Abstract
Background and Objectives
To determine whether cognitive reserve (CR) as measured by verbal intelligence quotient, educational length, and achievement protects amyotrophic lateral sclerosis (ALS) patients' verbal fluency, executive functioning, and memory against brain volume loss over a period of 12 months.
Methods
This cohort study was completed between 2013 and 2016 with a follow‐up duration of 12 months. ALS patients were recruited from two specialist out‐patient clinics in Rostock and Magdeburg in Germany. Participants underwent cognitive testing and magnetic resonance imaging both at baseline and again after 12 months. The cognitive domains assessed included verbal memory in addition to executive functions such as verbal fluency, working memory, shifting and selective attention.
Results
Thirty‐eight ALS patients took part; 25 patients had no cognitive impairment (ALSni), and 13 were cognitively impaired (ALSci). On average, patients lost 294 mm3 in their superior frontal gyri, 225 mm3 in their orbitofrontal gyri, and 15.97 mm3 in their hippocampi over 12 months. There was strong evidence that CR protected letter fluency from further decline (Bayes factor [BF] >10) and moderate evidence that it supported learning effects in letter flexibility (BF >3). However, there is a lack of evidence supporting the notion that working memory, shifting, selective attention or verbal memory (BF = 1) are protected.
Discussion
As CR is easily determined and protects ALS‐specific cognitive domains over time, it should be regarded as a valuable predictive marker.
Introduction
The heterogeneous nature of amyotrophic lateral sclerosis (ALS) impedes predictions of individual disease progression. The large majority of studies so far has focused on the progression of motoric decline. 1 However, cognitive impairment is common in ALS as well and serves as a negative prognostic indicator of survival. 2 , 3 , 4 Recently, the long‐standing concept of cognitive reserve (CR) has been adapted to motor and cognitive decline in ALS. 5 , 6 , 7 , 8 CR refers to differences in ability to cope with age‐ or disease‐related brain changes, such as pathological burden, facilitating different levels of clinical impairment at similar levels of pathology. 9 , 10 Examples of key surrogate markers for CR include educational attainment, occupational complexity, and vocabulary size. 7 The CR hypothesis has recently been established in ALS‐frontotemporal spectrum disorders (ALS‐FTSD). 5 , 6 , 7 , 8 Canosa et al. adopted a cross‐sectional approach which provided evidence that higher education correlated with increased pathological burden in medial frontal regions, regardless of the level of cognitive impairment. 8 Consonni et al. showed that lifestyle factors – namely, high levels of educational and occupational attainment, social interactivity, physical activity and multilinguality – were associated with better‐preserved executive functions, verbal fluency and memory in ALS‐FTSD. 7 Costello et al. 6 provide the only longitudinal approach to‐date: greater educational attainment, occupational complexity, and physical activity correlated with better‐preserved cognitive functioning at baseline but did not protect cognition over 12 months. This was likely because Costello et al.’s cohort 6 showed no cognitive decline against which CR could have been protective; such stability is well‐established in ALS. 11 , 12 , 13 Our own previous work, which took a cross‐sectional approach, presented strong evidence that higher education and verbal intelligence protected cognitive functioning from regional atrophy. 5 Regional atrophy measured by magnetic resonance imaging (MRI) has been related to cognitive impairment in ALS‐FTSD. 14 , 15 , 16 , 17 , 18 The longitudinal study presented here expands on the previous literature 5 , 6 , 7 , 8 by investigating the extent to which CR protects cognitive functioning in patients with ALS and whether this protective effect is moderated by patients' impairment levels or regional atrophy. Based on our cross‐sectional findings, 5 we hypothesised that CR would be associated with better‐preserved verbal fluency and verbal memory performance and that this association would be stronger than the association between cognitive trajectories with volume loss, age, and sex. As evidence for CR's effect on executive functions was mixed, we explored the associations between executive functions and CR.
Methodology
Design
This was a prospective, longitudinal study. The predictor was CR (see Measurements). The outcomes were decline across the executive, verbal fluency, and memory domains. Our markers of neurodegeneration were volume loss in the superior frontal gyrus, the orbitofrontal gyrus, and the hippocampus over 12 months. These markers of neurodegeneration were accounted for by adding them to the null model, alongside age, sex, Strong profile at baseline, recruitment location, and total intracranial volume (TIV). This corrected null model supports the notion that age, sex, baseline Strong profile, recruitment location, and TIV have an effect on cognitive performance. Cognitive performance and MRI measurements were taken at baseline and 12 months' follow‐up.
Participants
The ALS‐FTD Intersite project recruited persons with ALS‐FTSD from specialist out‐patient clinics in Rostock and Magdeburg, Germany (Fig. 1). Persons with a history of brain injury, epilepsy, or psychiatric illness were excluded from the study.
Figure 1.
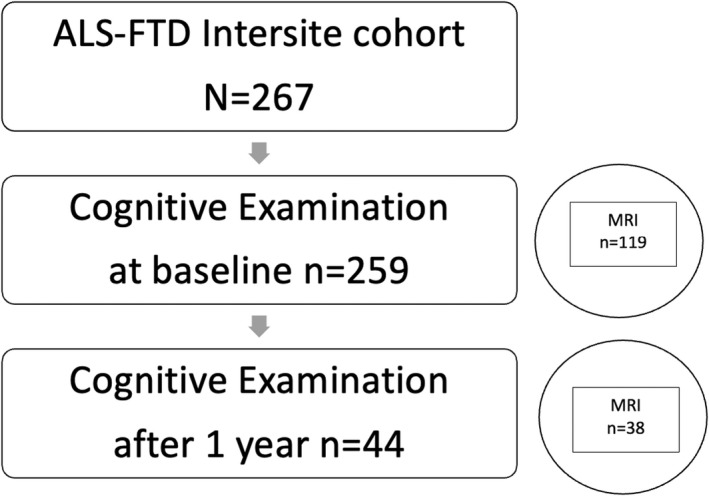
The flow of participants throughout this study.
Patients were diagnosed according to the revised El Escorial criteria 19 and cognitive‐behavioural profiles were established according to the most recent Strong and Rascovsky criteria. 20 , 21 Participants with ALS‐FTD were excluded from analysis. Details can be found in Table 1. In longitudinal imaging studies of ALS, high attrition rates of up to 94% are common due to the disease's rapid progression. 12 , 22 , 23 We had recruited 125 persons but only 38 participated at follow‐up. Figure 1 outlines the flow of participants throughout the study. At first examination, five patients had pure upper or lower motor neuron syndromes and did not meet the revised El Escorial criteria by Brooks et al. 19 However, these five patients were diagnosed with restrictive phenotypes of ALS (Ludolph et al.), 24 see Table 1. Of the 16 patients with possible ALS, three progressed to probable ALS. All patients underwent genetic testing: three had SOD1 mutations and one had a VAPB mutation. 25 , 26 A very low prevalence of C9orf72 has been documented in Northern German samples before. 27
Table 1.
Participants' background.
| Measure | ALS without cognitive impairment (n = 25) | ALS with cognitive impairment (n = 13) |
|---|---|---|
| Demographic background | ||
| Recruitment location (HRO/MD) | 20/5 | 4/9 |
| Sex (f/m) | 9/16 | 4/9 |
| Age (years) | 54.52 (10.20) | 60.77 (8.59) |
| Cognitive reserve measure | 9.16 (1.99) | 8.62 (0.60) |
| Education (years) | 13.24 (2.44) | 11,77 (1.74) |
| ISCED level | 4.80 (1.08) | 4.23 (0.60) |
| Verbal IQ | 92.56 (14.81) | 90.85 (7.96) |
| Clinical presentation | ||
| Disease duration until final MRI (months) | 49.75 (39.82) | 41.64 (30.44) |
| ALSFRS‐R at baseline | 39.00 (4.92) | 38.62 (4.81) |
| Progression speed δ between baseline & final MRI | 0.61 (0.51) | 0.54 (0.60) |
| SOD1 mutation | 3 (12%) | 0 |
| VAPB mutation | 1 (4%) | 0 |
| Tested, no mutation found | 21 (84%) | 13 (100%) |
| Disease onset (%) | ||
| Bulbar | 4 (16%) | 2 (15%) |
| Spinal | 15 (60%) | 10 (77%) |
| Unknown | 6 (24%) | 1 (8%) |
| El Escorial criteria (%) | ||
| Not applicable | 5 (20%) | 3 (23%) |
| Possible ALS | 10 (40%) | 6 (46%) |
| Probable ALS | 2 (24%) | 3 (23%) |
| Definite ALS | 4 (6%) | 1 (8%) |
| Phenotype (%) | ||
| Classical ALS | 14 (56%) | 6 (46%) |
| Predominant upper motor neuron | 2 (8%) | 3 (23%) |
| Primary lateral sclerosis | 3 (12%) | 0 |
| Progressive muscular atrophy | 3 (12%) | 2 (15%) |
| Flail arm syndrome | 1 (4%) | 0 |
| Flail leg syndrome | 1 (4%) | 2 (15%) |
| Uncertain | 1 (4%) | 0 |
ALS, amyotrophic lateral sclerosis; HRO, Rostock; MD, Magdeburg; ISCED, international standard classification of education; IQ, intelligence quotient.
Standard protocol approvals, registrations, and patient consents
The study was approved by the local medical ethics committees at Rostock University and Otto‐von‐Guericke University, Magdeburg, Germany (reference numbers A2010‐32 and A2011‐56) and conducted according to the declaration of Helsinki. All participants gave written, informed consent.
Measurements
Cognitive reserve
Cognitive reserve was constructed as a composite measure by summing up points assigned for: educational years, educational attainment, and verbal intelligence as a proxy for general intelligence. Verbal intelligence was measured by passive vocabulary; for details see our previous work. 5 , 28 In ALS, performance on this specific test has repeatedly been shown to remain intact and indistinguishable from healthy controls, 5 , 29 and unrelated to physical disability. 5 , 29 We assigned points for educational years following Consonni et al.’s strategy, 7 and according to the international standard classification of education (ISCED) to reflect educational length and achievement. Crucially, the former – educational years – assigns points purely based on the length of time spent in formal education while the latter – ISCED levels – reflects educational achievement. For example, it is possible to obtain 13 years of education by primary and secondary education (“Realschule”) followed by 3 years of vocational training (“Berufsausbildung”), resulting in ISCED level 4, or instead of “Realschule” by completing secondary education (“Gymnasium”) without any further tertiary education or vocational training –resulting in ISCED level 3. By combining both measures, our CR measure reflects standard educational lengths, levels and achievements.
Neuropsychological assessment
Our participants underwent full neuropsychological examination, consisting of letter fluency and flexibility, category fluency and flexibility, 30 shifting ability 31 ; selective attention within the Stroop paradigm; short‐term and working memory; and verbal memory. 32 We derived the following outcomes from these assessments.
The verbal fluency tasks were limited to 2 minutes, with the conditions of letter S, H/G switching, animals, and sports/fruit switching. We indexed all four verbal fluency tasks by subtracting reading time from production time, then dividing the result by the number of correctly produced words. These verbal fluency indices correct for the effects of dysarthria on speech tempo, with a higher index signifying a diminished word production or a slower reading speed. 33 Shifting ability was measured by the ratio between trail making tests B and A to correct for motor‐related deceleration. 31 A lower ratio indicates a slower shifting ability. Response inhibition was examined using the Stroop task, with the outcome operationalised as the ratio of the inverse efficiency between naming and reading: the time needed to name divided by 1 minus the error naming rate, divided by the time needed to read, divided by 1 minus the error reading rate. 34 Short‐term and working memory were assessed using digit‐span‐forward and digit‐span‐backward tasks, respectively. Verbal memory was assessed using the German equivalent of the Raven auditory verbal learning test. 32 From this test, we derived the outcomes of material learnt (the sum of words learnt over trials 1–5, as a percentage of max. 75 words); the material acquired (trial 1 subtracted from trial 5, divided by trial 5); immediate recall (trial 6 divided by trial 5); delayed recall (trial 7 divided by trial 5), and recognition (correctly recognised words minus incorrectly recognised words). To compare between‐outcome performance more easily, we standardised our participants' raw scores to z‐scores based on healthy age, sex and education‐matched controls.
MRI acquisition
MRI scanning was performed with two 3 T Siemens Magnetom VERIO scanners (Erlangen, Germany) using a 32‐channel head coil; one single scanner at each site (Rostock and Magdeburg, Germany). High‐resolution T1‐weighted anatomical images were acquired using the magnetization‐prepared rapid gradient echo (MPRAGE) sequence with the following parameters: 256 × 256 image matrix with 192 sagittal slices, FOV 250 × 250 × 192 mm, voxel size 1 × 1 × 1 mm3, echo time 4.82 msec, repetition time 2500 msec, and flip angle 7°. The anatomical T1‐weighted images from all time points were co‐registered to each other, segmented into grey matter, white matter and cerebrospinal fluid partitions using the CAT12 toolbox longitudinal pipeline in Matlab 2019ab. Then, the Diffeomorphic Anatomical Registration Through Exponentiated Lie (DARTEL) algebra algorithm 35 was used in combination with the default CAT12 brain template to normalise the mean T1‐weighted image to the Montreal Neurological Institute (MNI) reference coordinate system. The estimated deformation field was subsequently applied to the grey matter segments of all time points to bring them in MNI space as well, followed by modulation to preserve the total amount of grey matter and smoothing with an 8 mm Gaussian kernel. In phantom tests according to the American College of Radiology guidelines, 36 both sites' scanners met the criteria for geometric accuracy, high contrast spatial resolution, slice thickness accuracy, slice position accuracy, image intensity uniformity, percent signal ghosting and low contrast object detectability. We subtracted baseline regional volume from that at 12 months' follow‐up to determine loss of volume (see Table 2). We assumed that CR's protective effect would be most decisive against volume loss in brain regions where an association with cognitive functioning has previously been documented. Consequently, we focused our hypothesis‐driven testing on the superior frontal gyrus atrophy for fluency 37 , 38 and working memory functions 37 ; in addition to orbitofrontal gyrus atrophy for shifting, selective attention 39 and planning 37 , 40 ; and to hippocampal atrophy for verbal memory. 22
Table 2.
The means and standard deviations of the covariates, and the mean with 95% credible intervals of the outcomes.
| Measure | ALSnci | ALSci | N |
|---|---|---|---|
| Total intracranial volume (TIV) | 1458.60 (146.03) | 1438.77 (152.14) | 38 |
| Volume loss over 12 months (mm3) | |||
| Superior frontal gyrus | −251.38 (850.83) | −376.72 (1666.63) | 38 |
| Orbitofrontal gyrus | −260.01 (553.69) | −158.92 (449.58) | 38 |
| Hippocampus | −28.48 (116.70) | 8.09 (98.50) | 38 |
| Cognitive performance (z scores) | Baseline | Follow up | Baseline | Follow up | |
|---|---|---|---|---|---|
| Verbal fluency | |||||
| Letter fluency | −0.22 (−0.58, 0.13) | −0.06 (−0.40, 0.27) | −2.41 (−3.57, −1.25) | −2.23 (−3.91, −0.54) | 30 |
| Letter flexibility | −0.25 (−0.71, 0.22) | −0.23 (−0.93, 0.46) | −6.31 (−9.59, −3.03) | −3.56 (−6.02, −1.11) | 29 |
| Category fluency | 0.34 (−0.10, 0.79) | 0.61 (0.21, 1.01) | −1.49 (−2.32, −0.66) | −2.67 (−3.75, −1.59) | 30 |
| Category flexibility | 0.08 (−0.35, 0.51) | 0.46 (−0.19, 1.10) | −2.42 (−4.53, −0.31) | −3.92 (−7.22, −0.62) | 29 |
| Executive functions | |||||
| Working memory | −0.23 (−0.77, 0.32) | −0.08 (−0.70, 0.55) | −0.90 (−0.70, 0.32) | −0.90 (−1.82, 0.03) | 33 |
| Short‐term memory | −0.14 (−0.64, 0.37) | −0.17 (−0.68, 0.35) | −0.88 (−1.40, −0.36) | −0.82 (−1.43, −0.22) | 32 |
| Shifting | 0.35 (−0.02, 0.73) | 0.10 (−0.11, 0.32) | 0.18 (−0.45, 0.82) | 0.22 (−0.59, 1.02) | 32 |
| Selective attention | −0.33 (−0.60, −0.06) | −0.13 (−0.51, 0.25) | 0.67 (−0.24, 1.58) | 0.41 (−0.51, 1.33) | 22 |
| Verbal memory | |||||
| Learning | 0.59 (0.03, 1.16) | 0.43 (−0.15, 1.01) | −1.30 (−2.21, −0.40) | −1.44 (−2.85, −0.03) | 31 |
| Acquired | 0.04 (−0.49, 0.58) | 0.33 (−0.23, 0.89) | −0.18 (−1.42, 1.05) | −1.06 (−3.06, 0.95) | 31 |
| Immediate recall | 5.60e‐3 (−0.42, 0.43) | 0.03 (−0.31, 0.38) | −1.00 (−2.23, 0.23) | −1.18 (−2.43, 0.08) | 31 |
| Delayed recall | −0.08 (−0.45, 0.28) | −0.11 (−0.50, 0.27) | −0.81 (−1.35, −0.27) | −0.63 (−1.60, 0.35) | 31 |
| Correct recognition | −0.05 (0.67, 0.56) | −0.73 (−1.61, 0.14) | −0.84 (−1.87, 0.19) | −2.57 (−4.45, −0.68) | 31 |
Statistical analysis
As we were interested in establishing support for the CR hypothesis, we chose Bayes factor (BF) hypothesis testing. This permitted us to compare multiple hypotheses and select the most plausible one in accordance with our data. 41 , 42 To compare hypotheses, we conducted mixed‐measures analyses of covariance with the within‐subjects independent variable of time, the between‐subjects independent variables Strong criteria at baseline, sex and recruitment location, andgenetic the covariates CR, age and regional brain volume loss with the outcomes of executive, verbal fluency, and memory performance (see “Design”). Independent variables and covariates must not be inter‐correlated. We verified this using Kendall's tau correlation coefficients. CR and volume loss did not, notably, correlate with one another, indicating that the influence of either on cognitive ability would be independent from the other. We compared the following hypotheses: (1) the null hypothesis, including all relevant potential confounding variables, represented by the corrected null model including age, sex, Strong profile at baseline, recruitment location, TIV, regional volume loss, time; (2) the CR hypothesis, represented by the sole main effect of CR; and (3) the hypothesis that patients with different Strong profiles benefit distinctly from CR, represented by the interaction between Strong profiles and CR. For statistical purposes, we combined the ALSbi and ALSni patients into “ALSnci” as both groups had no cognitive impairment, and there were only three ALSbi patients in our sample. Analysing them separately did not seem to affect our conclusions in a meaningful way, as indicated by the respective results file on the Open Science Framework (see Data availability statement). We compared these ALSnci patients to ALSci patients. Broadly speaking, three conclusions are possible within the Bayesian framework 41 : support for either alternative hypothesis (BF ≥3), support for the null hypothesis (BF <0.33), or inconclusive evidence (BF between 0.3 and 3). We will consider the CR hypothesis supported by our data if the evidence in its favour is at least moderate compared to the null hypothesis (BF ≥3) 41 because this would mean that the effects of CR were supported against all these confounds, including atrophy and onset type. In BFHT, there is no sample size below which our inferences become untrustworthy. 42
Modelling took place in Jeffreys's Amazing Statistics Program (JASP). 43 We set JASP to place the best model on top, and report BF01 for all models compared to it, quantifying the top model's performance over those listed below. Numerical accuracy was established over 10,000 iterations using a Markov chain Monte Carlo (MCMC) algorithm.
We report our results according to published guidelines. 44 “P(M)” are the models' prior probabilities, i.e., how likely they were considered to be prior to observing our data. Prior probabilities were set to be equal so that the null model and the CR models were equally likely. “P(M|data)” indicates each model's posterior probability after observing our data. “BFM” indicates the degree to which our data have changed the prior model odds. 41 “BF01” indicates the evidence in favour of the best model, and error% indicates the numerical stability of BF01 over 10,000 MCMC iterations; the latter should be below 20%. 44 Effect sizes and coefficients are reported with a 95% credible interval.
Data availability statement
The original MRI files are not publicly available due to confidentiality restraints. A comma separated values file containing the data underlying the analyses and figures is available at: https://osf.io/8ng3p/, accompanied by an HTML file containing our reported JASP output, and the JASP output when ALSci and ALSbi patients were analysed separately.
Results
For brevity's sake, we report details on those analyses that produced decisive evidence in any direction. We summarise the prior and posterior probabilities of analyses with inconclusive evidence, details may be obtained from our online supplement. Average volume loss, cognitive performance scores and the final number of participants included in each analysis may be found in Table 2. We found at least moderate evidence that ALSci patients did not exhibit greater volume loss than ALSnci patients (unidirectional Mann–Whitney U tests, all BF >3).
Hypotheses with conclusive evidence
Our data were sufficiently informative to provide conclusive evidence for letter fluency, letter flexibility and immediate recall of verbally acquired material. Model comparisons, and the best models' R 2 and the coefficients are in Table 3. The 95% CI of the volume loss coefficients, notably, always included zero, meaning that a correlation coefficient with size zero between volume loss and cognitive ability was always plausible.
Table 3.
Comparisons for models with conclusive evidence: letter fluency, letter flexibility, immediate recall and recognition.
| Function | Name | Model summary | Best model inference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P(M) | P(M|data) | BFM | BF01 | Error% | R 2 | CR | ALSci | Volume loss | ||
| Letter fluency | CR × Profile | 0.33 | 0.57 | 2.66 | 1.00 | 0.40 (0.24, 0.74) | 0.45 (0.06, 0.89) | −0.76 (−1.21, −0.27) | −3.53e‐5 (−3.86e‐4, 3.09e‐4) | |
| CR | 0.33 | 0.33 | 0.97 | 1.75 | 11.63 | |||||
| Null | 0.33 | 0.10 | 0.23 | 5.55 | 5.91 | |||||
| Letter flexibility | CR × Strong | 0.33 | 0.76 | 6.38 | 1.00 | 0.72 (0.59, 0.80) | 0.58 (−0.08, 1.23) | −1.74 (−2.73, −0.66) | −5.19e‐3 | |
| CR | 0.33 | 0.19 | 0.46 | 4.05 | 11.76 | |||||
| Null | 0.33 | 0.11 | 0.11 | 15.07 | 6.59 | |||||
| Immediate recall | CR × Strong | 0.33 | 0.65 | 3.66 | 1.00 | 0.55 (0.52, 0.63) | −0.63 (−1.24, −0.04) | −0.37 (−0.73, −6.81e‐3) | −8.55e‐5 (−3.53e‐3, 1.67e‐3) | |
| Null | 0.33 | 0.22 | 0.58 | 2.89 | 5.81 | |||||
| CR | 0.33 | 0.13 | 0.30 | 4.98 | 9.42 | |||||
| Recognition | Null | 0.33 | 0.59 | 2.87 | 1.00 | 0.66 (0.52, 0.77) | Not applicable | |||
| CR | 0.33 | 0.26 | 0.72 | 2.23 | 2.61 | |||||
| CR × Strong | 0.33 | 0.15 | 0.34 | 4.02 | 2.98 | |||||
Bold face indicates the best model for our data. Model names: CR = cognitive reserve, main effect‐only; CR × Strong = interaction effect between CR and Strong profile; Null = null hypothesis, corrected for age, sex, TIV, regional volume loss and recruitment location.
Letter fluency
Our data were approximately five times more likely under the interaction between CR and Strong profile compared to the null model (BF01 = 5.55, Table 3) with an error rate of 6% indicating that the BF01 ranged from 5.5 to 6.4 over the 10,000 MCMC iterations. Analysing our data has increased our belief in this hypothesis from 33% to 57%. The CR model explained approximately 40% of the variance in letter fluency. In this model, CR's influence was stronger than that of volume loss in the superior frontal gyrus (Fig. 2A).
Figure 2.
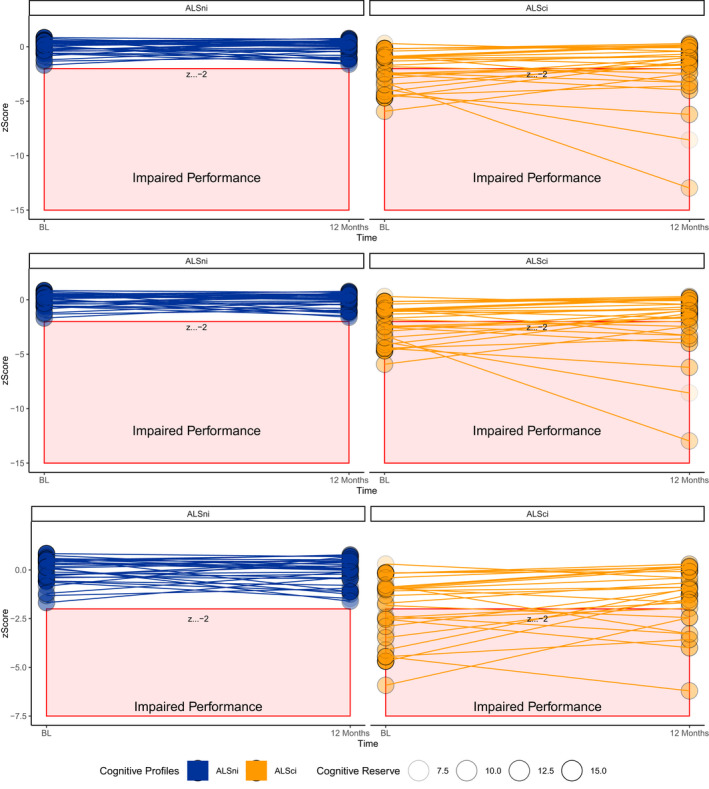
The effect of cognitive reserve over 12 months. (A) Higher CR protected letter fluency over 12 months. (B) ALSci patients with higher CR improved their letter flexibility over 12 months, but ALSnci patients did not. (C) ALSci patients with lower CR experienced worse immediate recall than ALSnci patients.
Letter flexibility
Our data were approximately 15 times more likely under the interaction between CR and Strong profile at baseline compared to the null hypothesis (BF01 = 15.07, Table 3). The error rate of 6.59% indicates that the BF01 ranged from 14.07 to 16.07 (BF01 = 15.07 ± 1.00). Analysing our data has increased our belief in the interaction model from 33% to 76%; it explained 72% of the variance in letter flexibility. In it, CR's effect exceeded that of atrophy in the superior frontal gyrus but not that of ALSci. Figure 2B shows that ALSci patients with higher CR were able to improve their performance, while ALSnci patients performed steadily. In this model, CR's influence was stronger than that of volume loss in the superior frontal gyrus.
Immediate recall
Our data were approximately five times more likely under the interaction model compared to the pure CR model (BF01 = 4.98). The error rate of 9.42% indicates that the BF01 varied by ±0.46 over 10,000 MCMC iterations. Analysing our data has increased our belief in the interaction model from 33% to 65% but reduced our belief in the pure CR model to 13%. The interaction model was nearly three times better than the corrected null model (BF01 = 2.89 ± 0.99) The interaction model explained 55% of variance in immediate recall. In this model, CR's influence was stronger than that of volume loss in the hippocampus (Fig. 2C).
Recognition
Here, we found conclusive evidence that the corrected null model with the combined main effects of time, sex, recruitment location, Strong profile, age, TIV and change in hippocampal volume was four times better than the interaction between CR and Strong profile (BF01 = 4.02 ± 0.12). The null model explained 66% of the variance in recognition performance.
Hypotheses with absent evidence
For the following cognitive functions, our data were insufficiently informative as indicated by small increases in posterior probability. This means that there is an absence of evidence within these data, i.e., no support for any hypothesis.
Category fluency
The best model was the main effect of CR. However, our data only increased our belief in this model from 33% to 40%, thus providing insufficient information to facilitate a decision in favour of any hypothesis.
Category flexibility
The best model was the main effect of CR. However, our data only increased our belief in this model from 33% to 49%, meaning that this sample contained insufficient information to support or reject any hypothesis.
Working memory
Here, the null model was the best model. Our data increased our belief in the null model from 33% to 40%, indicating that they were insufficiently informative to prompt a decision favouring any hypothesis.
Short‐term memory
The best model was the null model, but our belief in it increased from 33% to 35%, signifying that our data were insufficiently informative to facilitate a decision between the competing hypotheses.
Shifting
The null model was the best model, but our belief in it increased from 33% to only 40%, suggesting that our data were insufficiently informative to allow any conclusions about the competing hypotheses.
Selective attention
The null model was the best model, but our belief in this model increased only from 33% to 44%, indicating that our data provided insufficient information to support any hypothesis.
Learning
The CR model was the best model, but our data only increased our belief from 33% to 43%, meaning that they were insufficiently informative to facilitate decisive evidence.
Material acquired
The corrected null model was the best model, but our belief in it was only increased from 33% to 44%, indicating that our data did not provide enough information to support any hypothesis.
Delayed recall
The null model was the best model; our data increased our belief in it by 10%: from 33% to 47% – indicating that they were insufficiently informative to support any model.
Figure 3 summarises the evidence in favour of CR and its interaction with Strong profile compared to the corrected null model: there was decisive evidence in our data to support that CR protects letter fluency and flexibility, but for the other functions, the data were insufficiently informative to facilitate a decision in favour of the CR or the null hypothesis.
Figure 3.
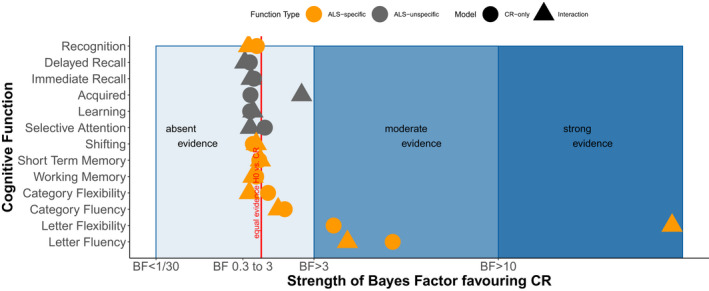
A summary of evidence favouring the CR hypothesis over the null hypothesis.
Discussion
Based on our previous work, 5 we had hypothesised that specific cognitive functions would be protected by CR: verbal fluency, verbal memory and working memory. Our data provided evidence that letter fluency and flexibility were protected from decline moderated by superior frontal atrophy over 12 months. In letter fluency, patients with larger reserve were protected from further decline. In letter flexibility, ALSci patients benefitted more strongly from CR than ALSnci patients: with higher CR, their performance improved (Fig. 2B). However, the evidence in our data was not informative enough to support the CR hypothesis or the null hypothesis for executive functions and verbal memory. Importantly, this signifies an absence of evidence, not evidence that CR's effect is absent. Verbal fluency – letter fluency in particular – is the most frequent, disease‐specific cognitive impairment in ALS. 45 Our data thus generate the hypothesis that CR's long‐term protection is limited to disease‐specific functions in ALS. As patients with PMA and PLS were part of our cohort (Table 1), this could be said to undesirably increase the heterogeneity of our cohort and affect the results; however, having re‐ran the analyses to confirm the results without both ALS‐variants, our findings were sustained.
Cross‐sectionally, Canosa et al. 8 applied the Edinburgh Cognitive and Behavioural ALS Screening's total score to show that longer education protected overall cognitive performance from lower fronto‐medial glucose metabolism, indicative of higher pathological burden. Our previous, cross‐sectional work 5 provided strong evidence that CR had small, protective effects on verbal fluency, working memory, verbal memory and visuo‐constructive abilities against regional atrophy, but not shifting and planning abilities. Further studies without measures of neurological damage indicated that while patients with high and low CR may differ in cognitive performance at baseline, 6 , 7 they decline at similar rates. 6 This indicates that CR was not protective of functions over time when neurological damage was not considered. 6
Cross‐sectional evidence has suggested that cognitive profiles according to Strong did not interact with CR. 5 , 8 The presented longitudinal analyses indicate that ALSci patients were most likely to benefit from CR, and provided moderate to strong evidence of medium‐sized protective effects for letter fluency/flexibility against superior frontal atrophy. Our data extend previous findings 6 by showing that ALSci patients benefit from high CR especially in flexibility tasks. Our patients – like Costello et al.’s patients – remained cognitively stable over time despite the late stage of their ALS (mean disease duration 47 months, Table 2). 6 Cognitive stability in ALS is well‐documented. 11 , 13
The CR hypothesis has been applied to explain delayed disease onset in Alzheimer's disease 46 and slower decline of global cognitive functions, not just disease‐specific memory. 47 Whereas previous, cross‐sectional evidence suggested that CR may protect a wide range of functions in ALS, 5 , 7 , 8 our study indicates that CR was exclusively protective of disease‐specific letter fluency functions. Our previous work 5 , 48 further lent support for the brain reserve hypothesis: between‐person differences in brain size might facilitate better coping with neuronal damage and in turn, better cognition. While brain atrophy presents along a continuum ranging from ALSni with less atrophy in non‐primary motor areas to ALS‐FTD patients with severe atrophy, 14 , 18 , 48 functional impairment presents as a spectrum with distinct, mutually exclusive profiles of brain perfusion, 49 functional connectivity, 50 brain glucose metabolism 51 and cognition. 20 However, this present study provides stronger support for the longitudinal benefit of CR over that of brain reserve: the effects of brain atrophy were minuscule, and centered on zero (Table 3). This suggests that CR's influence on cognition exceeds that of brain reserve because the aforementioned functional impairments are not entirely reliant on atrophy. However, future research needs to determine conclusive evidence for executive, language, social cognition and memory functions across the ALS‐FTSD. Given that the architecture of cognition remains a matter of debate 52 , 53 , 54 and that atrophy is widespread, 18 future studies should direct their attention towards cognitive networks to investigate the interrelationships between cognitive functions in ALS‐FTSD. Establishing cognitive networks may reveal structures underlying these vulnerabilities.
The absence of evidence does not warrant the rejection of the CR hypothesis in these functions (Fig. 2). This absence is likely because neuroscience overall still struggles to map cognitive functions onto brain areas 53 and to establish the architecture of cognitive functions overall. Factor analyses have suggested that memory and reasoning could be one domain 54 and that verbal fluency might be a language function rather than an executive one as it relates to both domains. 52 This necessitates future network analyses, to see if ALS‐FTSD patients and healthy persons differ in their cognitive architecture. Furthermore, small samples such as ours only serve to detect large effect sizes, medium to small effect sizes may be undetectable. Finally, verbal memory and executive domains were unimpaired in our sample, and remained stable over time (Table 2; Fig. 2C). While CR may support this stability, there can be no protection against changes. We propose that our analyses be replicated in larger, independent samples. Bayesian modelling techniques are particularly suitable for such replication analyses. Limitations of our study include the lack of language and social cognition tasks and additional measurements of reserve, such as occupational status, 55 or physical activity. Additionally, our operationalization of brain pathology rested on regional atrophy but – as discussed above – there are metabolic changes also worthy of inclusion when assessing the CR hypothesis. While our attrition rate of 81% is not uncommon in longitudinal ALS studies owing to both physical deterioration and waning willingness, the resulting relatively small sample size may introduce bias towards the number of patients on the ALS‐FTD spectrum who present with milder neurodegeneration.
Overall, recent endeavours to establish the CR hypothesis across the ALS‐FTSDs should be considered successful. We now know that a considerable subset of cognitive functions can be protected by CR cross‐sectionally, and that longitudinally, ALSci patients benefit strongly from high CR in their ALS‐specific letter fluency functions. Across other cognitive domains, further evidence is necessary to support the CR hypothesis.
Author Contributions
AGMT conceptualised the research question; chose the data analysis design; prepared, cleaned and analysed the data; interpreted the results; then wrote the original draft. EK conceptualised the study design, designed the neuropsychological battery, collected the data, interpreted the results; reviewed the draft. JM conceptualised the study design, designed the neuropsychological battery, collected the data, interpreted the results; reviewed the draft. SV conceptualised the study design; collected the neurological data; reviewed the draft. ST conceptualised the study design; reviewed the statistical analysis; reviewed the draft. AH conceptualised the research question; interpreted the results; acquired the Boris Canessa Foundation's funding; reviewed the draft. JP conceptualised the study design; conceptualised the research question; collected the neurological data; interpreted the results; acquired the DZNE's funding and André Greipel's support; reviewed the draft.
Conflicts of Interest
S. T. declares the following (all in Germany): MSD Sharp & Dohme GmbH, Lindenplatz 1, 85549 Haar; 11/09/2018: talk “Dementia and Diabetes–current report” at the quality circle for physicians in Kühlungsborn; 14/11/2018: MSD expert forum NAB Alzheimer in Munich, participant as consultant; 13/08/2019: talk “Dementia and Diabetes–current report” at the event “Dementia and Diabetes” in Rostock; ROCHE Pharma AG, Emil‐Barell‐Str. 1, 79639 Grenzach‐Wyhlen; 12/09/2019: 3rd National Advisory Board for ROCHE Pharma AG, Frankfurt (Main), participant as consultant; 27/09/2019: talk “Amyloid as target for diagnosis and treatment in Alzheimer's disease” at the ROCHE Symposium at the DGN Congress in Stuttgart; 09/2020: Advisory board Biogen, Biogen GmbH, Riedenburger Straße 7, 81677. Munich; 01/22/2021: online expert discussion “Mild Cognitive Impairment”, talk “MCI Epidemiology”, Dr. Willmar Schwabe GmbH & Co. KG, Willmar‐Schwabe‐Str. 4, 76227 Karlsruhe; 25/03/2021: virtual advisory board “National Alzheimer Advisory Board”, consultant, Roche Pharma AG, Emil‐Barell‐Str. 1, 79639 Grenzach‐Wyhlen. A.H. received. royalties from BIOGEN and DESITIN for advisory board meetings. A. H. has received honoraria from Biogen and Desitin as a consultant.
Acknowledgements
The authors would like to thank the patients and their families for their participation. They would further like to thank professional cyclist André Greipel and his “Fight ALS” initiative for the continued support of the “Cognition in ALS” working group at DZNE Rostock. A.G.M.T. is supported by the Boris Canessa Foundation. A.H. is supported by the Hermann and Lilly Schilling‐Stiftung für medizinische Forschung im Stifter[1]verband.
Annals of Clinical and Translational Neurology 2022;9(8): 1212–1223
Funding Information
Dr Anna G. M. Temp was supported by donations from professional cyclist André Greipel's “Fight ALS” Initiative and the Boris Canessa ALS Foundation. Professor Andreas Hermann is supported by the Hermann und Lilly Schilling‐Stiftung für medizinische Forschung im Stifterverband.
Funding Statement
This work was funded by Boris Canessa Foundation; Deutsches Zentrum für Neurodegenerative Erkrankungen grant RO010; Hermann und Lilly Schilling‐Stiftung für medizinische Forschung im Stifterverband ; André Greipel's “Fight ALS” Initiative.
References
- 1. Beswick E, Park E, Wong C, et al. A systematic review of neuropsychiatric and cognitive assessments used in clinical trials for amyotrophic lateral sclerosis. J Neurol. 2020;268:4510‐4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon PH, Delgadillo D, Piquard A, et al. The range and clinical impact of cognitive impairment in French patients with ALS: a cross‐sectional study of neuropsychological test performance. Amyotroph Lateral Scler. 2011;12:372‐378. [DOI] [PubMed] [Google Scholar]
- 3. Elamin M, Bede P, Montuschi A, Pender N, Chio A, Hardiman O. Predicting prognosis in amyotrophic lateral sclerosis: a simple algorithm. J Neurol. 2015;262:1447‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chio A, Vignola A, Mastro E, et al. Neurobehavioral symptoms in ALS are negatively related to caregivers' burden and quality of life. Eur J Neurol. 2010;17:1298‐1303. [DOI] [PubMed] [Google Scholar]
- 5. Temp AGM, Prudlo J, Vielhaber S, et al. Cognitive reserve and regional brain volume in amyotrophic lateral sclerosis. Cortex. 2021;139:240‐248. [DOI] [PubMed] [Google Scholar]
- 6. Costello E, Rooney J, Pinto‐Grau M, et al. Cognitive reserve in amyotrophic lateral sclerosis (ALS): a population‐based longitudinal study. J Neurol Neurosurg Psychiatry. 2021;92:460‐465. [DOI] [PubMed] [Google Scholar]
- 7. Consonni M, Dalla Bella E, Bersano E, Telesca A, Lauria G. Cognitive reserve is associated with altered clinical expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:237‐247. [DOI] [PubMed] [Google Scholar]
- 8. Canosa A, Palumbo F, Iazzolino B, et al. The interplay among education, brain metabolism, and cognitive impairment suggests a role of cognitive reserve in amyotrophic lateral sclerosis. Neurobiol Aging. 2021;98:205‐213. [DOI] [PubMed] [Google Scholar]
- 9. Stern Y, Arenaza‐Urquijo EM, Bartres‐Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16:1305‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasper E, Zydatiss K, Schuster C, et al. No change in executive performance in ALS patients: a longitudinal neuropsychological study. Neurodegener Dis. 2016;16:184‐191. [DOI] [PubMed] [Google Scholar]
- 12. Elamin M, Bede P, Byrne S, et al. Cognitive changes predict functional decline in ALS: a population‐based longitudinal study. Neurology. 2013;80:1590‐1597. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber H, Gaigalat T, Wiedemuth‐Catrinescu U, et al. Cognitive function in bulbar‐ and spinal‐onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J Neurol. 2005;252:772‐781. [DOI] [PubMed] [Google Scholar]
- 14. Agosta F, Ferraro PM, Riva N, et al. Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp. 2016;37:1614‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bede P, Iyer PM, Schuster C, et al. The selective anatomical vulnerability of ALS: ‘disease‐defining’ and ‘disease‐defying’ brain regions. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:561‐570. [DOI] [PubMed] [Google Scholar]
- 16. Omer T, Finegan E, Hutchinson S, et al. Neuroimaging patterns along the ALS‐FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:611‐623. [DOI] [PubMed] [Google Scholar]
- 17. Bede P, Hardiman O. Longitudinal structural changes in ALS: a three time‐point imaging study of white and gray matter degeneration. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:232‐241. [DOI] [PubMed] [Google Scholar]
- 18. van der Burgh HK, Westeneng HJ, Walhout R, et al. Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology. 2020;94:e2592‐e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron D . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293‐299. [DOI] [PubMed] [Google Scholar]
- 20. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis ‐ frontotemporal spectrum disorder (ALS‐FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machts J, Keute M, Kaufmann J, et al. Longitudinal clinical and neuroanatomical correlates of memory impairment in motor neuron disease. Neuroimage Clin. 2021;29:102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benbrika S, Doidy F, Carluer L, et al. Longitudinal study of cognitive and emotional alterations in amyotrophic lateral sclerosis: clinical and imaging data. Front Neurol. 2021;12:620198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria-2015. Amyotroph lateral scler frontotemporal degener. 2015;16:291‐292. [DOI] [PubMed] [Google Scholar]
- 25. Temp AGM, Dyrba M, Kasper E, Teipel S, Prudlo J. Case report: cognitive conversion in a non‐Brazilian VAPB mutation carrier (ALS8). Front Neurol. 2021;12:668772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Funke AD, Esser M, Kruttgen A, et al. The p.P56S mutation in the VAPB gene is not due to a single founder: the first European case. Clin Genet. 2010;77:302‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krüger S, Battke F, Sprecher A, et al. Rare variants in neurodegeneration associated genes revealed by targeted panel sequencing in a German ALS cohort. Front Mol Neurosci. 2016;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt K‐H, Metzler P. Wortschatztest. Hogrefe testzentrale; 1992. [Google Scholar]
- 29. Osmanovic A, Wieselmann G, Mix L, et al. Cognitive performance of patients with adult 5q‐spinal muscular atrophy and with amyotrophic lateral sclerosis. Brain Sci. 2020;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aschenbrenner S, Tucha O. Regensburger Wortflüssigkeits Test. Hogrefe Testzentrale; 2001. [Google Scholar]
- 31. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271‐276. [Google Scholar]
- 32. Helmstaedter C, Lendt M, Lux S. Verbaler Vern‐ und Merkfähigkeitstest Weinheim: Beltz Test. 2001.
- 33. Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grisé D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000;38:734‐747. [DOI] [PubMed] [Google Scholar]
- 34. Khng KH, Lee K. The relationship between Stroop and stop‐signal measures of inhibition in adolescents: influences from variations in context and measure estimation. PLoS One. 2014;9:e101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95‐113. [DOI] [PubMed] [Google Scholar]
- 36. Radiology; ACo . Phantom Test guidance for use of the large MRI phantom for the ACR MRI accreditation program. 2018.
- 37. Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, Abrahams S. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain. 2013;136:3290‐3304. [DOI] [PubMed] [Google Scholar]
- 38. Chenji S, Ishaque A, Mah D, et al. Neuroanatomical associations of the Edinburgh cognitive and behavioural ALS screen (ECAS). Brain Imaging Behav. 2021;15:1641‐1654. [DOI] [PubMed] [Google Scholar]
- 39. Goldstein LH, Newsom‐Davis IC, Bryant V, Brammer M, Leigh PN, Simmons A. Altered patterns of cortical activation in ALS patients during attention and cognitive response inhibition tasks. J Neurol. 2011;258:2186‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meier SL, Charleston AJ, Tippett LJ. Cognitive and behavioural deficits associated with the orbitomedial prefrontal cortex in amyotrophic lateral sclerosis. Brain. 2010;133:3444‐3457. [DOI] [PubMed] [Google Scholar]
- 41. Wagenmakers E‐J, Love J, Marsman M, et al. Bayesian inference for psychology. Part II: example applications with JASP. Psychon Bull Rev. 2018;25:58‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wagenmakers E‐J, Marsman M, Jamil T, et al. Bayesian inference for psychology. Part I: theoretical advantages and practical ramifications. Psychon Bull Rev. 2018;25:35‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. JASP (version 0.11.1) [computer program]. 2019.
- 44. Van Doorn J, Van den Bergh D, Böhm U, et al. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon Bull Rev. 2019;28:813‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abrahams S, Goldstein LH, Simmons A, et al. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507‐1517. [DOI] [PubMed] [Google Scholar]
- 46. Alladi S, Bak TH, Duggirala V, et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81:1938‐1944. [DOI] [PubMed] [Google Scholar]
- 47. Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life‐span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schuster C, Kasper E, Dyrba M, et al. Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35:240‐246. [DOI] [PubMed] [Google Scholar]
- 49. Shen D, Hou B, Xu Y, et al. Brain structural and perfusion signature of amyotrophic lateral sclerosis with varying levels of cognitive deficit. Front Neurol. 2018;9:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Temp AGM, Dyrba M, Büttner C, et al. Cognitive profiles of amyotrophic lateral sclerosis differ in resting‐state functional connectivity: an fMRI study. Front Neurosci. 2021;15:682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canosa A, Pagani M, Cistaro A, et al. 18F‐FDG‐PET correlates of cognitive impairment in ALS. Neurology. 2016;86:44‐49. [DOI] [PubMed] [Google Scholar]
- 52. Whiteside DM, Kealey T, Semla M, et al. Verbal fluency: language or executive function measure? Appl Neuropsychol Adult. 2016;23:29‐34. [DOI] [PubMed] [Google Scholar]
- 53. Poldrack RA, Yarkoni T. From brain maps to cognitive ontologies: informatics and the search for mental structure. Annu Rev Psychol. 2016;67:587‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eich T, Parker D, Gazes Y, Razlighi Q, Habeck C, Stern Y. Towards an ontology of cognitive processes and their neural substrates: a structural equation modeling approach. PLoS One. 2020;15:e0228167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dodich A, Carli G, Cerami C, Iannaccone S, Magnani G, Perani D. Social and cognitive control skills in long‐life occupation activities modulate the brain reserve in the behavioural variant of frontotemporal dementia. Cortex. 2018;99:311‐318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original MRI files are not publicly available due to confidentiality restraints. A comma separated values file containing the data underlying the analyses and figures is available at: https://osf.io/8ng3p/, accompanied by an HTML file containing our reported JASP output, and the JASP output when ALSci and ALSbi patients were analysed separately.


