disease. J Psychiatr Res. 2015;60:187. [DOI] [PubMed] [Google Scholar]
- 18. Huang HC, Tsai CH, Muo CH, et al. Risk of Parkinson's disease following zolpidem use: a retrospective, population‐based cohort study. J Clin Psychiatry. 2015;76:e104‐e110. [DOI] [PubMed] [Google Scholar]
- 19. Sarwar AI. Trazodone and parkinsonism: the link strengthens. Clin Neuropharmacol. 2018;41:106‐108. [DOI] [PubMed] [Google Scholar]
- 20. Girgus JS, Yang K, Ferri CV. The gender difference in depression: are elderly women at greater risk for depression than elderly men? Geriatrics. 2017;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas KM, Redd LA, Wright JD, Hartos JL. Sleep and mental health in the general population of elderly women. J Prim Prev. 2017;38:495‐503. [DOI] [PubMed] [Google Scholar]
- 22. Virameteekul S, Phokaewvarangkul O, Bhidayasiri R. Profiling the most elderly parkinson's disease patients: does age or disease duration matter? PLoS One. 2021;16:e0261302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan W, Beaulieu‐Jones B, Krolewski R, et al. Accelerating diagnosis of Parkinson's disease through risk prediction. BMC Neurol. 2021;21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5‐S17. [DOI] [PubMed] [Google Scholar]
- 25. Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18‐S77. [DOI] [PubMed] [Google Scholar]
- 26. Cauley JA, Hovey KM, Stone KL, et al. Characteristics of self‐reported sleep and the risk of falls and fractures: the Women's Health Initiative (WHI). J Bone Miner Res. 2019;34:464‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grieshober L, Wactawski‐Wende J, Hageman Blair R, et al. A cross‐sectional analysis of telomere length and sleep in the Women's Health Initiative. Am J Epidemiol. 2019;188:1616‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boursiquot BC, Larson JC, Shalash OA, Vitolins MZ, Soliman EZ, Perez MV. Vitamin D with calcium supplementation and risk of atrial fibrillation in postmenopausal women. Am Heart J. 2019;209:68‐78. [DOI] [PubMed] [Google Scholar]
- 29. Cavanaugh AM, Rauh MJ, Thompson CA, et al. Racial/ethnic disparities in physical function before and after Total knee arthroplasty among women in the United States. JAMA Netw Open. 2020;3:e204937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavanaugh AM, Rauh MJ, Thompson CA, et al. Rehabilitation after Total knee arthroplasty: do racial disparities exist? J Arthroplasty. 2020;35:683‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou EL, Pettinger M, Haring B, et al. Lipoprotein(a) levels and risk of abdominal aortic aneurysm in the Women's Health Initiative. J Vasc Surg. 2020;73:1245‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Progovac AM, Pettinger M, Donohue JM, et al. Optimism may moderate screening mammogram frequency in Medicare: a longitudinal study. Medicine. 2019;98:e15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yung R, Ray RM, Roth J, et al. The association of delay in curative intent treatment with survival among breast cancer patients: findings from the Women's Health Initiative. Breast Cancer Res Treat. 2020;180:747‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beydoun HA, Williams M, Beydoun MA, Eid SM, Zonderman AB. Relationship of physical intimate partner violence with mental health diagnoses in the Nationwide emergency department sample. J Womens Health (Larchmt). 2017;26:141‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vin‐Raviv N, Akinyemiju TF, Galea S, Bovbjerg DH. Sleep disorder diagnoses and clinical outcomes among hospitalized breast cancer patients: a nationwide inpatient sample study. Support Care Cancer. 2018;26:1833‐1840. [DOI] [PubMed] [Google Scholar]
- 36. Burstyn I, LaCroix AZ, Litvan I, Wallace RB, Checkoway H. Occupation and Parkinson disease in the Women's Health Initiative observational study. Am J Ind Med. 2019;62:766‐776. [DOI] [PubMed] [Google Scholar]
- 37. Creasy SA, Crane TE, Garcia DO, et al. Higher amounts of sedentary time are associated with short sleep duration and poor sleep quality in postmenopausal women. Sleep. 2019;42:zsz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McMurry TL, Hu Y, Blackstone EH, Kozower BD. Propensity scores: methods, considerations, and applications in the journal of thoracic and cardiovascular surgery. J Thorac Cardiovasc Surg. 2015;150:14‐19. [DOI] [PubMed] [Google Scholar]
- 39. Williamson EJ, Forbes A. Introduction to propensity scores. Respirology. 2014;19:625‐635. [DOI] [PubMed] [Google Scholar]
- 40. Arruda WO, Silva MS, Bertholdo DB, Munhoz RP, Teive HA. Zolpidem in movement disorders after cardiac arrest. Parkinsonism Relat Disord. 2017;37:114‐115. [DOI] [PubMed] [Google Scholar]
- 41. Bhattacharjee S, Goldstone L, Warholak T. Prevalence, patterns and predictors of psychotropic polypharmacy among elderly individuals with Parkinson's disease in long term care settings in the United States. J Parkinsons Dis. 2016;6:247‐255. [DOI] [PubMed] [Google Scholar]
- 42. Kashihara K, Nomura T, Maeda T, et al. Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson's disease ‐ results of a multicenter open trial. Intern Med. 2016;55:231‐236. [DOI] [PubMed] [Google Scholar]
- 43. Behl T, Makkar R, Sehgal A, et al. Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. Int J Mol Sci. 2021;22:7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang KH, Chen CM. The role of oxidative stress in Parkinson's disease. Antioxidants (Basel). 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Picca A, Calvani R, Coelho‐Junior HJ, Landi F, Bernabei R, Marzetti E. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants (Basel). 2020;9:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Circadian rhythms, neuroinflammation and oxidative stress in the story of Parkinson's disease. Cell. 2020;9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frandsen R, Baandrup L, Kjellberg J, Ibsen R, Jennum P. Increased all‐cause mortality with psychotropic medication in Parkinson's disease and controls: a national register‐based study. Parkinsonism Relat Disord. 2014;20:1124‐1128. [DOI] [PubMed] [Google Scholar]
- 48. Katsunuma H, Shimizu T, Ogawa K, Kubo H, Ishida H, Yoshihama A. Treatment of insomnia by concomitant therapy with zopiclone and aniracetam in patients with cerebral infarction, cerebroatrophy, Alzheimer's disease and Parkinson's disease. Psychiatry Clin Neurosci. 1998;52:198‐200. [DOI] [PubMed] [Google Scholar]
- 49. Schrag A, Choudhury M, Kaski D, Gallagher DA. Why do patients with Parkinson's disease fall? A cross‐sectional analysis of possible causes of falls. NPJ Parkinson's Disease. 2015;1:15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 International Classification of Diseases Codes.
Table S2. Assigned Therapeutic Classes.
Table S3. Psychotropic medication use as a risk factor for Parkinson's disease according to time‐to‐event, age and Women's Health Initiative components—Women's Health Initiative.
Table S4. Psychotropic medication use as a risk factor for Parkinson's disease amongst individuals with depression, anxiety and/or sleep disorder diagnoses at baseline according to time‐to‐event, age and Women's Health Initiative components—Women's Health Initiative.
Table S5. Psychotropic medication use as a risk factor for Parkinson's disease (PD) using PD definition that does not cover ICD‐9‐CM/ICD‐10 diagnostic codes—Women's Health Initiative.
Table S6. Antidepressant, anxiolytic and hypnotic medication use as a risk factor for Parkinson's disease (PD) using baseline medications data only—Women's Health Initiative.
Table S7. Detailed definition of transitions in psychotropic medication use as risk factors for Parkinson's disease – Women's Health Initiative.
Table S8. Antidepressant and anxiolytic use as a risk factor for Parkinson's disease (PD) amongst individuals with sleep disorders only—Women's Health Initiative.
Table S9. Psychotropic medication use, self‐rated health and their interactions in relation to Parkinson's disease (PD) in the overall sample—Women's Health Initiative.


