Abstract
Hypertension guidelines recommend measuring blood pressure (BP) in both arms at least once. However, this is seldom done due to uncertainties regarding measurement procedure and the implications of finding a clinically important inter‐arm BP difference (IAD). This study aimed to provide insight into the prevalence of clinically important IADs in a large Indian primary care cohort.
A number of 134 678 (37% female) unselected Indian primary care participants, mean age 45.2 (SD 11.9) years, had BP measured in both arms using a standardized, triplicate, automated simultaneous measurement method (Microlife WatchBP Office Afib).
On average, there were clinically minor differences in right and left arm BP values: systolic BP 134.4 vs 134.2 mmHg (p < .01) and diastolic BP 82.7 vs 82.6 mmHg (p < .01), respectively.
Prevalence of significant mean systolic IAD between 10 and 15 mmHg was 7,813 (5.8%). Systolic IAD ≥ 15 mmHg 2,980 (2.2%) and diastolic IAD ≥ 10 mmHg 7,151 (5.3%). In total, there were 7,595 (5.6%) and 8,548 (6.3%) participants with BP above the 140/90 mmHg threshold in only the left or right arm, respectively. Prevalence of participants with elevated BP on one arm only was highest in patients with a systolic IAD ≥ 15 mmHg; 19.1% and 13.7%, for left and right arm, respectively.
This study shows that a substantial prevalence of IAD exists in Indian primary care patients. BP is above the diagnostic threshold for hypertension in one arm only for 6% of participants. These findings emphasize the importance of undertaking bilateral BP measurement in routine clinical practice.
Keywords: blood pressure, cardiovascular disease, hypertension, inter‐arm blood pressure difference
1. INTRODUCTION
Recent hypertension management guidelines for Europe, USA, UK, and Canada recommend blood pressure (BP) measurement in both arms when assessing a patient for hypertension at a first visit. 1 , 2 , 3 , 4 Inter‐arm differences (IAD) in BP have been associated with peripheral arterial disease and increased cardiovascular and all‐cause mortality. 5 , 6 Despite these recommendations, bilateral BP measurements are often not performed in routine clinical practice. 7 , 8 , 9 This may be due to uncertainties around appropriate methods of bilateral BP measurement and correct interpretation and management of an IAD. 10
There is some discrepancy between hypertension guidelines in different parts of the world with regard to what is considered as a clinically relevant IAD. UK National Institute of Health and Care Excellence (NICE) guidelines and European Society of Hypertension (ESH) guidelines recommend, based on findings from meta‐analyses, 5 , 10 that a systolic IAD ≥ 15 mmHg is ‘suggestive of atheromatous disease 11 ’ The recently published American College of Cardiology (ACC) guidelines suggest that clinicians should ‘verify that left/right inter‐arm differences are insignificant’ and, if an IAD is present, the higher reading arm should be used for subsequent BP readings. 1 The Canadian hypertension program specifies that a systolic IAD of 10 mmHg or more is significant. 12 Both the Indian and Japanese Society of Hypertension guidelines state that BP must be checked in both arms, without further specifications. 13
The measurement method for detecting an IAD is also unclear, despite guidelines highlighting its important role in hypertension measurement and ongoing management. 14 ESC guidelines suggest that IADs should be determined preferably by simultaneous, double‐arm BP measurement. This recommendation is based on the findings of some studies showing that sequential measurements can overestimate IAD compared with simultaneous BP measurements, risking unnecessary referrals and unnecessary procedures. 14 , 15 , 16 , 17 However, recent evidence has confirmed the prognostic value of sequentially measured arm BPs. 6
The purpose of this study was to 1) determine the prevalence of absolute IADs in BP in a large representative primary care cohort in India, 2) compare prevalence's of IAD with clinically significant IAD cut‐off values recommended in international BP guidelines and existing literature, and 3) determine the relationship between IADs and patient characteristics.
2. METHODS
2.1. Study design and participants
In this cross‐sectional study, participants were randomly selected patients from 2400 primary practice centers across India from January 2018 to February 2019. BP was measured by physician assistants, immediately prior to consultation with primary care physicians, after a minimum of 5 minutes of seated rest, with appropriately sized cuffs and both arms supported and positioned at the level of the heart. Both upper arms were measured simultaneously using a validated, automatic, electronic sphygmomanometer (Microlife WatchBP Office AFIB, Microlife AG, Switzerland). 18 , 19 , 20 , 21 This device incorporates a novel algorithm for BP measurement in AF to overcome some of the inherent inaccuracy in oscillometric measurement in atrial fibrillation (AF) 22 and also issues ‘alerts’ when potential AF is detected. Recommendations from current ESH guidelines for preparation and positioning for BP measurement were followed. 11 Using the device, three consecutive bilateral BP readings were automatically taken at 1‐minute intervals, and the mean of the three systolic and diastolic BPs were reported for both arms.
2.2. Patient data
After written informed consent was obtained, the following patient data were extracted from participant medical records: age, sex, alcohol use, lipid values, smoking status, and diagnosis of diabetes mellitus (Type 1 and 2) or hypertension. The study protocol was approved by Rippon Independent Ethics Committee (registration number: ECR/299/Indt/TN/2018/RR‐21), Chennai, Tamil Nadu, India.
2.3. Statistical analysis
Systolic and diastolic IAD were calculated as mean right arm minus mean left arm BP. Mean and standard deviation (SD) values of IAD (ie, right minus left arm difference) and absolute IAD values were calculated and reported with other baseline demographics. Subsequently, participant characteristics were compared according to predefined commonly cited cut‐off points of absolute systolic and diastolic inter‐arm differences, namely, 5, 10, and 15 mmHg. Proportions of participants with systolic BP higher on the right and on the left arms were calculated, and the prevalence of hypertension (defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg) in one arm, but normotension (< 140/90 mmHg) in the other arm, was determined.
An additional separate analysis was performed to compare patients' characteristics of patients who received an AF alert during BP measurement with those who did not receive such an alert.
Multivariable linear regressions were performed to explore association of absolute systolic and diastolic IADs. Candidate variables were pre‐specified as age, sex, hypertension, diabetes mellitus, highest SBP of both arms, highest DBP of both arms, heart rate, dyslipidemia, and AF alert. Results are presented as β coefficients and associated 95% confidence intervals (CI); statistical significance was accepted at p < .05.
Main demographic and clinical data were summarized according to systolic thresholds ≥ 5, 10, and 15 mmHg reporting means ± SD for continuous variables and the absolute (n) and relative (%) frequencies for categorical variables. Differences across groups were evaluated by analysis of variance or Chi‐square test, depending on the type of variable. Data analysis was performed using RStudio Version 1.2.5033 for Windows.
3. RESULTS
3.1. Demographics and prevalence of inter‐arm differences in blood pressure
The study enrolled 134 678 persons with a mean age of (standard deviation, SD) 45.2 (11.9) years; 49 757 (36.9%) were females (Table 1). Overall, both systolic BP (134.4 (16.3) vs 134.2 (16.2) mmHg; p < .01) and diastolic BP (82.7 (10.0) vs 82.6 (10.2) mmHg; p < .01) were marginally higher on the right arm compared to the left arm. Mean IAD (right minus left arm) was .2 (6.2) mmHg and .1 (5.4) mmHg for systolic and diastolic BP (Figure 1A and 1B). Approximately 36% of participants had a systolic IAD ≥ 5 mmHg and 8.0 % had a systolic IAD ≥ 10 mmHg. On average, absolute systolic and diastolic IAD were 4.4 (4.4) mmHg and 3.5 (4.1) mmHg, respectively. Comparison of participants grouped according to 5 mmHg increments of systolic IAD showed a trend towards rising age, systolic and diastolic BPs, rising heart‐rate, and rising prevalence of diabetes and hypertension (p < .001 for all comparisons). An IAD ≥ 5 mmHg was associated with more frequent AF alerts during blood pressure measurement, in comparison to those with a systolic IAD < 5 mmHg (Table 1).
TABLE 1.
Patient characteristics separated for systolic inter‐arm difference (IAD) cut‐off levels
| <5 mmHg (No. = 85 705) | 5‐10 mmHg (No. = 38 180) | 10‐15 mmHg (No. = 7813) | > = 15 (No. = 2980) | Total (No. = 134 678) | p value | |
|---|---|---|---|---|---|---|
| Gender | <.001 | |||||
| Female | 31 892 (37.2%) | 13 838 (36.2%) | 2841 (36.4%) | 1186 (39.8%) | 49 757 (36.9%) | |
| Male | 53 813 (62.8%) | 24 342 (63.8%) | 4972 (63.6%) | 1794 (60.2%) | 84 921 (63.1%) | |
| Age (yrs) | <.001 | |||||
| 44.7 (11.9) | 45.7 (11.8) | 47.2 (12.3) | 48.3 (13.2) | 45.2 (11.9) | ||
| SBP left (mmHg) | <.001 | |||||
| 132.7 (15.0) | 136.3 (17.0) | 138.8 (19.0) | 140.2 (22.6) | 134.2 (16.2) | ||
| SBP right (mmHg) | <.001 | |||||
| 133.0 (15.1) | 136.5 (16.8) | 138.1 (19.1) | 140.0 (26.2) | 134.4 (16.3) | ||
| DBP left (mmHg) | <.001 | |||||
| 81.9 (9.5) | 83.5 (10.1) | 84.8 (11.7) | 87.6 (18.0) | 82.6 (10.2) | ||
| DBP right (mmHg) | <.001 | |||||
| 82.1 (9.5) | 83.6 (10.0) | 84.1 (11.5) | 85.3 (17.3) | 82.7 (10.0) | ||
| Heart rate (BPM) | <.001 | |||||
| 82.1 (12.0) | 84.1 (12.3) | 84.6 (13.4) | 87.0 (14.2) | 82.9 (12.3) | ||
| IAD Absolute SBP (mmHg) | <.001 | |||||
| 2.2 (1.2) | 6.3 (1.3) | 11.2 (1.3) | 22.7 (12.5) | 4.4 (4.4) | ||
| IAD SBP (mmHg) | <.001 | |||||
| 0.2 (2.5) | 0.3 (6.4) | −0.6 (11.2) | −0.1 (25.9) | 0.2 (6.2) | ||
| IAD Absolute DBP (mmHg) | <.001 | |||||
| 2.7 (2.9) | 4.1 (3.5) | 6.1 (5.9) | 10.0 (14.2) | 3.5 (4.1) | ||
| IAD DBP (mmHg) | <.001 | |||||
| 0.3 (4.0) | 0.1 (5.4) | −0.7 (8.5) | −2.3 (17.2) | 0.1 (5.4) | ||
| IAD DBP (categorized) | <.001 | |||||
| < 10 mmHg | 83 439 (97.4%) | 36 015 (94.3%) | 6131 (78.5%) | 1942 (65.2%) | 127 527 (94.7%) | |
| ≥ 10 mmHg | 2266 (2.6%) | 2165 (5.7%) | 1682 (21.5%) | 1038 (34.8%) | 7151 (5.3%) | |
| Diagnosis based on both arms | <.001 | |||||
| normotension | 52 068 (60.8%) | 17 154 (44.9%) | 2549 (32.6%) | 776 (26.0%) | 72 547 (53.9%) | |
| left hypertension | 2954 (3.4%) | 2876 (7.5%) | 1196 (15.3%) | 569 (19.1%) | 7595 (5.6%) | |
| right hypertension | 3695 (4.3%) | 3399 (8.9%) | 1046 (13.4%) | 408 (13.7%) | 8548 (6.3%) | |
| hypertension both arms | 26 988 (31.5%) | 14 751 (38.6%) | 3022 (38.7%) | 1227 (41.2%) | 45 988 (34.1%) | |
| Arm with higher SBP | <.001 | |||||
| identical | 6420 (7.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6420 (4.8%) | |
| left higher | 34 836 (40.6%) | 18 387 (48.2%) | 4132 (52.9%) | 1564 (52.5%) | 58 919 (43.7%) | |
| right higher | 44 449 (51.9%) | 19 793 (51.8%) | 3681 (47.1%) | 1416 (47.5%) | 69 339 (51.5%) | |
| Smoking | <.001 | |||||
| No | 80 765 (94.2%) | 35 550 (93.1%) | 7306 (93.5%) | 2846 (95.5%) | 126 467 (93.9%) | |
| Yes | 4940 (5.8%) | 2630 (6.9%) | 507 (6.5%) | 134 (4.5%) | 8211 (6.1%) | |
| Alcohol | <.001 | |||||
| No | 81 032 (94.5%) | 35 466 (92.9%) | 7088 (90.7%) | 2781 (93.3%) | 126 367 (93.8%) | |
| Yes | 4673 (5.5%) | 2714 (7.1%) | 725 (9.3%) | 199 (6.7%) | 8311 (6.2%) | |
| Diabetes | <.001 | |||||
| No | 75 634 (88.2%) | 32 792 (85.9%) | 6753 (86.4%) | 2533 (85.0%) | 117 712 (87.4%) | |
| Yes | 10 071 (11.8%) | 5388 (14.1%) | 1060 (13.6%) | 447 (15.0%) | 16 966 (12.6%) | |
| Dyslipidemia | .269 | |||||
| No | 85 103 (99.3%) | 37 902 (99.3%) | 7772 (99.5%) | 2960 (99.3%) | 133 737 (99.3%) | |
| Yes | 602 (0.7%) | 278 (0.7%) | 41 (0.5%) | 20 (0.7%) | 941 (0.7%) | |
| AF alert | <.001 | |||||
| No | 84 956 (99.1%) | 36 878 (96.6%) | 7602 (97.3%) | 2918 (97.9%) | 132 354 (98.3%) | |
| Yes | 749 (0.9%) | 1302 (3.4%) | 211 (2.7%) | 62 (2.1%) | 2324 (1.7%) |
FIGURE 1.
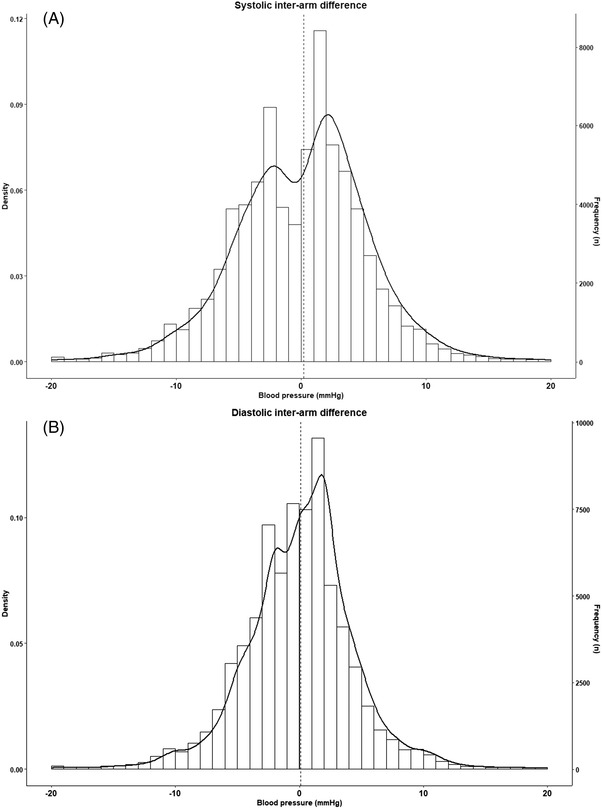
Histogram of systolic (A) and diastolic (B) inter‐arm difference. Interarm difference was calculated as right‐arm blood pressure minus left‐arm blood pressure
In total, there were 16 143 (11.9%) patients who had elevated BP (ie, > 140/90 mmHg) on one arm only; 8548 (6.3%) patients had right arm hypertension and 7595 (5.6%) had left arm hypertension. These patients had mean BP values close to the threshold values of 140/90 mmHg (Supplementary table 1).
3.2. Multivariable modeling
Multivariable analyses included all 134 678 participants with complete data in the final model. Factors positively associated with absolute systolic IAD were female sex, highest SBP and highest DBP, heart rate, alcohol use, and having an AF alert. Factors negatively associated with systolic IAD were male sex, smoking, diabetes, and dyslipidemia (Table 2). For absolute diastolic IAD, the model was similar apart from highest SBP and heart rate, which were negatively instead of positively associated with it. Adjusted R2 for absolute systolic and diastolic IAD were .070 and .114, respectively.
TABLE 2.
Factors associated with inter‐arm difference in multivariate models
| Systolic Absolute | Systolic | Diastolic absolute | Diastolic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | B | 95% CIs | p‐values | B | 95% CIs | p‐values | B | 95% CIs | p‐values | B | 95% CIs | p‐values |
| (Intercept) | −6.29 | −6.52 – −6.05 | <.001 | −1.15 | −1.50 – −0.80 | <.001 | −6.49 | −6.71 – −6.27 | <.001 | 2.84 | 2.53 – 3.14 | <.001 |
| Gender (Male) | −0.25 | −0.30 – −0.20 | <.001 | −0.04 | −0.11 – 0.03 | .259 | −0.30 | −0.34 – −0.25 | <.001 | 0.15 | 0.09 – 0.21 | <.001 |
| Age (Years) | −0.00 | −0.01 – −0.00 | <.001 | −0.01 | −0.02 – −0.01 | <.001 | 0.01 | 0.01 – 0.01 | <.001 | −0.01 | −0.01 – −0.00 | <.001 |
| Highest SBP (mmHg) | 0.06 | 0.06 – 0.06 | <.001 | −0.00 | −0.01 – −0.00 | .024 | −0.02 | −0.02 – −0.02 | <.001 | 0.01 | 0.01 – 0.01 | <.001 |
| Highest DBP (mmHg) | 0.02 | 0.02 – 0.02 | <.001 | 0.03 | 0.03 – 0.03 | <.001 | 0.16 | 0.15 – 0.16 | <.001 | −0.05 | −0.05 – −0.04 | <.001 |
| Heart rate (bpm) | 0.02 | 0.01 – 0.02 | <.001 | −0.00 | −0.00 – 0.00 | .385 | −0.01 | −0.01 – −0.00 | <.001 | −0.00 | −0.00 – 0.00 | .061 |
| Smoking (Yes) | −0.31 | −0.42 – −0.20 | <.001 | 0.04 | −0.13 – 0.21 | .650 | −0.25 | −0.36 – −0.15 | <.001 | −0.02 | −0.17 – 0.13 | .792 |
| Diabetes (Yes) | −0.12 | −0.19 – −0.05 | .001 | −0.15 | −0.25 – −0.05 | .004 | −0.22 | −0.29 – −0.16 | <.001 | −0.09 | −0.18 – −0.00 | .042 |
| Alcohol (Yes) | 0.60 | 0.49 – 0.71 | <.001 | −0.44 | −0.60 – −0.27 | <.001 | 0.43 | 0.32 – 0.53 | <.001 | 0.02 | −0.12 – 0.17 | .779 |
| Dyslipidemia (Yes) | −0.51 | −0.78 – −0.24 | <.001 | 0.35 | −0.05 – 0.74 | .087 | −0.32 | −0.58 – −0.07 | .012 | 0.44 | 0.09 – 0.78 | .014 |
| AF alert (Yes) | 1.27 | 1.10 – 1.45 | <.001 | −2.30 | −2.55 – −2.04 | <.001 | 0.83 | 0.67 – 1.00 | <.001 | −1.48 | −1.71 – −1.26 | <.001 |
| Observations | 134 678 | 134 678 | 134 678 | 134 678 | ||||||||
| R 2 / R2 adjusted | 0.071 / 0.070 | 0.005 / 0.005 | 0.114 / 0.114 | 0.007 / 0.007 | ||||||||
Additional analysis confirmed the positive association of a monitor generated AF alert with IAD: Patients who received an AF alert (n = 2234 [1.7%]) had significantly higher IAD values than those without such an alert for both systolic (6.2 ± 3.9 vs 4.4 ± 4.4) and diastolic (4.7 ± 3.5 vs 3.4 ± 4.0) BP, respectively. Patients with an AF alert were older, had higher BP and were relatively more often male. Half the patients with an AF alert showed an IAD value between 5 and 10 mmHg as compared to 20% for the patients without such an alert. From the latter group 75% had an IAD less than 5 mmHg as compared to only 20% of the group without an AF alert. However, due to the low prevalence of patients with an AF alert in the present study, this group had no significant influence on IAD values and/ or category prevalence values of the total population (Supplementary table 2).
4. DISCUSSION
4.1. Summary of main findings
This large cohort study demonstrates the importance of measuring BP in both arms; a systolic IAD ≥ 10 mmHg was found in more than 8% of persons. In total, 11.9% of participants had a BP > 140/90 mmHg in one arm but not in the other, suggesting that 6% of the cohort could be denied referral for further investigation to diagnose hypertension against this threshold if BP was only measured in one arm. Prevalence of recognized cardiovascular risk factors such as age, baseline BP, diabetes mellitus, and hypertension rose according to magnitude of systolic and diastolic IADs. A systolic IAD ≥ 5 mmHg was also associated with increased prevalence of BP monitor generated AF alerts independent of BP level.
4.2. Strengths and limitations of the approach
To our knowledge, this is the largest cohort study published to date examining the prevalence and associations of an IAD in BP. There is uncertainty as to any ethnic influence on magnitude of IADs. 17 Although many previous studies have reported IAD prevalence estimates we have found relatively little previous data from the Indian subcontinent. 23 , 24 , 25
Due to the observational design of the study, and practical limitations due to the cohort size, only a minimal amount of data was collected per patient. Therefore, the present study could only explore the relationship of IAD with a limited number of cardiovascular risk factors, and estimation of cardiovascular risk using recognized approaches such as the Framingham score and investigating the relation of IAD to hypertension‐mediated organ damage was not possible. 26 Although the WatchBP Office device collects separate BP values for each of the three automated pairs of measurements, for practical reasons, only the mean BP values were extracted for research. Therefore, analysis of the impact of BP variability on IAD was not possible. This also led to the fact that the present data did not allow to verify how many persons had consistent IAD of more than 10 or 20 mmHg as is recommended by the 2021 European Society of Hypertension guidelines. 27 Nevertheless, we are of the opinion that the average BP values provide a reliable indication of the true IAD because it is based on the average of three automated BP measurements. Recent evidence showed that concordance of the higher BP arm‐side was 63% overall, rising to 73% and 92% when the mean IAD was equal to or higher than 5 and 10 mmHg, respectively. This confirms consistency of IADs, especially where the average IAD was equal to or higher than 10 mmHg. 28 In addition, Clark and associates found in their meta‐analysis that an IAD of 10 mmHg or more was associated with peripheral vascular disease. This finding was based on 20 studies of which the majority did not use the consistency method. 5 ECG confirmation of AF was not obtained when an AF alert was reported by the Watch BP Office device. Previous reports have shown high specificity but variable sensitivity of the device for AF confirmed by ECG. 29 , 30 Consequently, the associations of IAD with AF alerts are of interest but we are careful with drawing conclusions on the associations of AF with IAD.
4.3. Relationship to existing literature
In the Indian literature, one community‐based cross‐sectional study carried out among 1634 adults in Anakaputhur, an urban area in Kancheepuram district of Tamil Nadu, reported much higher prevalences of systolic and diastolic IAD ≥ 10 mmHg (43.5% and 20.3%, respectively), and one other smaller study of 100 healthy medical students found that 29% had a systolic IAD ≥ 10 mmHg. 23 , 24 Both studies used a sequential manual measurement method. The lower prevalences reported in the current study are consistent with previous findings from our groups and others that observed IADs at any cut‐off are less prevalent when simultaneous rather than sequential measurement methods are used, when automated devices are used in place of manual assessment, and when measures are repeated. 14 , 17 , 31 , 32
The Microlife WatchBP Office device has been used in previous cohort studies finding comparable prevalences for a systolic IAD ≥ 10 mmHg of 9.1% (Denmark) and 10% (Germany) but 18% in an older age population in the Netherlands. 15 , 16 , 33 One other community study from Italy found 16.8% of participants had an IAD ≥ 10 mmHg for either systolic or diastolic BP. 34 The range of these previous reports is in line with the systolic IAD prevalence ≥ 10 mmHg of 8.1% found in the present study.
Clinical evidence has shown that a BP difference of 10 mmHg or more between arms identifies patients who need further vascular assessment. 5 However, this was mainly based on IAD taken sequentially, whereas simultaneous blood pressure measurement generally leads to lower IAD values. 14 , 15 Therefore, a systolic IAD as low as 5 mmHg may indicate additional cardiovascular risk, which rises with magnitude of the IAD. 6 Thus, measuring both arms could reveal clinically important information for about a third of people in this cohort.
Higher systolic IADs are strongly and consistently associated with presence of peripheral arterial disease and with cardiovascular risks and events. 5 , 6 , 35
Despite the fact that most guidelines recommend the performance of double arm BP measurement little is known about the reproducibility of IAD on different occasions. Regarding the method used in the present study, Krogager and associates previously investigated IAD reproducibility using the same device by performing two successive sets of three individual measurements. The authors concluded that the method as was also used in the present study is acceptable for evaluating IAD of more than 10 mmHg and that an extra measurement session does little to improve detection of more than 10 mmHg. 33 Although this study suggests that IAD is reproducible, it must be considered that two measurement sessions were performed shortly after each other.
4.4. Implications for clinical practice
International hypertension guidelines have recommended checking both arms as part of a BP assessment for more than 80 years. 36 However, this is not consistently undertaken in current practice. We found, in 2017, that 52% of UK practises reported measuring BP in both arms when considering a diagnosis of hypertension, but a quarter of GPs surveyed would not adopt the higher reading arm BP for diagnosis and management. 8 Meanwhile, in an online survey of 743 UK patients, only 88 (11.8%) recalled ever having their BP measured in both arms at any previous appointment, although this was more likely for those with hypertension (16.5%) than those without (6.7%). 9 Similarly, in the USA only 10% of first year and 31% of second to fourth year medical students checked BP in both arms during a clinical skill assessment. 37
The mean systolic and diastolic IADs were close to zero, and too small to be clinically meaningful differences, with near equal proportions overall having a higher BP on the right or on the left arm. It has previously been suggested that higher BP in one arm might be related to hand‐dominance and associated greater muscle mass in the upper‐arm of the dominant arm. There is some evidence for abolition of previously observed mean differences in favor of the right arm being about 1 mmHg higher than the left when handedness is considered. 34 , 38 Anatomy of the cardiovascular system may also play a part and subclavian stenosis occurs more often on the left side than the right. 39 , 40 , 41 The explanation, in the absence of overt peripheral artery disease, is likely to be multifactorial. The current data reinforce the point that no clear prediction of which arm is higher can be made, therefore both arms need to be measured during assessment of BP. Failure to do so risks underdiagnosing and therefore under‐treating arterial hypertension.
Like peripheral artery disease and cardiovascular disease, age is also a strong predictor of rising prevalence of IAD. 6 , 10 , 42 , 43 The present study showed a similar pattern with systolic and diastolic IADs ≥ 10 mmHg and ≥ 15 mmHg increasing with each decade in life.
Prevalence of smoking and dyslipidemia in this cohort was low (6.1% and .7% respectively), therefore we are cautious in interpreting the apparent inverse relationship of these variables with IAD in our models. We recently found a positive hazard for smoking in our individual participant data modeling of all‐cause mortality taking account of IAD, although total cholesterol appeared to have a negative relationship. 6 , 43
The presence of AF is associated with peripheral artery disease. 44 A systolic IAD ≥ 15 mmHg has previously been labelled subclavian stenosis, and it indicates a 2.5 times risk of presence of peripheral artery disease in comparison with lower IADs. 5 , 45 In this study, the presence of a monitor generated AF alert was significantly and independently associated with a higher IAD value. In addition, a more than 2 times greater chance of an AF alert was observed with an IAD ≥ 15 mmHg than with an IAD < 5 mmHg. One might think that the higher IAD values in patients with a monitor generated AF alert were caused by difficulties related to BP measurement in AF in general and, especially when using an oscillometric BP device. 46 However, it may be reasonably expected that this effect was minimal for this study because the BP monitor used is designed to accurately measure BP in AF patients 22 and IAD values were averaged from three BP readings. Finally, the presence of AF is related to higher BP variability which may explain the higher IAD values in patients with AF. 47
AF was not confirmed following alerts in this study, however the implications of an IAD for checking further for vascular disease are supported by our findings.
5. CONCLUSION
The current paper presents the largest clinical study performed thus far to investigate IAD using a simultaneous BP measurement method. More than 8% of all persons have a significant systolic IAD ≥ 10 mmHg and more than 2% of all patients have a significant systolic IAD ≥15 mmHg. This may indicate increased cardiovascular risk and, therefore justifies further clinical investigation. The prevalence of IAD increases with age. Large IADs are associated with a higher frequency of device generated AF alerts, independent of BP. Double‐arm BP measurement should be undertaken during, at least, initial BP assessment. In the present study, almost 12% of all patients who were normotensive in one arm were hypertensive in the other, so up to 6% may be classified as normotensive if both arms are not taken into account during assessment for hypertension. Overall, the right arm showed slightly higher BP than the left. However, within the group having a systolic IAD ≥ 15 mmHg, over 50% of the persons had higher left‐arm BP. As this systolic IAD ≥15 mmHg group consisted of a quarter of normotensive persons, more research is needed to investigate the reproducibility of this outcome and/or the cardiovascular risk of these persons.
CONFLICTS OF INTEREST
CEC has previously received a Microlife Watch BP Office from Microlife for unrestricted evaluation outside of the current study. He has received honoraria from Bayer AG (for unrelated work) and ReCor Medical. No company has had, or will have, any involvement in the design, conduct, or reporting of this study.
WJV is an employee of Microlife corporation
NG is an employee of Eris Lifesciences
Supporting information
Supplementary information
Supplementary information
ACKNOWLEDGEMENTS
STJMcD's position at the University of Exeter is partially supported by a National Institute for Health Research (NIHR) School for Primary Care Research Fellowship. STJMcD is also supported by the British Heart Foundation Hope for Hearts fund and a NIHR Programme Development Grant (NIHR202040). CEC is funded by the NIHR School for Primary Care Research (SPCR) (Grant Ref: 512).
Wander GS, McDonagh STJ, Rao MS, et al. Clinical relevance of double‐arm blood pressure measurement and prevalence of clinically important inter‐arm blood pressure differences in Indian Primary Care. J Clin Hypertens. 2022;24:993–1002. 10.1111/jch.14497
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the american College of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):2199‐2269. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the european society of hypertension and the european society of cardiology: eSH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36(12):2284‐2309. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence . Hypertension in adults: diagnosis and management (NG 136). 28/8/19 ed. 2019. [Google Scholar]
- 4. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34(5):506‐525. [DOI] [PubMed] [Google Scholar]
- 5. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta‐analysis. Lancet. 2012;379(9819):905‐914. [DOI] [PubMed] [Google Scholar]
- 6. Clark CE, Warren FC, Boddy K, et al. Associations between systolic interarm differences in blood pressure and cardiovascular disease outcomes and mortality: individual participant data meta‐analysis, development and validation of a prognostic algorithm: the INTERPRESS‐IPD collaboration. Hypertension. 2021;77(2):650‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heneghan C, Perera R, Mant D, Glasziou P. Hypertension guideline recommendations in general practice: awareness, agreement, adoption, and adherence. Br J Gen Pract. 2007;57(545):948‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mejzner N, Clark CE, Smith LF, Campbell JL. Trends in the diagnosis and management of hypertension: repeated primary care survey in south west england. Br J Gen Pract. 2017;67(658):e306‐e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens SL, McManus RJ, Stevens RJ. Current practice of usual clinic blood pressure measurement in people with and without diabetes: a survey and prospective ‘mystery shopper’ study in UK primary care. BMJ Open. 2018;8(4):e020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomiyama H, Inoguchi T, Munakata M, et al. Simultaneously measured interarm blood pressure difference and stroke: an individual participants data meta‐analysis. Hypertension. 2018;71(6):1030‐1038. [DOI] [PubMed] [Google Scholar]
- 11. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the european society of hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the european society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 12. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension canada's 2016 canadian hypertension education program guidelines for blood pressure Measurement, Diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569‐588. [DOI] [PubMed] [Google Scholar]
- 13. Shimamoto K, Ando K, Fujita T, et al. The japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253‐390. [DOI] [PubMed] [Google Scholar]
- 14. Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter‐arm differences: a meta‐analysis. Am J Hypertens. 2011;24(11):1201‐1208. [DOI] [PubMed] [Google Scholar]
- 15. Lohmann FW, Eckert S, Verberk WJ. Interarm differences in blood pressure should be determined by measuring both arms simultaneously with an automatic oscillometric device. Blood Press Monit. 2011;16(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 16. van der Hoeven NV, Lodestijn S, Nanninga S, van Montfrans GA, van den Born BJ. Simultaneous compared with sequential blood pressure measurement results in smaller inter‐arm blood pressure differences. J Clin Hypertens (Greenwich). 2013;15(11):839‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark C, Taylor R, Shore A, Campbell J. Prevalence of systolic inter‐arm differences in blood pressure varies for different primary care populations: systematic review and meta‐analysis. Br J Gen Pract. 2016;66(11):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palatini P, Fania C, Gasparotti F. Accuracy of the WatchBP office ABI device for office blood pressure measurement over a wide range of arm sizes. Blood Press Monit. 2018;23(2):117‐119. [DOI] [PubMed] [Google Scholar]
- 19. Saladini F, Benetti E, Masiero S, Palatini P. Accuracy of microlife WatchBP office ABI monitor assessed according to the 2002 european society of hypertension protocol and the british hypertension society protocol. Blood Press Monit. 2011;16(5):258‐261. [DOI] [PubMed] [Google Scholar]
- 20. Stergiou GS, Lin CW, Lin CM, et al. Automated device that complies with current guidelines for office blood pressure measurement: design and pilot application study of the microlife WatchBP OFFICE device. Blood Press Monit. 2008;13(4):231‐235. [DOI] [PubMed] [Google Scholar]
- 21. Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the microlife watch BP office professional device for office blood pressure measurement according to the international protocol. Blood Press Monit. 2008;13(5):299‐303. [DOI] [PubMed] [Google Scholar]
- 22. Stergiou GS, Kyriakoulis KG, Bountzona I, et al. Automated blood pressure measurement in atrial fibrillation: validation process modification and evaluation of a novel professional device which detects atrial fibrillation and adapts its blood pressure measurement algorithm. J Hypertens. 2021;39(4):614‐620. [DOI] [PubMed] [Google Scholar]
- 23. Gopalakrishnan S, Savitha A, Rama R. Evaluation of inter‐arm difference in blood pressure as predictor of vascular diseases among urban adults in kancheepuram district of tamil nadu. J Family Med Prim Care. 2018;7(1):142‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma N, Raju S, Kumar P, Kumai R, Bhardwaj K. Inter arm systolic blood pressure difference is associated with a high prevalence of cardio vascular diseases. Int J Res Med Sci. 2016;4(4):1177‐1180. [Google Scholar]
- 25. Sadasivam K, Sundari M, Bhavsar NR, Ramraj B, Sampath A, Saravanan A. Relationship between inter‐arm blood pressure difference (IAD) and severity of coronary artery disease. Ann Trop Med & Public Health. 2020;23(19). [Google Scholar]
- 26. D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation. 2008;117(6):743‐753. [DOI] [PubMed] [Google Scholar]
- 27. Stergiou GS, Palatini P, Parati G, et al. 2021 European society of hypertension practice guidelines for office and out‐of‐office blood pressure measurement. J Hypertens. 2021;39(7):1293‐1302. [DOI] [PubMed] [Google Scholar]
- 28. Clark CWF, Boddy K, McDonagh S, Moore S, Cloutier L. Which arm to measure blood pressure: does the higher or lower reading arm best reflect systolic blood pressure? An individual participant data meta‐analysis from the INTERPRESS‐IPD collaboration; 2021 annual scientific meeting of the british and irish hypertension society (BIHS). J Hum Hypertens. 2021:1‐19. [Google Scholar]
- 29. Omboni S, Verberk WJ. Opportunistic screening of atrial fibrillation by automatic blood pressure measurement in the community. BMJ Open. 2016;6(4):e010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kearley K, Selwood M, Van den Bruel A, et al. Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single‐lead ECG and modified BP monitors. BMJ Open. 2014;4(5):e004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz CL, Clark C, Koshiaris C, et al. Interarm difference in systolic blood pressure in different ethnic groups and relationship to the “White Coat Effect”: a cross‐sectional study. Am J Hypertens. 2017;30(9):884‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnett DK, Tang W, Province MA, et al. Interarm differences in seated systolic and diastolic blood pressure: the hypertension genetic epidemiology network study. J Hypertens. 2005;23(6):1141‐1147. [DOI] [PubMed] [Google Scholar]
- 33. Krogager C, Laugesen E, Rossen NB, Poulsen PL, Erlandsen M, Hansen KW. Evaluation of interarm blood pressure differences using the microlife WatchBP office in a clinical setting. Blood Press Monit. 2017;22(3):161‐165. [DOI] [PubMed] [Google Scholar]
- 34. Omboni S, Verberk WJ. Simultaneous double arm automated blood pressure measurement for the screening of subjects with potential vascular disease: a community study. Blood Press. 2019;28(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 35. Clark CE, Taylor RS, Shore AC, Campbell JL. The difference in blood pressure readings between arms and survival: primary care cohort study. BMJ. 2012;344:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Heart A, Cardiac Society of Great BI , Committee of standardisation of blood pressure readings. standard methods for taking blood pressure readings. JAMA 1939;113:294. [Google Scholar]
- 37. Rakotz MK, Townsend RR, Yang J, et al. Medical students and measuring blood pressure: results from the american medical association blood pressure check challenge. J Clin Hypertens (Greenwich). 2017;19(6):614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joshi S, Clark C, Campbell J. What is the normal inter‐arm difference? It depends on left or right handedness: systematic review and meta‐analysis. J Hum Hypertens. 2016;30:651‐652. [Google Scholar]
- 39. Amsterdam B, Amsterdam AL. Disparity in blood pressure in both arms in normals and hypertensives and its clinical significance. NY State J Med. 1943;43:6. [Google Scholar]
- 40. Caesar‐Peterson SQE, Subclavian Artery Stenosis. [Updated 2019 Feb 13] In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2019 Jan‐ Available from: https://wwwncbinlmnihgov/books/NBK470221/ [PubMed]
- 41. Ochoa VM, Yeghiazarians Y. Subclavian artery stenosis: a review for the vascular medicine practitioner. Vasc Med. 2011;16(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 42. Savji N, Rockman CB, Skolnick AH, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. 2013;61(16):1736‐1743. [DOI] [PubMed] [Google Scholar]
- 43. Clark C, Warren F, Boddy K, et al. Inter‐arm blood pressure difference: insights into aetiology from the INTERPRESS‐IPD collaboration. J Hypertens. 2019;37:e34. [Google Scholar]
- 44. Proietti M, Farcomeni A. Association between peripheral artery disease and incident risk of atrial fibrillation: strong evidence coming from population‐based cohort studies. J Am Heart Assoc. 2018;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aboyans V, Criqui MH, McDermott MM, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007;49(14):1540‐1545. [DOI] [PubMed] [Google Scholar]
- 46. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62(3):579‐584. [DOI] [PubMed] [Google Scholar]
- 47. Olbers J, Gille A, Ljungman P, Rosenqvist M, Ostergren J, Witt N. High beat‐to‐beat blood pressure variability in atrial fibrillation compared to sinus rhythm. Blood Press. 2018;27(5):249‐255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information


